Abstract
Background
Lifetime occurrence of intimate partner violence (IPV) in women has been associated with increased prevalence of aging-related chronic diseases, including those with a pathophysiology involving inflammation. To begin to identify potential biologic mediators of this relationship, this cross-sectional study examined associations between past IPV and circulating levels of C-reactive protein (CRP) and interleukin-6 (IL-6)—measures linked with emergence of aging-related diseases—along with in vitro IL-6 production by peripheral blood mononuclear cells (PBMC) stimulated with either phytohemagglutinin A (PHA) or lipopolysaccharide (LPS).
Methods
Apparently healthy, midlife women with divorce histories were recruited from the community. Histories of intimate partner psychological aggression, physical assault, sexual coercion, and stalking were assessed, along with current depression, posttraumatic stress symptoms, and health-related characteristics. At two visits, blood was drawn for assessment of biologic measures; measures were averaged across visits.
Results
In this sample (n=68), a history of being stalked was significantly positively correlated with CRP levels; in a multiple regression analysis that included body mass index (BMI) and current symptoms, this association was attenuated by adjusting for BMI. Physical assault history was significantly negatively correlated with PHA-stimulated IL-6 production. This was most apparent for severe assault and was not accounted for by BMI or symptoms.
Conclusions
IPV histories remitted for an average of 10 years were associated with biologic mediators of inflammation. The profile was not uniformly proinflammatory, suggesting that in situations of traumatic or chronic stress, different aspects of the inflammatory response are differentially regulated and subjected to diverse compensatory mechanisms.
Introduction
Intimate partner violence (IPV), as defined by the Centers for Disease Control and Prevention (CDC), involves physical or sexual violence or threat of such violence between current or former spouses or intimate partners. Acts of psychological aggression and emotional maltreatment also comprise IPV, particularly when there has been a history of threats or physical or sexual aggression in a relationship.1 Women's lifetime IPV rates sampled from 10 nations range from 15% (Japan) to 71% (Ethiopia), with a rate of 25% in the United States.2,3 Compared with other examples of interpersonal victimization, such as a physical assault by a stranger, the assaultive and aggressive acts in situations of IPV can sometimes occur repeatedly over years. These acts may cause injury, fear, and perceived life threat, the elements of traumatic stress. Further, between occurrences of discrete aggressive incidents, a context of fear may persist. Thus, as a stressor, IPV can be both chronic and traumatic.4 In data from a nationally representative U.S. sample, for example, two thirds of women physically assaulted by an intimate partner reported an average of 6.9 assaultive acts over 4.5 years.3 In a survey of female enrollees of a U.S. health maintenance organization, nearly 50% reported ≥6 years of psychological aggression.5
Two population-based, cross-sectional studies reveal the significance of IPV for women's health. Lifetime IPV occurrence predicted elevated prevalence of aging-related chronic medical conditions, including conditions where the pathophysiology involves inflammation (e.g., heart disease, stroke, cancer, joint disease). These associations held after adjusting for demographic and behavioral risks6 and after establishing the temporal precedence of IPV by excluding women for whom disease onset preceded IPV.7
Compared to other significant life stressors (e.g., caregiving for a relative with dementia), there have been few efforts to identify biologic mediators connecting IPV and poorer health. One exception focused on stimulated in vitro production of the cytokine interferon-γ (IFN-γ), an early mediator of the immunologic process that promotes inflammation.8 IFN-γ production was significantly greater in a group comprising women who reported either past or present partner violence, compared to women reporting no abuse. This association was fully mediated by current posttraumatic stress disorder (PTSD) symptoms that were largely comorbid with depression. The present study approaches this problem by focusing exclusively on women with past IPV and by considering the biologic measures C-reactive protein (CRP) and interleukin-6 (IL-6).
Although neither CRP nor IL-6 has been examined within the context of IPV, both could plausibly contribute to its association with multiple chronic conditions. CRP and IL-6 are biologically linked, but they have distinct functions. CRP is classified as an acute-phase protein; it assists in the recognition and elimination of pathogens and damaged cells9 and is widely used to index systemic inflammation. IL-6 is a cytokine, a protein that facilitates communication between cells. It stimulates CRP synthesis and is, therefore, generally considered proinflammatory, but it also has anti-inflammatory properties.10,11 Although there are exceptions,12 some prospective studies of apparently healthy persons reveal that elevated IL-6 and CRP circulating levels predict emergence of multiple aging-related chronic conditions and mortality.13–15 Circulating CRP and IL-6 levels are also stress reactive. For example, CRP and IL-6 elevations have been shown to accompany ongoing chronic stressors, such as fear of terrorism16 and caregiving for a relative with dementia,17 although again there are exceptions.18,19 The stress-reactive quality of IL-6 and CRP is consistent with data showing that key biologic stress mediators, glucocorticoids and catecholamines, influence cytokine production.20 Despite having some shared correlates and despite being biologically linked, IL-6 and CRP sometimes independently predict health risk,21 and other times they show divergent associations with both health outcomes22 and stress.19
Especially pertinent to the present study is evidence that chronic stress, even after it has remitted, is associated with accelerated age-related increases in circulating IL-6.17 Despite daunting obstacles, many women leave violent relationships. Paradoxically, violence sometimes continues or escalates after separation.3,23 This threat intensification, combined with the possibility of enduring biologic consequences of remitted chronic stress, underscores the relevance of research with women who have experienced past IPV.
Accordingly, the present study evaluated CRP and IL-6 levels in women with histories of divorce or separation. All women, midlife and postmenopausal, were at a biologic transition characterized by increased risk for aging-related chronic diseases. A prior report on this sample focused on relations among circulating inflammatory mediators from multiple biologic fluids.24 CRP levels were higher among women with histories of IPV (defined as one or more severe instances of physical assault or sexual coercion) compared to those without. The present article extends this prior report in three ways.
First, specific IPV types—physical assault, sexual coercion, stalking, and psychological aggression—are the focus here. The impetus for this is the multidimensional nature of IPV and evidence from preclinical research for stressor-specific biologic correlates.25 This exploratory aim is designed to move beyond a global, dichotomous measure of IPV and generate new information about IPV types potentially most relevant for biologic mediators of inflammation.
Second, in addition to CRP and IL-6 circulating levels, which are products of multiple cell types, this article reports on in vitro IL-6 production by stimulated peripheral blood mononuclear cells (PBMCs), a model of the capacity of immune system cells to produce IL-6 when challenged. To our knowledge, this has not been evaluated in the context of remitted chronic stress, but ongoing interpersonal stressors persistent for 1–6 months show positive associations with stimulated IL-6 production from PBMCs or whole blood.18,26
Third, this article evaluates whether relations between past IPV and biologic measures are accounted for by current symptoms of depression and PTSD. Symptoms of both disorders have been associated with elevations in stimulated IL-6 production and IL-6 and CRP circulating levels in some studies.27–30 Despite this and despite the fact that such symptoms arise in the context of life stressors, very few studies of IL-6 and CRP have addressed stressors and symptoms simultaneously.28,31,32 In addition to its conceptual advantages, this approach has practical relevance because associations with symptoms could suggest potential avenues for intervention.
Materials and Methods
Participant recruitment and selection
Mailings and community advertisements recruited women ever divorced or separated from a stressful relationship. Inclusion criteria were history of divorce or permanent separation from a cohabitating partner, age (between 45 and 60), and postmenopausal status (12-month cessation of menses and follicle-stimulating hormone [FSH] levels ≥25 mIU/mL). Exclusion criteria were absence of English language skills, ongoing divorce-related legal issues, psychiatric hospitalization in the preceding 6 months, active suicidal ideation, current IPV (IPV involving an ex-partner in the preceding year or any IPV history with a current partner, defined by a score >1 on three-item STaT33 [slapped, threatened, and throw things]), chronic disease other than unmedicated hypertension evidenced by self-report or eligibility laboratory tests, use of prescription or over-the-counter (OTC) medications with inflammatory effects (including psychotropics and botanicals), blood or needle phobia, and current alcohol use disorder or use of street drugs evidenced by self-report (≥5 on the derived Alcohol Use Disorders Identification Test [AUDIT-C] for alcohol use disorders34) or eligibility laboratory tests.
Procedure
A phone interview assessed initial inclusion and exclusion criteria. Eligible participants were scheduled for research visit 1. This visit included mental status interviews to confirm eligibility and a nursing evaluation to assess acute medical conditions, signs of illness or infection, systolic (SBP) and diastolic blood pressure (DBP), and body measurements. Blood was drawn via antecubital venipuncture and collected with appropriate anticoagulants for assessment of IL-6 and CRP, and the following eligibility laboratory tests: comprehensive metabolic profile, FSH, thyroid -stimulating hormone (TSH), ethanol, hemoglobin A1c (HbA1c), and complete blood count (CBC). A urine sample was obtained for a toxicology screen and urinalysis. Women then completed computer-administered questionnaires and were compensated $60.00.
Eligible women were scheduled for research visit 2. After mental status interviews and a nursing evaluation, blood was drawn via antecubital venipuncture for IL-6 and CRP assays. Women then completed computerized questionnaires and interviews about anxiety symptoms and were compensated $80.00. Data from interviews are not reported here.
Biologic measures
Isolation of plasma and PBMCs
Peripheral blood (10 mL) was centrifuged for 10 min at 350 g; the plasma was then separated by aspiration, aliquoted (0.25 mL) into cryovials, and stored frozen at −80°C until assay. Cells were resuspended to the original volume with Hank's Balanced Salt Solution (HBSS) containing 10% fetal bovine serum (FBS) and layered on top of a 10-mL cushion of Histopaque (Sigma, St. Louis, MO) in a 50-mL centrifuge tube. The tubes were centrifuged at ambient temperature for 20 min at 350 g, and the cells at the resulting interface (PBMC) were aspirated and washed twice with HBSS containing 1% FBS. After counting and viability assessment (trypan blue), the PBMCs were resuspended to a cell density of 4×106 cells/mL and used for the in vitro stimulation cultures.
Culture and stimulation of PBMCs
PBMCs were cultured in 24-well plates at a final cell density of 2.5×106 cells/mL using RPMI 1640 medium supplemented with 10% heat-inactivated FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM glutamine, 1 mM sodium pyruvate, and 0.1 mM nonessential amino acids. Phytohemagglutinin A (PHA, 1 μg/mL) and lipopolysaccharide (LPS, 5 μg/mL) were added separately to the cultures (1 mL final volume) to stimulate the PBMCs. Cell mixtures were then cultured for 48 hours at 37°C/5% CO2. Supernatants were collected by aspiration and centrifuged 10 min at 300 g to remove cells and debris. Supernatants were aliquoted (0.25 mL) into cryovials and stored frozen at −80°C until assayed by enzyme-linked immunosorbent assay (ELISA).
Measurement of IL-6 and CRP
Concentrations of IL-6 in plasma or tissue culture supernatants were measured by two-site ELISAs using Opti-EIA kits reagents (BD Pharmingen, San Diego, CA) specific for human IL-6 according to the manufacturer's instructions. Assay sensitivity was 0.3 pg/mL. Each sample was tested in triplicate. To minimize and control for interassay variability, analysis of the samples was deferred until a minimum of plasma and supernatants from 15 participants could be run together. Intra-assay and interassay coefficients of variation (CV) for the IL-6 ELISA were 6.9% and 9.6%, respectively. The minimum detection threshold was 0.3 pg/mL.
CRP was measured by turbidimetry on a Roche Integra 800 Analyzer. For the Roche Integra CRP assay, within-run coefficients of variation are 0.9% and 0.7% at 3.3 and 8.0 mg/L, respectively, and between-run coefficients of variation are 3.5% and 2.2%, respectively. The limit of detection is 0.1 mg/L.
Psychosocial measures
Relationship experiences
The Revised Conflict Tactics Scale (CTS2)35 assessed physical assault (12 items; Cronbach's α=0.86), sexual coercion (7 items; α=0.81), psychological aggression (8 items; α=0.85), and IPV-related injury (6 items; α=0.68). For each item, women rated frequency of occurrence from 0 (never) to 6, (>20 times) with respect to their prior intimate relationships collectively. Variety scores were computed for each subscale by counting positively endorsed items.36
Items from the National Violence Against Women Survey3 assessed lifetime occurrence of stalking by an intimate partner. These behaviorally specific items, based on the U.S. federal government's model antistalking code,37,38 are designed to identify events that constitute legally defined forms of stalking. Specifically, women reported yes/no if (1) a partner or former partner had ever perpetrated any of eight events (e.g., unsolicited phone calls, unsolicited correspondence, vandalism, following, or spying) and if these events caused (2) fear, or (3) fear of bodily harm, threat of harm, or threat to one's own or another's life. Following convention, women meeting components 1–3 were classified as stalking victims.3,39 Finally, for up to four relationships, women reported duration, age at separation/divorce, and whether, to stay safe, they had ever contacted police or filed an emergency protective order (EPO) or sought shelter in the community or with friends or family.
Psychological symptoms
The 9-item depression module of the Patient Health Questionnaire (PHQ-9)40 assessed depression symptoms in the past 2 weeks at research visits 1 (α=0.78, mean±standard deviation [SD]=4.57±4.19) and 2 (α=0.82, 4.21±4.11). Because symptom levels did not differ by visit (p=0.12) and were positively correlated (rS=0.64, p<0.0001), they were averaged to form one score. The 17-item Posttraumatic Stress Disorder Checklist-Civilian version (PCL-C), administered at visit 2 (α=0.93), assessed past month posttraumatic stress symptom severity.41 The PCL-C shows excellent reliability and validity in samples characterized by a variety of lifetime traumatic stressors (e.g., IPV, physical and sexual assault, childhood maltreatment, motor vehicle accidents)42–44 and also has been used in samples selected exclusively for IPV exposure.45
Sociodemographics and health-related characteristics
Women reported their age, marital and employment status, ethnicity, educational attainment, annual household income, and whether they were regular smokers. A 48-item checklist assessed past year stressful life events,46 and the 4-item Perceived Stress Scale (α=0.70) assessed perceptions of stress in the past month.47 Height and weight were used to calculate body mass index (BMI). The Cook-Medley Hostility Scale short-form48 (α=0.80) assessed trait hostility and aggression, and the National Women's Study Event History Module49 assessed adversity before age 12 (e.g., attempted sexual molestation, physical attack), both of which have been associated with IPV and systemic inflammation.31
Data analysis
Unstimulated IL-6 production levels were subtracted from PHA-stimulated and LPS-stimulated levels. These change-scores and circulating CRP and IL-6 levels were log-transformed to reduce right skewness. Linear regression analyses were conducted using the open-source R software package (R Foundation for Statistical Computing, Vienna, Austria, 2009, www.r-project.org/). Hypothesis tests were conducted at the 0.05 significance level. Spearman rank (rS) and rank biserial (rrb) correlation coefficients are presented throughout.
Results
Sample characteristics
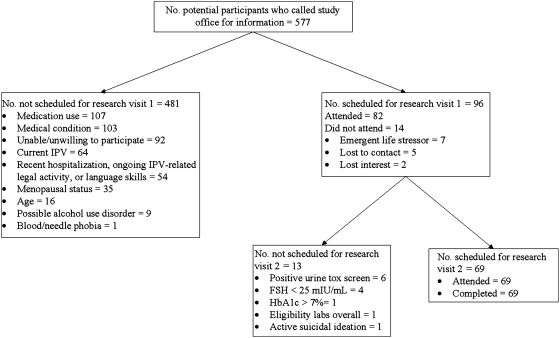
Figure 1 shows that 96 women (16.64% of callers) met initial study eligibility criteria, were able and willing to participate, and were scheduled for research visit 1. An average of 3.49 weeks (SD-2.27) later, 69 women (100% of those eligible) completed research visit 2. One participant unexpectedly failed to show any PHA stimulation in her PBMC cultures, resulting in technically improbable PHA-stimulated IL-6 change-scores that were lower than the baseline (−38.00 and −160.00 pg/mL), thus raising suspicion about the integrity of the blood sample. Plasma from this woman also had IL-6 levels ≥5.52 SD from the sample mean (101.7 and 106.9 pg/mL). Because of the unusual profile, this participant was removed from the sample, leaving 68 cases for analysis.
Fig. 1.
Study eligibility and enrollment diagram. FSH, follicle-stimulating hormone; HbAlc, hemoglobin Alc; IPV, intimate partner violence.
Sociodemographics and health-related characteristics
As shown in Table 1, participants were midlife women who self-identified as European American or African American. Few were currently partnered. Almost half had completed at least a college degree; most were employed, with an annual household income <$40,000.00. Levels of FSH confirmed women's postmenopausal status. Average TSH values and white blood cell counts were within their respective reference ranges; average HbA1c was in the normal range. Blood pressure was in the prehypertensive (SBP) or normotensive (DBP) range, and BMI was in the overweight range. Women reported an average of 4.59 past year stressful life events; perceived stress scores were about 1 point higher than age-matched U.S. norms.47 Childhood adversity was reported by 29.41%. Few women were current smokers, and a minority reported exclusionary conditions, signs of acute conditions, or a urinary tract infection at visit 1.
Table 1.
Sociodemographics and Health-Related Characteristics for Total Sample
| Variable | Mean (SD) or % (n) | Median |
|---|---|---|
| Age (years) | 54.68 (3.23) | |
| European American or white | 85.29% (58) | |
| Currently partnered | 23.53% (16) | |
| College graduate or beyond | 45.59% (31) | |
| Employed full-time or part-time | 83.83% (57) | |
| Annual household income <$40,000.00 | 61.19% (41) | |
| Follicle-stimulating hormone (mIU/mL) | 77.09 (29.12) | 69.36 |
| Years postmenopausal | 7.24 (5.65) | 5.61 |
| Thyroid stimulating hormone (μIU/mL) | 1.94 (1.26) | 1.59 |
| White blood cell count (103/μL) | 5.79 (1.48) | |
| HbA1c (%) | 5.60 (0.37) | 5.52 |
| Systolic blood pressurea (mm Hg) | 124.43 (14.79) | 120.75 |
| Diastolic blood pressurea (mm Hg) | 77.45 (8.86) | |
| Body mass index | 29.55 (6.71) | 28.62 |
| Stressful life events in past year | 4.59 (3.19) | 4.00 |
| Perceived stress in past month | 5.20 (3.01) | 5.00 |
| Childhood adversity | 29.41% (20) | |
| Trait hostility | 9.66 (5.04) | |
| Current regular smoker | 13.24% (9) | |
| Exclusionary conditionsb | 14.71% (10) | |
| Signs of acute conditionc | 16.18% (11) | |
| Urinary tract infection at visit 1 | 26.47% (18) |
n=68, except for annual household income and follicle-stimulating hormone (n=67), years postmenopausal, and hemoglobin A1c (HbA1c) (n=66). Medians are provided for skewed variables.
Average of measurements taken during visits 1 and 2.
One woman was enrolled with an Alcohol Use Disorder Identification Test (AUDIT-C) score of 5 due to a scoring error during a phone interview, and 4 were taking exclusionary medications—estradiol (n=2), testosterone (n=1), and a biophosphonate (n=1). Assessments of health behaviors preceding both research visits served as additional checks on exclusionary medications; 4 women reported morning use of such medications (i.e., acetaminophen, both acetaminophen and a “cholesterol medication,” baclofen, and loratadine). Finally, 1 woman disclosed an exclusionary medical condition during a research visit, and because of an emergent health condition, another was prescribed daily aspirin and a statin drug between visits 1 and 2. Collectively, 10 participants accounted for these cases.
Eleven women showed signs of skin rash, upper respiratory tract infection, allergic reaction, or enlarged submandibular nodes or glands at one of the research visits.
SD, standard deviation.
Relationship characteristics
Women experienced their most recent divorce or separation an average of 10.73±7.61 years before study participation and had been in distressed marriages or partnerships an average of 17.51±9.01 years. As shown in Table 2, all women reported histories of psychological aggression. Physical assault and sexual coercion were prevalent, as were IPV-related injury. Stalking, police contact or filing an EPO, and sheltering for safety were each reported by at least half of the women.
Table 2.
Descriptive Statistics for Relationship Experiences and Correlations Among Intimate Partner Violence Types
| Prevalencea% (n) | Variety score Mean (SD) | Physical assaultb | Sexual coercionb | Stalkingc | |
|---|---|---|---|---|---|
| Psychological aggression | 100 (68) | 5.81 (1.73) | 0.62** | 0.41** | 0.39* |
| Physical assault | 77.94 (53) | 4.56 (3.43) | – | 0.43** | 0.68** |
| Sexual coercion | 64.71 (44) | 1.84 (2.03) | – | 0.35* | |
| Stalking | 52.94 (36) | – | |||
| Injury | 61.76 (42) | – | |||
| Police contact/EPO for safety | 52.94 (36) | – | |||
| Sheltered for safety | 60.29 (41) | – |
Prevalence refers to the percentage of women who endorsed at least one item on a given measure. Correlations for psychological aggression, physical assault, and sexual coercion are based on variety scores.
Spearman rank correlation coefficients.
Rank biserial correlation coefficients.
p<0.01; **p<0.0005.
EPO, emergency protective order.
Biologic measures
Biologic measures were positively correlated across visits 1 and 2 (rSCRP=0.88; rSIL-6=0.79; rSΔPHA-stimulated IL-6=0.52; rSΔLPS-stimulated IL-6=0.41; p<0.0005). Levels did not differ significantly between visits (Wilcoxon signed rank test, p≥0.15), except that the LPS-stimulated IL-6 change was greater at visit 1 than visit 2 (p=0.04). Descriptive statistics for biologic measures averaged across visits are shown in Table 3. For CRP, which allows for interlaboratory comparisons, the median level was comparable to that from studies of healthy, midlife Americans (median, interquartile range [IQR]: 1.50, 0.60–3.50 mg/L),13 while the IQR extended somewhat higher.
Table 3.
Descriptive Statistics for Biologic and Symptom Measures and Zero-Order Correlations with Intimate Partner Violence Types
| Median (IQR) | IL-6 | PHA | LPS | PCL-C | PHQ | BMI | Psychological aggression | Physical assault | Sexual coercion | Stalking | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. CRP, mg/L | 1.43 (0.78-4.15) | 0.22 | 0.37** | 0.43** | 0.06 | 0.11 | 0.56** | 0.06 | 0.22 | 0.06 | 0.37* |
| 2. IL-6, pg/mL | 1.46 (0.53-3.85) | – | 0.27* | 0.29* | −0.16 | −0.08 | 0.03 | −0.13 | −0.08 | −0.04 | −0.22 |
| 3. PHA, pg/mL | 6064.0 (3282.5-9113.75) | – | 0.35** | −0.06 | −0.06 | 0.26* | −0.23 | −0.26* | −0.11 | −0.07 | |
| 4. LPS, pg/mL | 24021.0 (16812.0-29849.8) | – | 0.19 | 0.17 | 0.23 | 0.07 | −0.11 | 0.13 | −0.07 | ||
| 5. PCL-C | 22.16 (18.5-32.0) | – | 0.65** | 0.14 | 0.32** | 0.18 | 0.25* | 0.14 | |||
| 6. PHQ | 3.50 (1.50-6.25) | – | 0.08 | 0.15 | 0.02 | −0.06 | 0.09 | ||||
| 7. BMI | 28.62 (24.58-33.08) | – | 0.19 | 0.26* | 0.20 | 0.13 |
Descriptive statistics for biologic measures are nontransformed and averaged across visits.
Correlations for IPV types other than stalking are based on variety scores. Except for stalking, where rank biserial correlation coefficients are used, Spearman rank correlation coefficients are presented.
p≤0.04; **p≤0.008.
BMI, body mass index; CRP, C-reactive protein; IL-6, interleukin-6; IQR, interquartile range; LPS, lipopolysaccharide; PCL-C, Posttraumatic Stress Disorder Checklist (posttraumatic stress symptoms); PHA, phytohemagglutinin A (LPS-stimulated production change-scores); PHQ, Patient Health Questionnaire (depression symptoms).
Psychological symptoms
Descriptive statistics for psychological symptoms are shown in Table 3. Median depression severity was minimal,40 and median posttraumatic stress symptom severity was lower than the average for women without current syndromal PTSD.44
Associations among IPV types, psychological symptoms, and biologic measures
Correlations
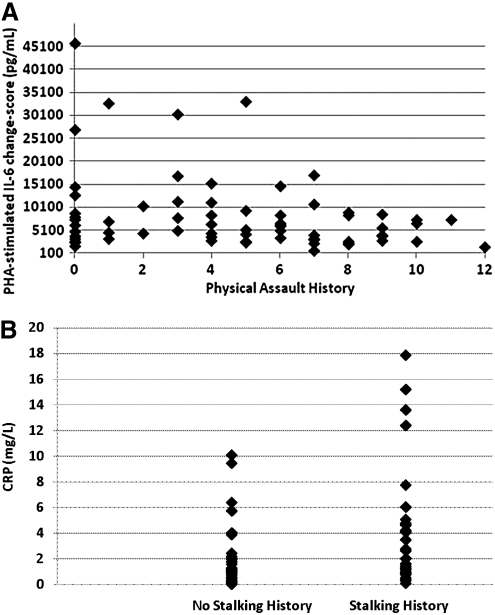
Table 3 shows that IPV types, but not psychological symptoms, were significantly correlated with biologic measures. As illustrated in Figure 2, PHA-stimulated IL-6 change-scores were significantly negatively associated with physical assault history (p=0.03), and circulating CRP levels were significantly positively associated with stalking history (p=0.008). This latter association was further evaluated by established CRP risk levels. Histories of being stalked were reported by 80% of women with very high CRP levels (>10 mg/L) and by 71% with high levels (>3 mg/L), compared to 52% and 35% with moderate (1–3 mg/L) or low levels (<1 mg/L), respectively (Fisher's exact test, p<0.09). Although very high CRP levels provide prognostic information for morbidity and mortality,50 some argue they could indicate acute inflammatory processes rather than habitual levels.51 To evaluate this possibility, between-visit percent change in CRP was calculated for the 5 women with very high levels. The degree of between-visit change (13% to 38%) was less than that which would be considered significant and possibly indicative of a developing or resolving acute inflammatory process (i.e., 117%).*
Fig. 2.
Associations between (A) raw (non-transformed) phytohemagglutinin A (PHA)-stimulated interleukin-6 (IL-6) change-scores and physical assault history and between (B) raw (nontransformed) C-reactive protein (CRP) circulating levels and stalking history.
Multiple regression analyses
For CRP levels and PHA-stimulated IL-6 change-scores, linear regression models of the visit averages evaluated relations with IPV type (stalking or physical assault history, respectively), depression symptom severity, and posttraumatic stress symptom severity. BMI, a well-recognized correlate of inflammatory markers, was included a priori. Because IL-6 has a circadian rhythm, correlations between time of blood draw (ranging from 8:11 am to 2:21 pm, except one draw at 4:30 pm) and PHA-stimulated IL-6 change-scores were computed; there were no significant associations at either visit 1(rs=0.03, p=0.79) or visit 2 (rs=−0.01, p=0.93), and, therefore, draw time was not included in the regression model.
Neither depression nor posttraumatic stress symptoms were associated with the two biologic measures, whereas BMI was positively associated with both (Table 4). After adjusting for BMI and symptoms, physical assault history was significantly negatively associated with PHA-stimulated IL-6 change-scores; stalking history was marginally positively associated with CRP. In a model excluding BMI, women with stalking histories had significantly higher CRP levels than those without (p=0.05). Thus, BMI attenuated the correlation between stalking history and CRP, but its lack of significant association with stalking (Table 3) indicates that this was not due to confounding or mediation.
Table 4.
Analyses Predicting Biologic Measures from Stalking History (for C-Reactive Protein) or Physical Assault History (for Phytohemagglutinin A-Stimulated Interlekin-6 Change-Score) After Adjusting for Body Mass Index and Current Posttraumatic Stress and Depression Symptoms
|
PHA-stimulated IL-6a |
C-reactive proteinb |
||||||
|---|---|---|---|---|---|---|---|
| Predictor | βc(95% CI) | pr2 | p value | Predictor | βc(95% CI) | pr2 | p value |
| Body mass index | 0.06 (0.03-0.09) | 0.19 | 0.0004 | Body mass index | 0.28 (0.16-0.41) | 0.25 | <0.0001 |
| Posttraumatic stress symptom severity | −0.003 (−0.03-0.02) | 0.001 | 0.79 | Posttraumatic stress symptom severity | −0.06 (−0.16-0.03) | 0.03 | 0.19 |
| Depression symptom severity | −0.01 (−0.08-0.06) | 0.002 | 0.73 | Depression symptom severity | 0.12 (−0.15-0.40) | 0.01 | 0.38 |
| Physical assault history | −0.08 (−0.14-−0.03) | 0.12 | 0.005 | Stalking history | 1.39 (−0.17-2.94) | 0.05 | 0.08 |
Model R2=0.24, p=0.001.
Model R2=0.33, p<0.0001.
β=standardized regression coefficient.
Follow-up analyses
Three follow-up analyses were conducted. First, the role of IPV severity was evaluated. For physical assault, separate variety scores were computed for severe (e.g., beat me up, 64.71% of women, 1.88±1.88) and minor assault (e.g., grabbed me, 76.47% of women, 2.68±1.87). Severe physical assault (rs=−0.32, p=0.007), not minor (rs=−0.15, p=0.21), was significantly correlated with PHA-stimulated IL-6 change-scores, a difference that approached statistical significance (p=0.06). When the adjusted regression model was refit with severe physical assault, it accounted for 14% of the variance in PHA-stimulated IL-6 change-scores (β=−0.17; p=0.0008). For stalking, a less severe form was defined as the occurrence of any stalking behavior regardless of fear or life threat; the zero-order correlation with CRP was not statistically significant (rrb=0.23, p=0.15).
Second, the role of potentially confounding health-related and sociodemographic characteristics was evaluated. Neither stalking nor physical assault history was significantly associated with years divorced or years in a distressed marriage or any of the sociodemographic or health-related characteristics (rrb or Fisher's exact test, p≥0.13). There were trends for associations between physical assault history and trait hostility (rs=0.21, p=0.09) and childhood adversity (rrb=0.29, p=0.06). When these variables were added to the regression model for PHA-stimulated IL-6 change-scores, the effect for physical assault history remained statistically significant (p=0.003); neither trait hostility (p=0.10) nor childhood adversity (p=0.62) reached statistical significance.
Third, the 10 women with exclusionary biomedical conditions (medication use, medical history, or possible substance use disorder) were omitted, and analyses with and without these women were compared. In terms of patterns of statistical significance, zero-order correlations among biologic measures, IPV types, and psychological symptoms were identical in both cases. The two regression models were also identical in both cases, with one exception: when the 10 women with exclusionary conditions were omitted, the effect for stalking history and CRP was statistically significant (β=1.80, p=0.04).
Discussion
This study examined correlations between remitted IPV and biologic mediators of inflammation in healthy midlife women. Women who reported intimate partner stalking histories showed higher CRP levels than those without. This was apparent only when stalking was defined as a traumatic stressor, engendering fear and perceived life threat. After adjusting for BMI, this effect was attenuated and was marginally statistically significant. Nonetheless, in the adjusted model, the magnitude of the relation between stalking history and CRP (r=0.22) exceeded a previously reported effect size for childhood maltreatment and CRP (r=0.14, converted from d=0.28).31 Sample size and consequent limitations in statistical power, plus the strong association between BMI and CRP, likely hampered the study's ability to detect this effect. Also, follow-up analyses showed that the presence of women with exclusionary conditions masked this effect.
Intimate partner stalking, with a lifetime prevalence of 5% among U.S. women,3 has been relatively neglected in IPV research. The few exceptions suggest that even among severely battered women, stalking intensifies and extends women's fear, anxiety, sadness, and sleep disruption and further dampens women's perceived safety.52,53 Although not exclusively a postrelationship event, intimate partner stalking is more likely to occur after, rather than before, relationship dissolution,3 making it particularly relevant for the health of postabused women. Because stalking was the only IPV type measured with appraisals of fear and perceived life threat, however, results attributed to it could be due to its assessment as a traumatic stressor. As recently argued, routine measurement of stress appraisals for all IPV types might benefit this research area.54
Because the cytokine IL-6 is often highlighted as a primary inducer of CRP synthesis, it is important to note that there was no statistically significant association between stalking history and circulating IL-6 levels. Other studies have similarly reported that the associations of CRP and IL-6 with chronic stress do not always converge,19 and some have reported null or inverse associations with psychosocial factors.18,55 Offering one possible explanation, other cytokines, such as IL-1β and tumor necrosis factor-α (TNF-alpha;), along with the adipokine resistin, play a role in CRP synthesis.56,57
Physical assault history was significantly correlated with in vitro production of the cytokine IL-6 by PBMCs, but only when stimulated with PHA. Similar to the results for stalking, this association was observed primarily for severe, rather than minor, physical assault. Further, it was not accounted for by BMI, trait hostility, or childhood adversity. Thus, in the present sample, a history of intimate partner physical assault emerged as an independent correlate of the capacity of immune system cells to produce IL-6 when stimulated in vitro. Although stimulated IL-6 production has not been identified as a risk factor for poorer health outcomes per se, these results nonetheless clearly link IPV history to a specific functional effect in the immune system.
Notably, this significant effect did not extend to LPS-stimulated IL-6 production, although the latter was also negatively correlated with physical assault. This somewhat different pattern could reflect the cell types being stimulated. At concentrations used in this study, LPS stimulates primarily monocytes, the main producers of proinflammatory cytokines, including IL-6. In contrast, PHA stimulates exclusively T lymphocytes, which can also produce IL-6. This suggests that the stress of intimate partner physical assault may have differential, rather than uniform, effects on the various cells of the immune system. It is interesting that in a population of PTSD patients, the sensitivity of PHA-stimulated but not LPS-stimulated cytokine production by whole blood to the inhibitory effects of glucocorticoids was found to be increased,32 further suggesting that traumatic stress and its emotional aftermath might affect immune system cells differentially.
In the present sample, psychological symptoms were not significantly associated with biologic measures. This is consistent with some prior research30,55,58 and might partially reflect the low symptom levels in the present sample. The more fundamental point, however, is that symptoms did not account for connections between remitted IPV and inflammation markers. Another plausible mediator, BMI, was similarly ruled out by the present results. It was not significantly correlated with stalking history, and despite its positive correlation with physical assault history, it did not account for the connection between this IPV type and PHA-stimulated IL-6. Overall, other mediators—psychological, biologic, or both—will need to be considered to explain the observed correlations between remitted IPV and markers of inflammation.
The present study has certain limitations. First, because many potential participants were disqualified for already having developed chronic diseases, women with sufficient stress-buffering resources to maintain good midlife health despite severe stress histories may comprise the present study sample. Therefore, these results should not be generalized to all women with IPV histories. Second, although women were carefully screened for medical conditions, subclinical disease cannot be ruled out as a factor in the results. Third, there is precedent for using IPV measures to assess cumulative lifetime IPV,3 but the reliability and validity of these retrospective reports are naturally a question. Finally, the cross-sectional study design limits causal inferences, and it is prudent to acknowledge trends for histories of physical assault and psychological aggression to correlate with CRP and PHA-stimulated IL-6, respectively, rather than to draw premature conclusions about the specificity of links between IPV types and biologic measures.
This article began by considering whether IL-6 and CRP might play a role in the relationship between IPV and aging-related chronic diseases. Results show that healthy midlife women with IPV histories present a complex inflammatory profile that may not be uniformly “proinflammatory” when all measures are taken into consideration. It may seem surprising for stressor history to be related to both increased CRP and decreased stimulated IL-6 production. Whereas plasma levels of CRP reflect chronic, systemic inflammation that is a cumulative downstream product of multiple mediators and cell types, stimulated IL-6 production reflects the acute in vitro response of a particular type of leukocyte (e.g., monocytes vs. T cells) in response to a given stimulation. The present results imply that in the context of chronic or traumatic stressors such as IPV, although the overall balance of the system may be biased in favor of increased systemic inflammation, different aspects of the inflammatory response may be differentially regulated and subjected to diverse compensatory mechanisms (such as glucocorticoids).32 Future studies should address the factors, such as specific stressor experiences,59 that might contribute to this differential regulation and to its persistence despite stressor remission.
Footnotes
Relative change value (95% confidence interval [CI]=(21/2)(1.96)(CVp2+CVi2+CVa2)1/2, where CVp is the preanalytical change value (considered to be negligible), CVi is the intraindividual change value (42.2%, from www.westgard.com/biodatabase1.htm), and CVa is the between-run analytical change value (2.2% at 8 mg/L).
Acknowledgments
This research was funded by the National Institute on Aging and conducted with support from the University of Louisville Hospital Clinical Research Center. We thank Heidi Resnick for her support and consultation, Kristen Allison, Jeanne Cundiff, Cristina Fernandez, and Rebecca Weigel for assisting with data collection, and the study participants.
Disclosure Statement
No competing financial interests exist.
References
- 1.Saltzman LE. Fanslow JL. McMahon PM. Shelley GA. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2002. Intimate partner violence surveillance: Uniform definitions and recommended data elements, version 1.0. [Google Scholar]
- 2.Garcia-Morena C. Jansen HAFM. Ellsberg M. Heise L. Watts C. Geneva: World Health Organization; 2005. WHO Multi-country study on women's health and domestic violence against women: Initial results on prevalence, health outcomes and women's responses. [Google Scholar]
- 3.Tjaden P. Thoennes N. Extent, nature, and consequences of intimate partner violence. Washington, DC: U.S. Department of Justice, National Institute of Justice; 2000. [Google Scholar]
- 4.Kaysen D. Resick PA. Wise D. Living in danger: The impact of chronic traumatization and the traumatic context on posttraumatic stress disorder. Trauma Violence Abuse. 2003;4:247–264. doi: 10.1177/1524838003004003004. [DOI] [PubMed] [Google Scholar]
- 5.Thompson RS. Bonomi AE. Anderson M, et al. Intimate partner violence: Prevalence, types, and chronicity in adult women. Am J Prev Med. 2006;30:447–457. doi: 10.1016/j.amepre.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Breiding MJ. Black MC. Ryan GW. Chronic disease and health risk behaviors associated with intimate partner violence—18 U.S. states/territories, 2005. Ann Epidemiol. 2008;18:538–544. doi: 10.1016/j.annepidem.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Coker AL. Davis KE. Arias I, et al. Physical and mental health effects of intimate partner violence for men and women. Am J Prev Med. 2002;23:260–268. doi: 10.1016/s0749-3797(02)00514-7. [DOI] [PubMed] [Google Scholar]
- 8.Woods AB. Page GG. O'Campo P. Pugh LC. Ford D. Campbell JC. The mediation effect of posttraumatic stress disorder symptoms on the relationship of intimate partner violence and IFN-gamma levels. Am J Community Psychol. 2005;36:159–175. doi: 10.1007/s10464-005-6240-7. [DOI] [PubMed] [Google Scholar]
- 9.Gabay C. Kushner I. Mechanisms of disease: Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 10.Maggio M. Guralnik JM. Longo DL. Ferrucci L. Interleukin-6 in aging and chronic disease: A magnificent pathway. J Gerontol Med Sci. 2006;61A:575–584. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steensberg A. Fischer CP. Keller C. Moller K. Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab. 2003;285:E433–E437. doi: 10.1152/ajpendo.00074.2003. [DOI] [PubMed] [Google Scholar]
- 12.Pai JK. Pischon T. Ma J, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351:2599–2610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- 13.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 14.Teunissen CE. van Boxtel MPJ. Bosma H, et al. Inflammation markers in relation to cognition in a healthy aging population. J Neuroimmunol. 2003;134:142–150. doi: 10.1016/s0165-5728(02)00398-3. [DOI] [PubMed] [Google Scholar]
- 15.Weaver JD. Huang MH. Albert M. Harris T. Rowe JW. Seeman TE. Interleukin-6 and risk of cognitive decline: MacArthur Studies of Successful Aging. Neurology. 2002;59:371–378. doi: 10.1212/wnl.59.3.371. [DOI] [PubMed] [Google Scholar]
- 16.Melamed S. Shirom A. Toker S. Berliner S. Shapira I. Association of fear of terror with low-grade inflammation among apparently healthy employed adults. Psychosom Med. 2004;66:484–491. doi: 10.1097/01.psy.0000130963.52755.b9. [DOI] [PubMed] [Google Scholar]
- 17.Kiecolt-Glaser JK. Preacher KJ. MacCallum RC. Atkinson C. Malarkey WB. Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis MC. Zautra AJ. Younger J. Motivala SJ. Attrep J. Irwin MR. Chronic stress and regulation of cellular markers of inflammation in rheumatoid arthritis: Implications for fatigue. Brain Behav Immun. 2008;22:24–32. doi: 10.1016/j.bbi.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Kanel R. Dimsdale JE. Mills PJ, et al. Effect of Alzheimer caregiving stress and age on frailty markers interleukin-6, C-reactive protein, and D-dimer. J Gerontol Med Sci. 2006;61A:963–969. doi: 10.1093/gerona/61.9.963. [DOI] [PubMed] [Google Scholar]
- 20.Elenkov IJ. Chrousos GP. Stress hormones, Th1/Th2 patterns, pro/anti-inflammatory cytokines and susceptibility to disease. Trends Endocrinol Metabol. 1999;10:359–368. doi: 10.1016/s1043-2760(99)00188-5. [DOI] [PubMed] [Google Scholar]
- 21.Bermudez EA. Rifai N. Buring J. Manson JE. Ridker PM. Interrelationships among circulating interleukin-6, C-reactive protein, and traditional cardiovascular risk factors in women. Arterioscler Thromb Vasc Biol. 2002;22:1668–1673. doi: 10.1161/01.atv.0000029781.31325.66. [DOI] [PubMed] [Google Scholar]
- 22.Ridker PM. Hennekens CH. Buring JE. Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 23.Campbell JC. Webster D. Koziol-McLain J, et al. Risk factors for femicide in abusive relationships: Results from a multisite case control study. Am J Public Health. 2003;93:1089–1097. doi: 10.2105/ajph.93.7.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandez-Botran R. Miller JJ. Burns VE. Newton TL. Correlations among inflammatory markers in plasma, saliva and oral mucosal transudate in post-menopausal women with past intimate partner violence. Brain Behav Immun. 2011;25:314–321. doi: 10.1016/j.bbi.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avitsur R. Powell N. Padgett DA. Sheridan JF. Social interactions, stress, and immunity. Immunol Allergy Clin North Am. 2009;29:285–293. doi: 10.1016/j.iac.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Miller GE. Rohleder N. Cole SW. Chronic interpersonal stress predicts activation of pro- and anti-inflammatory signaling pathways 6 months later. Psychosom Med. 2009;71:57–62. doi: 10.1097/PSY.0b013e318190d7de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maes M. Scharpe S. Meltzer HY, et al. Relationships between interleukin-6 activity, acute phase proteins, and function of the hypothalamic-pituitary-adrenal axis in severe depression. Psychiatry Res. 1993;49:11–27. doi: 10.1016/0165-1781(93)90027-e. [DOI] [PubMed] [Google Scholar]
- 28.Gill J. Vythilingam M. Page GG. Low cortisol, high DHEA, and high levels of stimulated TNF-α, and IL-6 in women with PTSD. J Trauma Stress. 2008;21:530–539. doi: 10.1002/jts.20372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howren MB. Lamkin DM. Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 30.Pace TWW. Heim CM. A short review on the psychoneuroimmunology of posttraumatic stress disorder: From risk factors to medical comorbidities. Brain Behav Immun. 2011;25:6–13. doi: 10.1016/j.bbi.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Danese A. Moffitt TE. Pariante CM. Ambler A. Poulton R. Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry. 2008;65:409–415. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Kloet CS. Vermetten E. Bikker A, et al. Leukocyte glucocorticoid receptor expression and immunoregulation in veterans with and without post-traumatic stress disorder. Mol Psychiatry. 2007;12:443–453. doi: 10.1038/sj.mp.4001934. [DOI] [PubMed] [Google Scholar]
- 33.Paranjape A. Rask K. Liebschutz J. Utility of STaT for the identification of recent intimate partner violence. J Natl Med Assoc. 2006;98:1663–1669. [PMC free article] [PubMed] [Google Scholar]
- 34.Dawson DA. Grant BF. Stinson FS. Zhou Y. Effectiveness of the Derived Alcohol Use Disorders Identification Test (AUDIT-C) in screening for alcohol use disorders and risk drinking in the US general population. Alcoholism Clin Exp Res. 2005;29:844–854. doi: 10.1097/01.alc.0000164374.32229.a2. [DOI] [PubMed] [Google Scholar]
- 35.Straus MA. Hamby SL. Warren WL. The Conflict Tactics Scales handbook. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- 36.Moffitt TE. Caspi A. Krueger RF, et al. Do partners agree about abuse in their relationship? A psychometric evaluation of interpartner agreement. Psychol Assess. 1997;9:47–56. [Google Scholar]
- 37.National Criminal Justice Association. Washington, DC: U.S. Department of Justice, National Institute of Justice; 1993. Project to develop a model anti-stalking code for states. [Google Scholar]
- 38.Tjaden P. Thoennes N. Allison CJ. Comparing stalking victimization from legal and victim perspectives. In: Davis KE, editor; Frieze IH, editor; Maiuro RD, editor. Stalking: Perspectives on victims and perpetrators. New York: Springer; 2002. pp. 9–30. [Google Scholar]
- 39.Baum K. Catalano S. Rand M. Rose K. Washington, DC: U.S. Department of Justice; 2009. Stalking victimization in the United States. Bureau of Justice Statistics Special Report. [Google Scholar]
- 40.Kroenke K. Spitzer RL. Williams JBW. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weathers F. Litz B. Herman D. Huska J. Keane T. San Antonio, TX: International Society for Traumatic Stress Studies; 1983. The PTSD Checklist (PCL): Reliability, validity, and diagnostic utility. [Google Scholar]
- 42.Dobie DJ. Kivlahan DR. Maynard C, et al. Screening for post-traumatic stress disorder in female Veteran's Affairs patients: Validation of the PTSD Checklist. Gen Hosp Psychiatry. 2002;24:367–374. doi: 10.1016/s0163-8343(02)00207-4. [DOI] [PubMed] [Google Scholar]
- 43.Ruggiero KJ. Del Ben K. Scotti JR. Rabalais AE. Psychometric properties of the PTSD Checklist—Civilian version. J Trauma. Stress. 2003;16:495–502. doi: 10.1023/A:1025714729117. [DOI] [PubMed] [Google Scholar]
- 44.Walker EA. Newman E. Dobie DJ. Ciechanowski P. Katon W. Validation of the PTSD Checklist in an HMO sample of women. Gen Hosp Psychiatry. 2002;24:375–380. doi: 10.1016/s0163-8343(02)00203-7. [DOI] [PubMed] [Google Scholar]
- 45.Krause ED. Kaltman S. Goodman LA. Dutton MA. Longitudinal factor structure of posttraumatic stress symptoms related to intimate partner violence. Psychol Assess. 2007;19:165–175. doi: 10.1037/1040-3590.19.2.165. [DOI] [PubMed] [Google Scholar]
- 46.Spurgeon A. Jackson CA. Beach JR. The Life Events Inventory: Re-scaling based on an occupational sample. Occup Med. 2001;51:287–293. doi: 10.1093/occmed/51.4.287. [DOI] [PubMed] [Google Scholar]
- 47.Cohen S. Williamson GM. Perceived stress in a probability sample of the United States. In: Spacapan S, editor; Oskamp S, editor. The social psychology of health. Beverly Hills, CA: Sage; 1988. pp. 31–67. [Google Scholar]
- 48.Barefoot JC. Dodge KA. Peterson BL. Dahlstrom WG. Williams RB. The Cook-Medley Hostility Scale: Item content and ability to predict survival. Psychosom Med. 1989;51:46–57. doi: 10.1097/00006842-198901000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Resnick H. Psychometric review of National Women's Study (NWS) Event History—PTSD module. In: Stamm BH, editor. Measurement of stress, trauma, and adaptation. Lutherville, MD: Sidran Press; 1996. [Google Scholar]
- 50.Hamer M. Chida Y. Stamatakis E. Association of very highly elevated C-reactive protein concentration with cardiovascular events and all-cause mortality. Clin Chem. 2010;56:132–135. doi: 10.1373/clinchem.2009.130740. [DOI] [PubMed] [Google Scholar]
- 51.Pearson TA. Mensah GA. Alexander RW, et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice. A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 52.Logan TK. Shannon L. Cole J. Walker R. The impact of differential patterns of physical violence and stalking on mental health and help-seeking among women with protective orders. Violence Women. 2006;12:866–886. doi: 10.1177/1077801206292679. [DOI] [PubMed] [Google Scholar]
- 53.Logan TK. Walker R. Toward a deeper understanding of the harms caused by partner stalking. Violence Vict. 2010;25:440–455. doi: 10.1891/0886-6708.25.4.440. [DOI] [PubMed] [Google Scholar]
- 54.Martinez-Torteya C. Bogat GA. von Eye A. Levendosky AA. Davidson WS. Women's appraisals of intimate partner violence stressfulness and their relationship to depressive and posttraumatic stress disorder symptoms. Violence Vict. 2009;24:707–722. doi: 10.1891/0886-6708.24.6.707. [DOI] [PubMed] [Google Scholar]
- 55.Steptoe A. Kunz-Ebrecht SR. Owen N. Lack of association between depressive symptoms and markers of immune and vascular inflammation in middle-aged men and women. Psychol Med. 2003;33:667–674. doi: 10.1017/s0033291702007250. [DOI] [PubMed] [Google Scholar]
- 56.Calabro P. Chang DW. Willerson JT. Yeh ETH. Release of C-reactive protein in response to inflammatory cytokines by human adipocytes: Linking obesity to vascular inflammation. J Am Coll Cardiol. 2005;46:1112–1113. doi: 10.1016/j.jacc.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 57.Eklund CM. Proinflammatory cytokines in CRP baseline regulation. Adv Clin Chem. 2009;48:111–136. doi: 10.1016/s0065-2423(09)48005-3. [DOI] [PubMed] [Google Scholar]
- 58.Cyranowski JM. Marsland AL. Bromberger JT. Whiteside TL. Chang Y. Matthews KA. Depressive symptoms and production of proinflammatory cytokines by peripheral blood mononuclear cells stimulated in vitro. Brain Behav Immun. 2007;21:229–237. doi: 10.1016/j.bbi.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 59.Kemeny ME. The psychobiology of stress. Curr Dir Psychol Sci. 2003;12:124–129. [Google Scholar]