Abstract
Aneurysmal bone cyst (ABC) is a locally recurrent bone lesion that has been regarded as a reactive process. Recently, a neoplastic basis in primary ABC was evidenced by demonstration of clonal chromosome band 17p13 translocations that place the USP6 (TRE2 or TRE17) oncogene under the regulatory influence of the highly active CDH11 promoter. Herein, we report CDH11 and/or USP6 rearrangements in 36 of 52 primary ABCs (69%), of which 10 had CDH11-USP6 fusion, 23 had variant USP6 rearrangements without CDH11 rearrangement, and three had variant CDH11 rearrangements without USP6 rearrangement. USP6 and CDH11 rearrangements were restricted to spindle cells in the ABC and were not found in multinucleated giant cells, inflammatory cells, endothelial cells, or osteoblasts. CDH11 and USP6 rearrangements did not correlate with recurrence-free survival, or with other clinicopathological features. CDH11 and USP6 rearrangements were not found in any of 17 secondary ABC associated with giant cell tumor, chondroblastoma, osteoblastoma, and fibrous dysplasia. These findings demonstrate that primary ABC are mesenchymal neoplasms exhibiting USP6 and/or CDH11 oncogenic rearrangements. By contrast, secondary ABC lack CDH11 and USP6 rearrangements, and although morphological mimics of primary ABC, appear to represent a non-specific morphological pattern of a diverse group of non-ABC neoplasms.
Aneurysmal bone cyst (ABC) is an intriguing bone lesion with the potential for local recurrence that has been regarded as a reactive process since its initial description in 1942 by Jaffe and Lichtenstein.1 Different theories have been proposed for the pathogenesis of ABC, and among the most widely accepted has been that a local circulatory abnormality leads to an increased venous pressure and resultant dilation of the vascular network.2–5 The reactive nature of ABC has also been suggested by the fact that a variety of benign and malignant bone neoplasms,5–8 including giant cell tumor of bone, chondroblastoma, osteoblastoma, fibrous dysplasia, and osteosarcoma may contain areas within the lesion that closely mimic ABC histologically. For these cases, the term “secondary ABC” has been coined.3,7 However, it is unclear whether primary and secondary ABC have a similar pathogenesis, or whether secondary ABC might rather be a common morphological pattern of growth that occurs as a non-specific phenomenon in a variety of primary bone tumors.
The purported reactive nature of ABC was refuted by the work of Panoutsakopoulos et al,9 which demonstrated chromosomal translocation t(16;17)(q22;p13) as a recurrent cytogenetic abnormality in primary ABC. Subsequently, other groups confirmed 17p13 rearrangement as a frequent cytogenetic aberration in primary ABC.10–15 Recently, we extended these studies by showing that the t(16;17)(q22;p13) fuses the promoter region of the osteoblast cadherin 11 gene (CDH11) on chromosome 16q22 to the entire coding sequence of the ubiquitin protease USP6 gene (also known as TRE2 or TRE17) on chromosome 17p13, suggesting that the pathogenesis of many primary ABC involves up-regulation of USP6 transcription.16
In this study we determined the frequency and the clinicopathologic consequences of CDH11 and USP6 genetic abnormalities in 52 primary ABC, and we also used these molecular markers to evaluate the pathogenetic relationship or lack thereof between primary and secondary ABC. Further, we identify the neoplastic cell in primary ABC by determining which cell components contained the genetic rearrangements.
Materials and Methods
Tumor Samples
Fifty-two primary ABC and 17 secondary ABC were histologically characterized according to established criteria.8 Metaphase preparations were available from eight of these cases, including two for which the cytogenetic findings have been reported previously (cases 19 and 29).16 Frozen tissue was available from 16 cases.
Fluorescence in Situ Hybridization (FISH)
BAC clones were obtained from Children’s Hospital Oakland Research Institute (Oakland, CA) and Research Genetics (Huntsville, AL). BAC minicontigs were assembled based on genomic mapping and sequence data from the Human Genome Working Draft. Minicontigs telomeric and centromeric to the USP6 locus were USP6. T (BACs RP11–124C16, RP11–111I16, and RP11–177H5) and USP6. C (CTD-2367F23 and RP11–457I18), respectively. Those telomeric and centromeric to CDH11 were CDH11. T (RP11–137A18, RP11–631H23, and RP11–351A20) and CDH11. C (RP11–615M9, RP11–730A21, and RP11–76J1), respectively. BAC DNA isolations and labeling were performed as described previously.16
FISH was performed using 4-μm paraffin-embedded tissue sections which were deparaffinized in xylene (3 × 10 minutes), dehydrated twice in 100% ethanol for 2 minutes, and treated with 100 mmol/L Tris and 50 mmol/L EDTA (pH 7.0) for 15 minutes at 93°C. Tissue sections were then rinsed once in 1X PBS and protein digested with Digest All-3 (Zymed, San Francisco, CA). After briefly washing in 1X PBS, the slides were sequentially dehydrated in alcohol (70%, 85%, 95%, and 100%), and air-dried for 1 hour at room temperature. Tissue sections were denaturated at 75°C for 2 minutes and BAC probe hybridization was carried out overnight in a humidified chamber at 37°C. Tissue sections were then washed in 0.5X SSC for 5 minutes at 73°C and treated with CAS block (Zymed) for 10 minutes. Probe detection was performed using FITC-anti-digoxigenin (1:500) and Alexa Fluor 594-streptavidin (1:500) (Molecular Probe, Eugene, OR)17 for 30 minutes. Slides were then mounted in VECTASHIELD mounting medium with 1.5 μg/ml of 4′,6-diamidino-2-phenylindole (DAPI). ABC were scored as positive for gene rearrangement if more than 5% of cells showed splitting apart of the flanking FISH probes. If fewer than 20% of cells showed rearrangement, the findings were corroborated by repeating the FISH assay in another section from the same paraffin block.
RNA Isolation and RT-PCR
RNA was isolated from frozen tumors after mechanical homogenization and overnight incubation in Trizol (Invitrogen, Carlsbad, CA) at 4°C. RNA reverse transcription into cDNA was performed using the GeneAmp RNA PCR Kit (Applied Biosystems, Foster City, CA) for 2 hours at 42°C using random hexamers. RNA isolation from paraffin sections were performed according to a previously described protocol.18
PCR reactions were performed using the Takara Ex Taq kit with the following parameters for 35 cycles: denaturation at 94°C for 30 seconds, annealing at 65°C for 30 seconds, and extension at 72°C for 1 minute. PCR primers for RT-PCR evaluation of the CDH11-USP6 fusion oncogene were CDH11+71F (5′-CGCCGCTGACTTGTGAAT-3′) and USP6+1781R (5′-CTCGGTGTCCCTTGTCATACTT-3′). Evaluation of low-abundance fusion transcripts in tumors where the first round PCR was negative was performed by nested PCR using primers CDH11+83F (5′-GTGAATGGGACCGGGACT-3′) and USP6+1736R (5′-CAGGAGCGGAAGGACATACTTA-3′) at the same cycling parameters as above for 25 cycles.
Statistical Analysis
Statistical analyses were performed with the S-PLUS 6.0 software package (Insightful Corp., 2001). The Fisher exact test was used to evaluate associations among categorical variables. The Mann-Whitney U-test was used to evaluate associations between categorical and continuous variables. Recurrence-free survival was calculated using the Kaplan-Meier product-limit method, and univariate survival analyses were calculated using the log-rank test. Multivariate survival analyses were performed using the Cox multivariate regression model. Proportionality assumption was evaluated using Kaplan-Meier and log-minus-log survival curves. Reported P values were two-sided and statistical significance was set at P ≤ 0.05.
Results
Overall Clinical and Morphological Features of Primary ABC
Clinical features are summarized in Table 1. Median patient age at diagnosis was 14 years (range, 2 to 42 years), and the genders were equally represented (27 females and 25 males). The most frequent primary sites were tibia (n = 11), femur (n = 8), fibula (n = 7), vertebra (n = 5), and humerus (n = 4). Thirty-nine tumors arose in peripheral locations, whereas 13 were central. Median tumor size was 4 cm (range, 1 to 8 cm). Treatment information was available for 49 patients, of whom 42 (86%) were treated by curettage and seven by local excision. No patient received radiotherapy. Clinical follow-up was available for 44 patients, of whom 17 (39%) had a local recurrence after a median interval of 35 months (range, 2 to 35 months). Two patients had more than one recurrence.
Table 1.
Clinical Features of Primary ABC
| Case | Age (years) | Sex | Location* | Size (cm) | Treatment | Follow-up (mo) | Recurrence | Time for recurrence (mo) |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | M | Femur | 4 | Curettage | 73 | Y | 30 |
| 2 | 2 | F | Femur | N/A | Curettage | 4 | N | |
| 3 | 5 | M | Pubis | 5 | Curettage | 9 | N | |
| 4 | 7 | M | Femur | 4 | Curettage | 9 | Y | 5 |
| 5 | 7 | F | Tibia | 5 | Curettage | 1 | N | |
| 6 | 8 | F | Tibia | 5 | Curettage | 54 | N | |
| 7 | 8 | F | Fibula | 2 | Curettage | N/A | N/A | |
| 8 | 10 | M | Calcaneous | 4 | Curettage | 25 | N | |
| 9 | 10 | M | Tibia | N/A | Curettage | 108 | N | |
| 10 | 10 | M | Femur | N/A | N/A | N/A | N/A | |
| 11 | 11 | F | Mandible | 1 | Excision | N/A | N/A | |
| 12 | 11 | M | Vertebra | 5 | Curettage | 18 | Y | 2 |
| 13 | 11 | M | Phalanx | 1 | Curettage | 9 | Y | 6 |
| 14 | 11 | M | Mandible | 3 | Curettage | 21 | Y | 11 |
| 15 | 11 | F | Humerus | 3 | Curettage | 94 | N | |
| 16 | 11 | M | Femur | 6 | Curettage | 25 | Y | 6 |
| 17 | 12 | M | Tibia | 4 | Curettage | 30 | N | |
| 18 | 12 | M | Fibula | 4 | Curettage | N/A | N/A | |
| 19† | 13 | F | Tibia | 4 | Curettage | 2 | N | |
| 20 | 13 | M | Femur | 4 | Curettage | 18 | N | |
| 21 | 13 | M | Femur | 7 | Curettage | 70 | N | |
| 22 | 13 | F | Fibula | 3 | Curettage | 18 | N | |
| 23 | 13 | F | Phalanx | 1 | Curettage | 30 | N | |
| 24 | 14 | M | Tibia | N/A | N/A | N/A | N/A | |
| 25 | 14 | F | Clavicle | 2 | Curettage | 21 | Y | 21 |
| 26 | 14 | M | Fibula | 4 | Curettage | 8 | N | |
| 27 | 14 | F | Tibia | 2 | Curettage | 51 | Y | 12 |
| 28 | 14 | F | Radius | 1 | Curettage | 24 | Y | 7 |
| 29† | 15 | F | Pubis | 4 | Curettage | 28 | Y | 6 |
| 30 | 15 | F | Fibula | 5 | Curettage | 49 | Y | 6 |
| 31 | 15 | F | Vertebra | 4 | Excision | 60 | N | |
| 32 | 15 | F | Tibia | N/A | N/A | N/A | N/A | |
| 33 | 16 | F | Vertebra | 6 | Excision | 12 | N | |
| 34 | 16 | F | Tibia | 2 | Curettage | 6 | N | |
| 35 | 17 | M | Vertebra | 5 | Excision | 12 | Y | 10 |
| 36 | 17 | F | Mandible | 4 | Curettage | 22 | N | |
| 37 | 17 | F | Tibia | 5 | Curettage | 46 | Y | 5 |
| 38 | 20 | F | Tibia | 4 | Curettage | 7 | N | |
| 39 | 26 | F | Phalanx | 4 | Excision | 33 | N | |
| 40 | 27 | M | Humerus | 3 | Curettage | 5 | N | |
| 41 | 27 | F | Clavicle | 2 | Curettage | 6 | Y | 6 |
| 42 | 29 | F | Calcaneous | N/A | Curettage | 44 | Y | 35 |
| 43 | 32 | M | Talus | 3 | Curettage | 12 | N | |
| 44 | 42 | M | Femur | 3 | Curettage | 12 | N | |
| 45 | 6 | M | Humeruss | 5 | Curettage | 31 | N | |
| 46 | 7 | F | Ulnas | 2 | Curettage | N/A | N/A | |
| 47 | 8 | M | Clavicles | 2 | Curettage | 78 | Y | 5 |
| 48 | 9 | F | Vertebras | 3 | Excision | 18 | N | |
| 49 | 9 | M | Calcaneouss | 4 | Curettage | 18 | Y | 6 |
| 50 | 15 | F | Fibulas | 4 | Curettage | 4 | N | |
| 51 | 18 | M | Fibulas | 3 | Curettage | 1 | N | |
| 52 | 8 | M | Shoulderst | 8 | Excision | N/A | N/A |
, s, denotes solid variant; st, denotes soft tissue tumor; N/A, not available.
, previously reported cases.16
All ABC were reviewed histologically and were classified according to contemporary criteria.8 Forty-five cases exhibited classic histology, featuring cavernous or slit-like hemorrhagic spaces surrounded by fibrous septa containing spindle cells and occasional osteoclast-like multinucleated giant cells. Osteoid formation with osteoblastic rimming was observed in all cases, and matrix calcification was observed in 17 cases (33%). Seven cases were solid variants of ABC, featuring a prominent solid growth and minimal or no cystic formation but otherwise histologically indistinguishable from the classic ABC.
Molecular Cytogenetics and Molecular Genetics of Primary and Secondary ABC
FISH analyses in 52 primary ABC showed that 36 (69%) had CDH11 and/or USP6 locus rearrangement (Table 2). Of these, 10 cases (28%) exhibited rearrangement of both loci, and a fusion transcript CDH11-USP6 was confirmed by RT-PCR in each of these cases. Notably, 23 ABC featured USP6 rearrangement without associated CDH11 rearrangement, consistent with variant USP6 activation mechanisms (Table 2). Only three ABC had CDH11 rearrangement without associated USP6 rearrangement (Table 2). CDH11-USP6 RT-PCR validations were performed in a representative group of the ABC with FISH rearrangements of USP6 only, CDH11 only, or neither USP6 or CDH11, and each of these tumors lacked the CDH11-USP6 fusion transcript (Table 2). Generally, the CDH11 and USP6 rearrangements appeared to be genomically balanced, although two ABCs had an unbalanced USP6 rearrangement with loss of chromosomal material telomeric to USP6, as evidenced by deletion of the FISH probe in that region. Another ABC had an unbalanced rearrangement of the CDH11 locus with loss of chromosome material centromeric to CDH11. Among seven solid variants of ABC, six cases had CDH11 or USP6 loci rearrangements (Table 2).
Table 2.
Molecular Genetic Features of Primary ABC
| Case* | FISH rearrangement |
RT-PCR |
||
|---|---|---|---|---|
| USP6 | CDH11 | % | CDH11-USP6 | |
| 1 | − | − | 0 | − |
| 2 | + | − | 18 | |
| 3 | + | + | 58 | + |
| 4 | + | − | 32 | − |
| 5 | + | − | 40 | − |
| 6 | − | − | 0 | |
| 7 | + | + | 60 | + |
| 8 | − | − | 0 | − |
| 9 | + | − | 52 | |
| 10 | + | + | 10 | + |
| 11 | + | + | 44 | + |
| 12 | + | − | 28 | |
| 13 | + | − | 82 | − |
| 14 | − | − | 0 | |
| 15 | − | − | 0 | |
| 16 | + | − | 60 | − |
| 17 | + | − | 52 | |
| 18 | − | − | 0 | |
| 19† | + | + | 36 | + |
| 20 | + | + | 27 | + |
| 21 | − | − | 1 | − |
| 22 | − | − | 0 | |
| 23 | + | − | 20 | |
| 24 | + | − | 33 | − |
| 25 | + | − | 35 | − |
| 26 | + | − | 80 | − |
| 27 | − | + | 30 | − |
| 28 | − | − | 0 | |
| 29† | + | + | 56 | + |
| 30 | + | − | 81 | − |
| 31 | + | + | 68 | + |
| 32 | − | − | 0 | |
| 33 | + | + | 32 | + |
| 34 | + | − | 60 | |
| 35 | + | − | 34 | − |
| 36 | + | − | 32 | |
| 37 | − | − | 0 | − |
| 38 | − | − | 0 | |
| 39 | + | − | 16 | − |
| 40 | − | − | 0 | − |
| 41 | − | − | 0 | |
| 42 | − | − | 0 | |
| 43 | + | − | 8 | |
| 44 | − | + | 50 | |
| 45s | + | − | 39 | |
| 46s | + | − | 35 | |
| 47s | + | − | 58 | |
| 48s | − | + | 30 | |
| 49s | + | − | 54 | |
| 50s | − | − | 1 | − |
| 51s | + | + | 7 | + |
| 52st | + | − | 38 | |
, s, denotes solid variant; st, denotes soft tissue tumor.
, previously reported cases.16
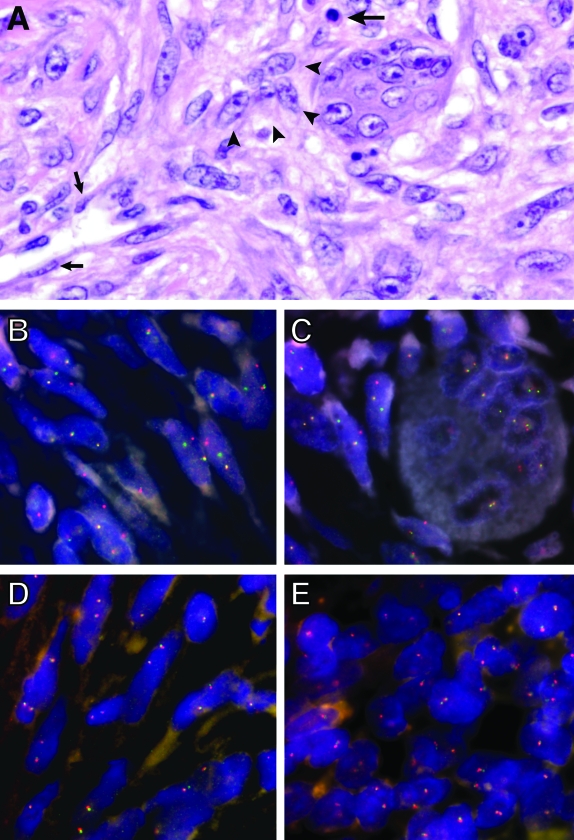
FISH and RT-PCR analysis were also performed in 17 secondary ABC, of which eight were associated with giant cell tumors, five with chondroblastomas, three with osteoblastoma, and one with fibrous dysplasia. In these lesions, both the primary tumor component as well as the secondary ABC lacked CDH11 and USP6 rearrangements by FISH (Figure 1, D and E), and lacked CDH11-USP6 fusion transcripts by RT-PCR.
Figure 1-4307.
Histological and molecular cytogenetic features in ABC. A: Histology (H&E) of primary ABC shows an inflammatory cell (large arrow), endothelial cells (small arrows), and a cluster of ovoid-to-spindled neoplastic cells (arrowheads) adjacent to a multinucleated giant cell. B–E: Dual-color “split-apart” USP6 fluorescence in situ hybridization (FISH) was performed in paraffin-embedded tissues using probes on the centromeric (green) and telomeric (red) sides of the USP6 locus. Splitting apart of a green-red probe signal indicates USP6 rearrangement. USP6 rearrangement is seen in spindle-shaped cells in a primary ABC (B) and in spindle cells clustered around a multinucleated giant cell (C), whereas the multinucleated cell lacks USP6 rearrangement. A secondary ABC occurring in association with a chondroblastoma lacks USP6 rearrangement in the ABC (D) and chondroblastoma (E) components.
Identification of Cell Types with USP6 Rearrangement
In primary ABC (Figure 1A) with USP6 or CDH11 rearrangement, the percentage of abnormal cells varied from 7% to 82% (median, 27%). These cells were usually spindled, wavy, or oval (Figure 1B), and were indistinguishable morphologically from surrounding spindle cells that lacked USP6 rearrangement. The cytogenetically abnormal cells were generally scattered diffusely throughout the lesion and were often grouped in small or large clusters adjacent to multinucleated giant cells (Figure 1C). USP6 and CDH11 rearrangement were never seen in the multinucleated giant cells, inflammatory cells, endothelial cells, and in the metaplastic bone-associated osteoblasts, corroborating their non-neoplastic nature. In addition, ABC cyst wall lining cells did not have USP6 or CDH11 locus rearrangement. Ki-67 antigen (MIB-1) median labeling index in ABC was 7% (range, 1 to 30%), and there was no association between the presence of USP6 locus rearrangement and MIB1 labeling index (P = 0.47).
Clinicopathologic Correlations of USP6 and CDH11 Rearrangements in ABC
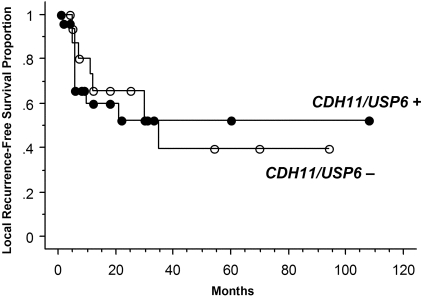
Clinical correlates were compared in primary ABC with molecular aberrations (USP6 or CDH11 rearrangement) versus those lacking demonstrable USP6 or CDH11 alterations. Patients with ABC with and without the molecular aberrations did not differ in age at diagnosis (P = 0.27), gender (P = 0.38), tumor size (P = 0.99), or location (central versus peripheral; P = 0.30). In addition, there was no difference in local recurrence-free survival after a median follow-up of 28 months (range, 1 to 108 months) (Figure 2 and Table 3). Cox multivariate regression analysis confirmed these findings and showed that only central tumor location was associated with an increased risk for local recurrence after adjusting for age, gender, tumor size, type of treatment (curettage or complete excision), and molecular aberrations (Table 3).
Figure 2-4307.
Kaplan-Meier curves for local recurrence-free survival in primary ABC stratified according to the presence or absence of CDH11 and USP6 loci rearrangements.
Table 3.
Clinicopathogic Correlates with Local Recurrence-Free Survival in ABC
| Variables | Univariate analysis p value | Multivariate analysis p value | HR | 95% CI |
|---|---|---|---|---|
| Age >= 14 years | 0.62 | |||
| Male Sex | 0.56 | |||
| Central location | 0.15 | 0.03 | 3.2 | 1.1–9.0 |
| Tumor size >= 4 | 0.54 | |||
| CDH11 or USP6 abnormalities | 0.89 | |||
| Curretage X excision | 0.25 | 0.1 | 6 | 0.7–49 |
Likelihood ratio for the model: p = 0.05.
p to exclude in the multivariate model: >0.10.
Discussion
ABC is a locally destructive bone lesion that has been regarded as a reactive process since its initial description more than 60 years ago.1,2,19 ABC generally occurs during the first two decades of life and females are affected slightly more often than males.19 The most frequent anatomical locations of ABC are the metaphyses of long bones, especially distal femur, proximal tibia, and posterior vertebral bodies.3,19,20 Radiologically, ABC presents typically as an eccentric and expansile lytic lesion that is often associated with a sclerotic margin.19,21 Clinically, ABC is characterized by a propensity to local recurrence,8,19 especially during the first 2 postoperative years.19 ABC is most often treated with curettage followed by bone grafting;3 wide excision is less often used, and radiation and amputation are reserved only for exceptional cases.
Histologically, ABC is characterized by hemorrhagic cystic and cavernous spaces of varying size surrounded by fibrous septa or more solid areas composed of mitotically active spindle or ovoid cells intermixed with inflammatory cells, scattered osteoclast-like multinucleated giant cells, extravasated red blood cells, and numerous capillaries. The histological growth is reminiscent of early granulation tissue. Trabecular osteoid formation with active osteoblasts and a characteristic matrix calcification with chondroid features are also frequently seen. Some tumors exhibit minimal or no cystic component, and these lesions have been designated as “solid variant of ABC” and “giant cell reparative granuloma”. The latter term is particularly used for lesions that occur in craniofacial bones and the small bones of the hand and feet.1,8,19,22–25
The pathogenesis of ABC has been a subject of debate since its original description.1 However, identification of recurrent chromosomal translocation t(16;17)(q22;p13) by Panoutsakopoulos et al9 strongly indicated a neoplastic pathogenesis in at least a subset of primary ABC. These findings were confirmed by additional cytogenetic reports,10–15 which also showed similar cytogenetic abnormalities in the solid variant of ABC and in soft tissue ABC.10,26 Furthermore, these studies showed that chromosome 17p13 is the most frequently rearranged chromosomal region in ABC.
Recently, we demonstrated that the chromosomal translocation t(16;17)(q22;p13) fuses the promoter region of the osteoblast cadherin 11 gene (CDH11) on chromosome 16q22 to the entire coding sequence of the ubiquitin protease USP6 gene on chromosome 17p13.16 We also found that CDH11 and USP6 loci were rearranged in ABCs with alternate translocations involving either chromosome 16q22 or 17p13. These studies suggested that the pathogenesis of some primary ABC involves transcriptional up-regulation of USP6. The transforming mechanisms of USP6 remain to be determined but could involve known USP6 roles in regulation of actin remodeling through interactions with the Rho GTPases Cdc42 and Rac1.27
In the present studies, we demonstrate CDH11 or USP6 rearrangements in 69% of primary ABC. Notably, 44% (23 of 52) had variant USP6 rearrangements, in the absence of CDH11 rearrangement. These findings suggest that USP6 could be the most prevalent fusion partner in ABC, with variant translocations providing alternative oncogenic mechanisms for USP6 transcriptional up-regulation. The identification of three cases in which only the CDH11 locus was rearranged, without evidence of USP6 rearrangement, also suggests that genes other than USP6 are up-regulated by juxtaposition with CDH11 in ABC.
The possibility of a genetic relationship between primary and secondary ABC was suggested recently by the finding of chromosome band 16q22 and 17p13 rearrangements in a giant cell tumor with secondary ABC.21 Nonetheless, in contrast to the situation in primary ABC, we found no evidence of CDH11 or USP6 rearrangements in secondary ABC associated with giant cell tumor, chondroblastoma, osteoblastoma, and fibrous dysplasia. Although chromosome 16 and 17 abnormalities have been found infrequently in giant cell tumors,21,28,29 it is unclear whether any of these involve CDH11 or USP6 loci. Based on the present evidence, we conclude that secondary ABC and primary ABC have a different pathogenesis. Secondary ABC, presumably, is a common endpoint of differentiation or a non-specific morphological pattern in various non-ABC tumors. Despite being a morphological mimic of primary ABC, secondary ABC likely has varied genetic features, corresponding to the specific bone tumors with which it is associated.
Presence of USP6 and CDH11 rearrangements did not correlate with ABC clinicopathological variables, nor, in univariate and multivariate analyses, with recurrence-free survival. The only variable that correlated with recurrence-free survival was tumor location, with central ABC being at greater risk for recurrence. However, we cannot exclude the possibility that specific USP6 or CDH11 rearrangement mechanisms are predictive of recurrence. Therefore, it would be worthwhile to evaluate relationships between molecular aberrations and ABC recurrence further, ideally in the context of a larger-scale and prospective clinical study.
The paraffin-section FISH analyses in this study enabled evaluation of ABC cell types which had the USP6 and CDH11 rearrangements. All cells with USP6 or CDH11 rearrangements were spindle or ovoid, and were diffusely admixed within the other ABC cell components, as well as being clustered around osteoclast-like multinucleated giant cells. The lack of cytogenetic aberrations in the inflammatory cells, endothelial cells, metaplastic bone-associated osteoblasts, and the multinucleated osteoclast-like giant cells supports their reactive nature. Conceivably, they are involved in a host response to the neoplastic ABC cells. It is also possible that one or more of the non-neoplastic cellular constituents release factors that stimulate division of the neoplastic ABC cells. The frequent clustering of neoplastic cells in the vicinity of multinucleated giant cells suggests a role for these cells in the neoplastic proliferation. Notably, the ABC cells with USP6 or CDH11 rearrangements were generally admixed with morphologically indistinguishable spindle cells lacking such rearrangements. We hypothesize that the latter group is composed by reactive fibroblasts/myofibroblasts, but it is also possible that some of these are committed ABC neoplastic cells at an earlier point in transformation, before acquisition of the USP6 or CDH11 rearrangements. Although the majority of cells in some ABC had USP6 or CDH11 rearrangements, there were also tumors in which fewer than 10% of cells had rearrangements (Table 2). The small numbers of apparently rearranged cells were not false-positive findings, as they were reproducibly present in these tumors when the FISH was repeated, and when scored by independent observers. Furthermore, RT-PCR reactions corroborated these molecular cytogenetic results showing the presence of CDH11-USP6 fusion transcripts in these tumors. In all, these findings suggest that the neoplastic ABC component induces a vigorous, reactive, host response mimicking young granulation tissue, including inflammatory, myofibroblastic, osteoclast-like giant cells, osteoblastic cells, and numeous capillaries, therefore accounting for the historical perception that these are largely reactive lesions.
In conclusion, primary ABC is a mesenchymal neoplastic disease characterized by a spindle cell proliferation exhibiting USP6 or CDH11 rearrangements in approximately two thirds of the cases. Secondary ABC is a morphological mimic of primary ABC, but lacks the hallmark USP6 or CDH11 rearrangements of primary ABC, and likely represents a common endpoint of differentiation in various non-ABC bone tumors.
Footnotes
Address reprint requests to Jonathan A. Fletcher, Department of Pathology, Brigham and Women’s Hospital, 75 Francis Street, Boston, MA 02115. E-mail: oliveira.andre@mayo.edu or jfletcher@partners.org.
Supported by Mayo Clinic and Mayo Clinic Foundation.
References
- Jaffe H, Lichtenstein L. Solitary unicameral bone cyst: with emphasis on the roentgen picture, the pathologic appearance, and the pathogenesis. Arch Surg. 1942;44:1004–1025. [Google Scholar]
- Fechner R, Mills S. Rosai J, editor. Armed Forces Institute of Pathology; Washington: Non-neoplastic lesions that mimic neoplasms: aneurysmal bone cyts. 1993:pp 253–258. [Google Scholar]
- Dorfman H, Czerniak B. Cystic lesions. Mosby; St. Louis: Bone Tumors. 1998 [Google Scholar]
- Clough JR, Price CH. Aneurysmal bone cyst: pathogenesis and long term results of treatment. Clin Orthop. 1973;97:52–63. doi: 10.1097/00003086-197311000-00009. [DOI] [PubMed] [Google Scholar]
- Kransdorf MJ, Sweet DE. Aneurysmal bone cyst: concept, controversy, clinical presentation, and imaging. AJR Am J Roentgenol. 1995;164:573–580. doi: 10.2214/ajr.164.3.7863874. [DOI] [PubMed] [Google Scholar]
- Levy WM, Miller AS, Bonakdarpour A, Aegerter E. Aneurysmal bone cyst secondary to other osseous lesions: report of 57 cases. Am J Clin Pathol. 1975;63:1–8. doi: 10.1093/ajcp/63.1.1. [DOI] [PubMed] [Google Scholar]
- Martinez V, Sissons HA. Aneurysmal bone cyst: a review of 123 cases including primary lesions and those secondary to other bone pathology. Cancer. 1988;61:2291–2304. doi: 10.1002/1097-0142(19880601)61:11<2291::aid-cncr2820611125>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Rosenberg AE, Nielsen GP, Fletcher JA. Aneurysmal bone cyst. Fletcher CDM, Unni KK, Mertens F, editors. IARC Press; Lyon: World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Soft Tissue and Bone. 2002:pp 338–339. [Google Scholar]
- Panoutsakopoulos G, Pandis N, Kyriazoglou I, Gustafson P, Mertens F, Mandahl N. Recurrent t(16;17)(q22;p13) in aneurysmal bone cysts. Genes Chromosomes Cancer. 1999;26:265–266. doi: 10.1002/(sici)1098-2264(199911)26:3<265::aid-gcc12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Dal Cin P, Kozakewich HP, Goumnerova L, Mankin HJ, Rosenberg AE, Fletcher JA. Variant translocations involving 16q22 and 17p13 in solid variant and extraosseous forms of aneurysmal bone cyst. Genes Chromosomes Cancer. 2000;28:233–234. [PubMed] [Google Scholar]
- Althof PA, Ohmori K, Zhou M, Bailey JM, Bridge RS, Nelson M, Neff JR, Bridge JA. Cytogenetic and molecular cytogenetic findings in 43 aneurysmal bone cysts: aberrations of 17p mapped to 17p13.2 by fluorescence in situ hybridization. Mod Pathol. 2004;17:518–525. doi: 10.1038/modpathol.3800090. [DOI] [PubMed] [Google Scholar]
- Baruffi MR, Neto JB, Barbieri CH, Casartelli C. Aneurysmal bone cyst with chromosomal changes involving 7q and 16p. Cancer Genet Cytogenet. 2001;129:177–180. doi: 10.1016/s0165-4608(01)00453-8. [DOI] [PubMed] [Google Scholar]
- Herens C, Thiry A, Dresse MF, Born J, Flagothier C, Vanstraelen G, Allington N, Bex V. Translocation (16;17)(q22;p13) is a recurrent anomaly of aneurysmal bone cysts. Cancer Genet Cytogenet. 2001;127:83–84. doi: 10.1016/s0165-4608(00)00422-2. [DOI] [PubMed] [Google Scholar]
- Winnepenninckx V, Debiec-Rychter M, Jorissen M, Bogaerts S, Sciot R. Aneurysmal bone cyst of the nose with 17p13 involvement. Virchows Arch. 2001;439:636–639. doi: 10.1007/s004280100449. [DOI] [PubMed] [Google Scholar]
- Wyatt-Ashmead J, Bao L, Eilert RE, Gibbs P, Glancy G, McGavran L. Primary aneurysmal bone cysts: 16q22 and/or 17p13 chromosome abnormalities. Pediatr Dev Pathol. 2001;4:418–419. doi: 10.1007/s10024-001-0035-0. [DOI] [PubMed] [Google Scholar]
- Oliveira AM, Hsi BL, Weremowicz S, Rosenberg AE, Dal Cin P, Joseph N, Bridge JA, Perez-Atayde AR, Fletcher JA. USP6 (Tre2) fusion oncogenes in aneurysmal bone cyst. Cancer Res. 2004;64:1920–1923. doi: 10.1158/0008-5472.can-03-2827. [DOI] [PubMed] [Google Scholar]
- Hibbard MK, Kozakewich HP, Dal Cin P, Sciot R, Tan X, Xiao S, Fletcher JA. PLAG1 fusion oncogenes in lipoblastoma. Cancer Res. 2000;60:4869–4872. [PubMed] [Google Scholar]
- Argani P, Zakowski MF, Klimstra DS, Rosai J, Ladanyi M. Detection of the SYT-SSX chimeric RNA of synovial sarcoma in paraffin-embedded tissue and its application in problematic cases. Mod Pathol. 1998;11:65–71. [PubMed] [Google Scholar]
- Vergel De Dios AM, Bond JR, Shives TC, McLeod RA, Unni KK. Aneurysmal bone cyst: a clinicopathologic study of 238 cases. Cancer. 1992;69:2921–2931. doi: 10.1002/1097-0142(19920615)69:12<2921::aid-cncr2820691210>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Biesecker JL, Marcove RC, Huvos AG, Mike V. Aneurysmal bone cysts: a clinicopathologic study of 66 cases. Cancer. 1970;26:615–625. doi: 10.1002/1097-0142(197009)26:3<615::aid-cncr2820260319>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Sciot R, Dorfman H, Brys P, Dal Cin P, De Wever I, Fletcher CD, Jonson K, Mandahl N, Mertens F, Mitelman F, Rosai J, Rydholm A, Samson I, Tallini G, Van den BH, Vanni R, Willen H. Cytogenetic-morphologic correlations in aneurysmal bone cyst, giant cell tumor of bone and combined lesions: a report from the CHAMP study group. Mod Pathol. 2000;13:1206–1210. doi: 10.1038/modpathol.3880224. [DOI] [PubMed] [Google Scholar]
- Sanerkin NG, Mott MG, Roylance J. An unusual intraosseous lesion with fibroblastic, osteoclastic, osteoblastic, aneurysmal, and fibromyxoid elements: “solid” variant of aneurysmal bone cyst. Cancer. 1983;51:2278–2286. doi: 10.1002/1097-0142(19830615)51:12<2278::aid-cncr2820511219>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Wold LE, Dobyns JH, Swee RG, Dahlin DC. Giant cell reaction (giant cell reparative granuloma) of the small bones of the hands and feet. Am J Surg Pathol. 1986;10:491–496. doi: 10.1097/00000478-198607000-00006. [DOI] [PubMed] [Google Scholar]
- Waldron CA, Shafer WG. The central giant cell reparative granuloma of the jaws: an analysis of 38 cases. Am J Clin Pathol. 1966;45:437–447. doi: 10.1093/ajcp/45.4.437. [DOI] [PubMed] [Google Scholar]
- Gipple JR, Pritchard DJ, Unni KK. Solid aneurysmal bone cyst. Orthopedics. 1992;15:1433–1436. doi: 10.3928/0147-7447-19921201-10. [DOI] [PubMed] [Google Scholar]
- Nielsen GP, Fletcher CD, Smith MA, Rybak L, Rosenberg AE. Soft tissue aneurysmal bone cyst: a clinicopathologic study of five cases. Am J Surg Pathol. 2002;26:64–69. doi: 10.1097/00000478-200201000-00007. [DOI] [PubMed] [Google Scholar]
- Masuda-Robens JM, Kutney SN, Qi H, Chou MM. The TRE17 oncogene encodes a component of a novel effector pathway for Rho GTPases Cdc42 and Rac1 and stimulates actin remodeling. Mol Cell Biol. 2003;23:2151–2161. doi: 10.1128/MCB.23.6.2151-2161.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge JA, Neff JR, Mouron BJ. Giant cell tumor of bone: chromosomal analysis of 48 specimens and review of the literature. Cancer Genet Cytogenet. 1992;58:2–13. doi: 10.1016/0165-4608(92)90125-r. [DOI] [PubMed] [Google Scholar]
- Molenaar WM, van den BE, Dolfin AC, Zorgdrager H, Hoekstra HJ. Cytogenetics of fine needle aspiration biopsies of sarcomas. Cancer Genet Cytogenet. 1995;84:27–31. doi: 10.1016/0165-4608(95)00068-2. [DOI] [PubMed] [Google Scholar]