Abstract
The molecular mechanisms that cause emphysema are complex but most theories suggest that an excess of proteinases is a crucial requirement. This paradigm is exemplified by severe deficiency of the key anti-elastase within the lung: α1-antitrypsin. The Z mutant of α1-antitrypsin has a point mutation Glu342Lys in the hinge region of the molecule that renders it prone to intermolecular linkage and loop-sheet polymerization. Polymers of Z α1-antitrypsin aggregate within the liver leading to juvenile liver cirrhosis and the resultant plasma deficiency predisposes to premature emphysema. We show here that polymeric α1-anti-trypsin co-localizes with neutrophils in the alveoli of individuals with Z α1-antitrypsin-related emphysema. The significance of this finding is underscored by the excess of neutrophils in these individuals and the demonstration that polymers cause an influx of neutrophils when instilled into murine lungs. Polymers exert their effect directly on neutrophils rather than via inflammatory cytokines. These data provide an explanation for the accelerated tissue destruction that is characteristic of Z α1-antitrypsin-related emphysema. The transition of native Z α1-antitrypsin to polymers inactivates its anti-proteinase function, and also converts it to a proinflammatory stimulus. These findings may also explain the progression of emphysema in some individuals despite α1-antitrypsin replacement therapy.
α-1 Antitrypsin (AT) is the main proteinase inhibitor within the lung. It is produced primarily by hepatocytes from where it is secreted into the plasma. It diffuses into the lung to act as the main inhibitor of neutrophil elastase.1,2 It is also produced to a lesser extent by macrophages, polymorphonuclear leukocytes, and bronchial epithelial cells.3–5 The normal AT protein is known as M-AT according to its isoelectric point.6 There are more than 70 variants described of which the Z variant is the most clinically relevant. Z-AT (Glu342Lys) homozygotes are found in 1 in 2000 of the North European population and are characterized by severe plasma deficiency of the protein.7
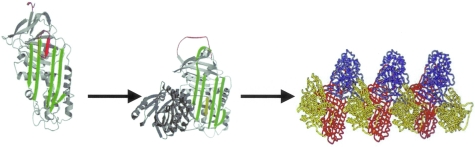
The structure of AT is characterized by a dominant β-sheet A and an exposed reactive center loop that is the bait for neutrophil elastase7–9(Figure 1). On cleaving the P1-P1′ bond in the reactive loop, the AT molecule undergoes a dramatic conformational change such that the reactive loop inserts into β-sheet A and the proteinase is translocated to the opposite end of the molecule and inactivated.10–14 The reactive loop-β-sheet A interaction is critical for effective proteinase inhibition yet it is also its Achilles heel. The Z protein has a partially inserted reactive loop that promotes aberrant loop-sheet interaction whereby the loop of one molecule inserts into the β-sheet A of another to form loop-sheet polymers (Figure 1).15–17 These polymers aggregate in the endoplasmic reticulum of hepatocytes resulting in neonatal hepatitis, juvenile cirrhosis, and hepatocellular carcinoma.18–22 The secretory defect results in severe plasma deficiency of AT exposing the lungs to the damaging effects of neutrophil elastase that results in premature panacinar emphysema.23 Thus, Z-AT is the only known genetic cause for emphysema and accounts for 1 to 2% of cases.24,25
Figure 1-4410.
Demonstration of the partially loop-inserted Z α1-AT (red) that opens up β-sheet A (green) to favor insertion of the reactive loop of another molecule to form an AT dimer (center) and polymers (right) (adapted from R. Mahadeva et al15).
The pathogenesis of emphysema in Z-AT homozygotes is thought to arise mainly from deficiency of the proteinase inhibitor. Instillation of proteinases with elastolytic properties into mammalian lungs, and the association of genetic deficiency of AT with emphysema together have formed a central pillar of the anti-proteinase-proteinase hypothesis of chronic obstructive pulmonary disease.26–30 There are however many other mechanisms and factors that are important in emphysema.31–33 Importantly, there are several differences between emphysema with normal and low levels of AT. The emphysema in Z-AT homozygotes develops earlier in life, initially at least predominantly affecting the basal areas and is of the panacinar rather than the centriacinar variety.34,35 One striking observation is that bronchoalveolar lavage fluid (BALF) from Z-AT homozygotes with emphysema contains more neutrophils than BALF from individuals with emphysema and M-AT.36 The reasons for this remain unclear, but may in part be because of an excess of interleukin-8 or leukotriene B4 in the BALF.37,38 However, one additional explanation may be the presence of polymers of α1-AT in the lungs of Z-AT homozygotes. Our preliminary studies have indicated that polymers of AT can be detected in BALF from Z-AT homozygotes, and, in vitro, polymers of AT are chemotactic to neutrophils.39,40 This data raised the novel hypothesis that Z-AT undergoes a conformational transition to polymers within the lungs that further depletes the local anti-proteinase protection. This would transform AT into a proinflammatory stimulus thus exacerbating the lung disease. We demonstrate here the co-localization of Z-AT polymers with neutrophils in the alveoli of individuals with Z-AT and show that these polymers are proinflammatory in cell and mouse models of disease. These data provide an explanation for the excessive number of neutrophils in the lungs of Z-AT homozygotes and the progression of disease despite adequate α1-AT replacement.
Materials and Methods
Preparation of Conformations of α1-AT
Native M-AT was purified from human plasma by ammonium sulfate fractionation, glutathione, and Q-Sepharose chromatography according to previous published methods.41 The protein migrated as a single band on 12% (w/v) sodium dodecyl sulfate-polyacrylamide gel electrophoresis (PAGE). The specific proteinase inhibitory activity of the AT was 80% as assessed by active site titration with bovine α-chymotrypsin.42 To prepare a polymer sample free of the monomeric (native) conformation, the native protein (1 mg/ml) was heated at 55°C for 16 hours in phosphate-buffered saline (PBS). Assessment was made on 7.5% (w/v) nondenaturing PAGE followed by Western blotting for α1-AT (see Figure 5). Biochemical, biophysical, and structural data previously performed has indicated that there are no major differences between polymers of AT generated by this method compared with polymers of Z-AT.15–17,34,40,41
Figure 5-4410.
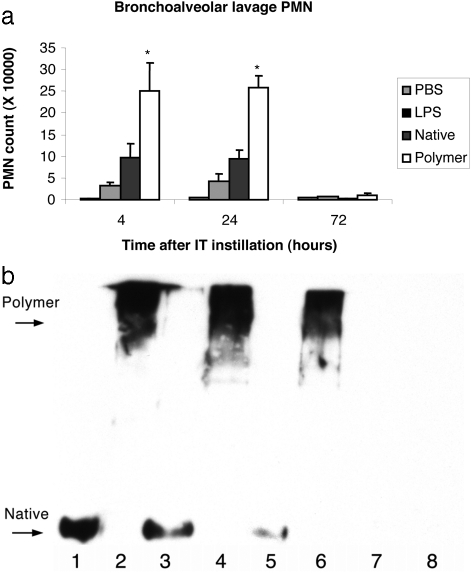
a: Graph demonstrating the effect of intratracheal instillation of native and polymeric α1-AT on polymorphonuclear leukocyte (PMN) numbers in BALF from C57BL/6J mice. Each bar graph represents the data from six mice. *, P < 0.01 for polymeric AT compared with native AT. b: C57BL/6J mice were anesthetized and intubated. Native or polymeric α1-AT in 40 μl of PBS was instilled via the intratracheal route. At 4, 24, and 72 hours after instillation, BAL was performed and aliquots were assessed on a 7.5% (w/v) nondenaturing PAGE followed by Western blot analysis for α1-AT using a polyclonal antibody that recognizes all forms of α1-AT. Lane 1: Native AT, starting material 0.1 μg; lane 2: polymeric AT, starting material 0.1 μg; lane 3: BAL fluid 4 hours after native AT instillation; lane 4: BAL fluid 4 hours after instillation of polymeric AT; lane 5: BAL fluid 24 hours after native AT instillation; lane 6: BAL fluid 24 hours after instillation of polymeric AT; lane 7: BAL fluid 72 hours after native AT instillation; lane 8: BAL fluid 72 hours after instillation of polymeric AT.
Enzyme-Linked Immunosorbent Assay for Total α1-AT and Polymers of α1-AT
Institutional review board, ethical committee, and tissue bank approval were obtained for the use of human lung tissue for these experiments. Lung tissue was collected prospectively at the time of transplantation from 10 Z-AT and 10 M-AT individuals with severe emphysema. Individuals were nonsmokers at the time of transplantation and all had a diagnosis of emphysema. By definition all had severe disease and at the time of the transplantation. Tissue was taken at least 2 cm from the periphery of the upper and lower lobes of each lung, and embedded either in paraffin or frozen.
Thirty mg of frozen emphysematous lung tissue from five Z-AT and six M-AT individuals were homogenized using Tissue Tearor (Biospec Products) in cell lysis buffer (CellLytic; Sigma, St. Louis, MO) on ice. The lung homogenates were centrifuged and proteinase inhibitor cocktail (Sigma, Poole, UK), 1 mmol/L phenylmethyl sulfonyl fluoride, and 1 mmol/L 1,10 phenanthroline were added to the supernatants. The supernatants were kept on ice and immediately assessed with an in-house enzyme-linked immunosorbent assay (ELISA) for the detection and quantification of total α1-AT and for polymers of AT.
Briefly, immunoplates (Nunc, Denmark) were coated with rabbit anti-human AT (Sigma) primary antibody overnight. This antibody detects all conformations of α1-AT. Unbound sites were blocked with PBS containing 1% (w/v) bovine serum albumin. Lung homogenates and polymer standards were added and incubated at room temperature for 1 hour. A goat anti-human AT antibody labeled with horseradish peroxidase (Abcam, UK) was added followed by the substrate 2,2′-azino-bis(3-ethybeniazoline-6-sulfonic acid) (Chemicon, Temecula, CA). The plate was read at OD 405 nm and the concentration of AT in the samples was calculated from the standard curve. The concentrations of AT recovered from the M-AT and the Z-AT lungs were compared using the Student’s t-test.
For the quantification of polymers, the above method was followed differing in that the primary coating antibody used was a monoclonal antibody—ATZII (referred to as the anti-polymer antibody) with polymer standards and lung homogenates. This latter antibody recognizes polymeric AT and the AT-elastase complex, but not the cleaved, or native form of AT.22 Homogenization of monomeric Z-AT in cell lysis buffer did not cause the Z-AT to polymerize.
Immunohistochemical Techniques
Formalin-fixed lung tissue from 10 individuals with M-AT and from 10 individuals with Z-AT with emphysema was stained for AT with the anti-polymer antibody. Briefly, sections were incubated in hydrogen peroxide to quench endogenous peroxidases and then washed in PBS. Sections were then incubated in primary antibody for 1hour at room temperature and then washed in PBS. Antibodies were labeled by a streptavidin biotin complex and visualized by 3′3 diaminobenzidine substrate, producing a brown reaction product (Chemate ABC detection system; DakoCytomation, UK). Sections were counterstained with Carazzi’s hematoxylin and examined by light microscopy. Two blinded observers (C.A. and S.S.) analyzed the tissue distribution of AT.
Assessment of the Specificity of Immunostaining
All immunohistochemical staining was accompanied by incubating sections with isotype control, mouse IgG (Vecta Laboratories, UK), and also by omission of the primary antibody. Incubation of the conformations with the anti-polymer antibody was undertaken to define the specificity of staining. Before immunohistochemistry, the anti-polymer antibody was incubated with a 10-fold molar excess of either native AT or polymeric AT at room temperature for 1 hour.
Assessment for α1-AT-Elastase Complexes and Polymorphonuclear Leukocyte Staining
The presence of neutrophil elastase, either as part of a neutrophil elastase complex or within neutrophils, was assessed using a mouse anti-human neutrophil elastase antibody (DakoCytomation). Antibodies were detected using a streptavidin biotin complex immunocytochemistry detection system according to the manufacturer’s recommendations (Chemate ABC detection system, DakoCytomation). Sections stained for neutrophil elastase for the demonstration of neutrophils were assessed by two observers who were blinded to the patient group (C.A. and R.P.). Each lung slide was examined using ×40 objective using a Leica DMLB microscope (Leica, UK). Six random fields were selected and the number of neutrophils within the alveolar walls recorded for each specimen. Differences in the mean numbers of neutrophils in the resected lung tissue from M- and Z-AT homozygotes were compared using the Mann-Whitney U-test and the Sigma Plot Statistical software.
Co-Localization of Polymers of AT and Polymorphonuclear Leukocytes
Co-localization of polymers and polymorphonuclear leukocytes was performed using double-immunocytochemistry analysis. Briefly, polymers were demonstrated as outlined above, with a streptavidin biotin complex immunocytochemistry technique and visualized using 3′3 diaminobenzidine. On completion of the polymer immunostaining technique, sections were washed in PBS and then incubated with an antibody directed against neutrophil elastase (DakoCytomation) for 1 hour. Sections were then washed again with PBS and visualized using DakoCytomation Envision system, which is a biotin-free dextran polymer secondary antibody. To allow for differentiation of the two antibodies demonstrated neutrophils were visualized using 3-amino-9-ethylcarbazole, producing a red reaction product. Sections were then counterstained with Carazzi’s hematoxylin.
Intratracheal Instillation of Conformers of AT into Murine Lungs
Animal studies were approved by the Harvard Animal Care Committee. Polymeric and native AT were prepared as above. The polymer and native AT preparations were tested for endotoxin content using the Limulus Amoebocyte assay (Associates of Cape Cod, USA). Residual endotoxin was removed from the samples using End-X beads (Associates of Cape Cod). Based on preliminary studies, samples were deemed satisfactory when the endotoxin content was less than 10 ng/ml.
C57BL/6J female mice 8 weeks of age and at least 20 g in size (Jackson Laboratories, Bar Harbor, ME) were anesthetized and intubated with a nonpyrogenic 22-gauge cannula (Terumo Medical Corp., USA). Mice received either 2.5 mg/ml of polymers or native AT diluted in 40 μl of PBS. PBS alone and 10 ng/ml of lipopolysaccharide (LPS) (Escherichia coli 0111:B4, Sigma) were used as controls. The total contaminating dose of LPS instilled into murine lungs was less than 0.8 ng. Instillation of a human protein into murine lungs may have an effect on neutrophil influx; therefore before undertaking these studies instillation of 2.5 mg/ml of low endotoxin (<0.1 ng/mg) human serum albumin (Sigma) was undertaken. There was however no effect on neutrophil influx into murine lungs after instillation of human albumin.
Assessment of BALF Neutrophil Content
After 4 hours, 24 hours, and 72 hours cohorts of mice (at least six in each group) were sacrificed by CO2 narcosis and were subject to bronchoalveolar lavage (BAL) with 8 aliquots of 0.5 ml of PBS. The BAL samples were centrifuged and protease inhibitor cocktail (Sigma) and 1 mmol/L phenylmethyl sulfonyl fluoride were added to the lavage fluid. The BAL fluid was then aliquoted and stored at −80°C until use. The cell pellet was resuspended in PBS without proteinase inhibitors. Red blood cells were lysed in 0.15 mol/L NH4Cl, 0.01 mol/L KHCO3, 1 μmol/L disodium-ethylenediaminetetraacetic acid, pH 7.2, and the total numbers of cells quantified by the mean of two hemocytometer counts. Cytospins were prepared from the BAL cellular fraction and were stained with Hema 3 (Fisher, Pittsburgh, PA). The remainder of the cellular fraction was lysed with 0.2 mol/L Tris, 0.15 mol/L NaCl, 0.02 mol/L CaCl2, 0.04% (w/v) Triton, pH 8.5, and stored at −80°C before assay for neutrophil elastase. The proportions of different inflammatory cells were determined by counting at least 300 cells. Differences in the mean neutrophil counts were assessed by the Student’s t-test.
Neutrophil elastase activity as an indicator of total polymorphonuclear cells was assayed as previously described.42 Briefly, neutrophil elastase (Athens Research, USA) was used to construct a standard curve using the fluorogenic substrate methoxysuccinyl-Ala-Ala-Pro-Val-7-amino-4-trifluoromethyl coumarin (MeOSuc-Ala-Ala-Pro-Val-AFC) (Enzyme System Products, USA) dissolved in 0.2 mol/L Tris, 0.15 mol/L NaCl, 0.02 mol/L CaCl2, 0.04% Triton, pH 8.5. This substrate is highly specific for neutrophil elastase. Standards and lysed BAL cell extracts were incubated in duplicate at 37°C for 4 hours and the quantity of the released product assessed at an excitation wavelength of 400 nm and an emission wavelength of 505 nm using an F-2500 fluorescence spectrophotometer (Hitachi, Japan). Values for standards were used to construct a standard curve and the concentration of neutrophil elastase assessed in samples by standard methods. The lower limit of detection using this assay was 0.2 ng/ml.
Assessment for the Presence of Conformers of AT in BALF
BALF was assessed for the presence of polymers and the native AT after intratracheal instillation by Western blot analysis. BAL fluid 4, 24, and 72 hours after instillation of the protein was assessed by 7.5% (w/v) nondenaturing PAGE and then transferred onto nitrocellulose Immobilon P membrane (Millipore, Bedford, MA) using a semidry blotter (Bio-Rad, Hercules, CA). Membranes were blocked with 5% (w/v) low-fat milk and were incubated with rabbit anti-human AT antibody (Sigma) followed by a donkey anti-rabbit-horseradish peroxidase (Amersham Biosciences, Buckinghamshire, UK), and visualized with photographic film after labeling with enhanced chemiluminescence (Amersham Biosciences).
Assessment of BALF Cytokine Content
BAL fluid was assessed in duplicate for KC and MIP-2 (murine homologues of IL-8) by specific ELISA (R&D Systems, Minneapolis, MN).
Chemotaxis Experiments
One ml of sterile 3% thioglycolate media (Sigma) was administered intraperitoneally to C57BL/6J mice. After 4 hours peritoneal lavage was undertaken with Dulbecco’s modified Eagle’s medium and 1% (w/v) human serum albumin and assessed for neutrophil numbers. The chemotactic ability of polymers and native AT to murine neutrophils was assessed using a 48-well modified Boyden chamber (Neuro Probe, Inc., MD). A suspension of neutrophils (50 μl, 5 × 106 cells/ml) in media was placed in the upper wells with 25 μl of test substance or media in the lower wells. Additional control test solutions included, formylated Met-Leu-Phe 10−4 mol/L, LPS (E. coli 0111:B4), and 4% zymosan-activated serum. The zymosan-activated serum was prepared by incubating rat serum with zymosan (20 mg zymosan in 1 ml of rat serum; Sigma Aldrich, St. Louis, MO) for 1 hour at 37°C, then 30 minutes at 56°C. Zymosan-activated serum was then obtained after centrifugation and aspiration of the supernatant. The upper and lower wells were separated by a nitrocellulose filter containing 3-μm diameter pores (Neuro Probe). The chamber was then incubated in a 5% CO2 incubator at 37°C for 45 minutes. Each test substance and controls were assessed together. Samples of native and polymeric AT had less than 10 ng/ml of endotoxin contamination, therefore a LPS control (10 ng/ml) was included in these experiments. After incubation, the membrane and cells were stained with Hema 3 (Fisher). Cell migration was assessed by manually counting the number of cells that had passed into the lower well. The results for each assay were expressed as the mean of five separate counts. Each experimental condition was repeated between three and six times. Differences in the numbers of neutrophil migration were assessed by Student’s t-test.
Results
The median FEV1 was 0.4 L (interquartile range, 0.375 to 0.525 L) for M-AT emphysema individuals, and 0.5 L (interquartile range, 0.4 to 0.84 L) for Z-AT individuals. There was no significant difference between the two groups in terms of FEV1 (P = 0.35). Immunostaining and ELISA of lung tissue and lung homogenates were performed to detect, localize, and quantify polymers of Z-AT.
ELISA of Lung Homogenates
ELISA for total AT revealed a 2.5-fold increase in the amount of AT recovered from M-AT lungs compared to Z-AT lungs. The mean (SD) concentration of AT was 59.6 (7.0) μg/ml and 23.8 (6.9) μg/ml for M-AT and Z-AT lungs, respectively (P < 0.01). Polymeric AT was detected in the Z-AT emphysematous lung homogenates mean 4.7 (SD 2.6) μg/ml, but not in the M-AT lung homogenates. Polymers constituted on average 20% (SD, 8%) of the total AT recovered from Z-AT lungs.
Immunohistochemistry for Polymers of α1-AT
After detection of polymers of AT in lung homogenates from Z-AT individuals, immunostaining was undertaken with the anti-polymer antibody on Z-AT and M-AT emphysematous lungs to localize polymeric AT. Immunostaining with this antibody demonstrated positive staining within the alveolar wall localizing around type II cells, and the endothelium in all 10 Z-AT lungs (Figure 2A). The specificity of immunostaining within the alveolar epithelium was demonstrated by the absence of signal in sections incubated with the isotype control (Figure 2B), and by omission of the primary antibody. To assess the specificity of staining further, the anti-polymer antibody was incubated with a 10-fold excess of native (monomeric) AT before immunostaining. This did not alter the staining in the Z-AT lungs (Figure 2C). However, incubation of polymers with the anti-polymer antibody before immunostaining abolished staining within the alveolar epithelium, but did not alter staining within the endothelium (Figure 2D). There was no difference in the immunostaining between samples taken from the upper and lower lobes of the Z-AT lungs.
Figure 2-4410.
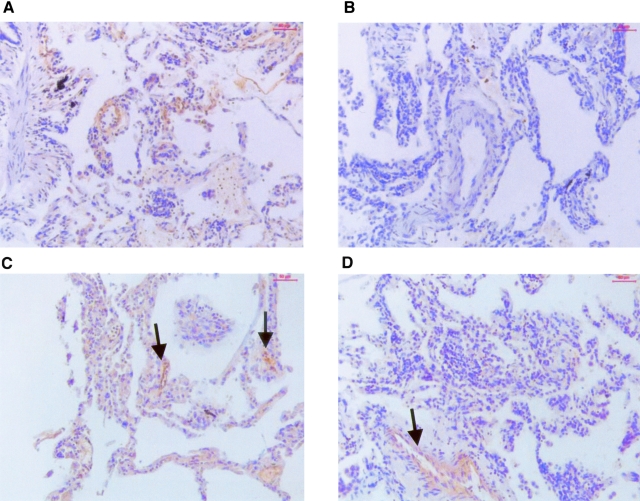
Immunohistochemistry of the anti-polymer antibody on emphysematous lung tissue from Z-AT individuals. A: Z-AT lungs stained with the anti-polymer antibody—note the staining within the alveolar walls, particularly around capillaries. B: Z-AT lungs stained with isotype control antibody—note the absence of staining within the alveolar walls and the lung vasculature. C: The anti-polymer antibody was preincubated with a 10-fold molar excess of native α1-AT before immunostaining Z-AT lungs. Staining was not inhibited, indicating that the anti-polymer antibody recognizes polymeric AT and not native AT. D: The anti-polymer antibody was preincubated with a 10-fold molar excess of polymers of α1-AT before immunostaining. Note the absence of staining within the alveolar walls. However, staining is not inhibited within the endothelium of the larger pulmonary vessels (arrow).
In contrast, none of the 10 M-AT lungs demonstrated immunostaining to polymeric AT in alveolar tissue. However there was endothelial staining of the larger vessels in a similar manner to that seen in the Z-AT lungs (Figure 3, A and B). The positive staining within the M-AT endothelium was also not affected by preincubation of the anti-polymer antibody with polymers before performing immunostaining (data not shown). The failure to abolish staining within the endothelium of the larger vessels in both Z-AT and M-AT lungs by incubating the anti-polymer antibody with polymers before immunostaining suggests that the staining within the endothelium is nonspecific and is unlikely to represent polymers of AT. These data suggest that polymers of AT are present in the alveolar wall of Z-AT emphysematous lungs, but not M-AT emphysematous lungs.
Figure 3-4410.
Immunohistochemistry of the anti-polymer antibody on emphysematous lung tissue from M-AT individuals. A: M-AT lungs stained with the anti-polymer antibody. There was an absence of staining within the alveolar walls, but there was however (B, arrow) positive staining within the endothelium of a pulmonary artery, a pattern also seen within Z-AT endothelium. C: IgG isotype control of M-AT patient. There was an absence of staining within the pulmonary artery and surrounding alveolar walls.
Immunostaining with Neutrophil Elastase Antibody
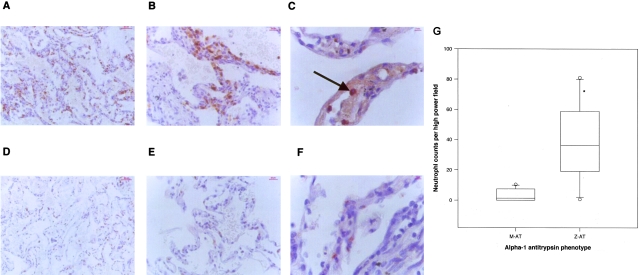
Although the anti-polymer antibody ATZ11 does not recognize the native or cleaved form of AT, it does recognize the α1-AT-elastase complex in addition to polymeric AT. Therefore to assess whether the positive staining could represent the α1-AT-elastase complex, the explanted lungs were also examined with an anti-neutrophil elastase antibody. In both M-AT and Z-AT emphysematous lungs, this antibody stained neutrophils and did not reveal any extracellular staining to suggest α1-AT-elastase complexes (Figure 4; A, B, D, E). Neutrophils were primarily localized within the alveolar wall in Z-AT homozygotes (Figure 4A). There was no evidence of concurrent bacterial infection. The mean counts per high-power field in the alveolar wall were at least fourfold higher in the Z-AT group than the M-AT group (P < 0.001) (Figure 4G). There was no difference in neutrophil numbers between the upper and lower lobes of the lungs. Double-staining for neutrophil elastase and polymeric AT was performed to assess for localization of neutrophils and polymeric AT, respectively. In Z-AT lungs, neutrophils within the alveolar wall were co-localized with polymers surrounding them (Figure 4C). However, in the M-AT lungs, only staining for neutrophils could be seen.
Figure 4-4410.
Staining of M-AT and Z-AT emphysematous lungs with an antibody to neutrophil elastase and double staining with the anti-polymer antibody. A–C: Z α1-AT emphysematous lungs. A: Neutrophils demonstrated with anti-neutrophil elastase antibody. There was an abundance of neutrophils within the alveolar walls at both low power (A) and high power (B). C: The polymers co-localized with neutrophils in Z-AT alveoli. Oil immersion image of neutrophils (red, arrows) and polymers (brown) stained with neutrophil elastase and the anti-polymer antibody, respectively. D–F: M α1-AT emphysematous lungs. Neutrophils demonstrated with neutrophil elastase staining (low power, D; high power, E). Neutrophils were present within the alveolar walls. F: M-AT lungs: co-localization of neutrophil elastase (red, arrows) and the anti-polymer antibody (brown). There was no polymer staining within the alveolar walls. G: Graph of PMN counts in alveoli from 10 M-AT and 10 Z-AT emphysematous lungs. Six random fields were counted in each individual and the number of neutrophils documented by two independent observers blinded to the patient phenotype. *, P < 0.01 for neutrophil counts in the alveolar wall in Z-AT versus M-AT emphysematous lung tissue. Original magnifications: ×10 (A, D); ×40 (B, E); ×100 (C).
Intratracheal Instillation of Conformers of AT into Murine Lungs
To assess for a direct relationship between polymeric AT and neutrophils within the lung, polymeric and native AT were instilled into murine lungs. Purified polymers and native AT dissolved in PBS were instilled into C57BL/6J mouse lungs by endotracheal intubation. Instillation of polymeric AT produced a significant neutrophil influx into the BAL fluid at 4 hours and 24 hours after instillation compared with PBS, LPS 0.8 ng, and native AT; P < 0.01 for all. By 72 hours neutrophil counts had returned to baseline (Figure 5A). There was no significant difference in the neutrophil influx after instillation of native AT compared with LPS control. Native and polymer samples of AT had a contaminating dose of 10 ng/ml of LPS that equates to 0.8 ng in 40 μl. Instillation of LPS control produced a small increase in neutrophil numbers compared with PBS control. To verify these results, neutrophil elastase activity was assessed after lysis of cells recovered from BAL using a specific substrate for neutrophil elastase-MeOSuc-Ala-Ala-Pro-Val-AFC. Neutrophil elastase levels were detectable only after polymer instillation at 24 hours (mean, 1.2 ng/ml; SD, 0.8), but not after intrapulmonary instillation of native AT or PBS. There were no differences in the numbers of alveolar macrophages seen compared with controls (data not shown).
Detection of Native and Polymeric α1-AT in BALF after Instillation of α1-AT
To assess the relationship between polymers and neutrophil influx Western blot analysis for AT in BALF after instillation of AT was performed on 7.5% (w/v) nondenaturing PAGE. Polymers and native AT were detected at similar times after instillation. They were both present at 4 and 24 hours after instillation with a suggestion that there was more polymeric AT remaining at 24 hours. Both native and polymeric AT were undetectable at 72 hours (Figure 5B). Thus, the time course of neutrophil influx paralleled the presence of polymers in the BALF. Furthermore, there was no change in the conformation of native AT, ie, it did not spontaneously form polymers within the murine lung.
BALF Cytokine Content
To assess whether the effect of polymeric AT was mediated via chemokines, the BAL fluid content for KC and MIP-2 (the murine homologues of human IL-8) were assessed. Concentrations of these cytokines were extremely low. Mean values after native and polymeric AT instillation were 0.6 versus 0.4 ng/ml for KC, and 0.5 versus 0.4 ng/ml for MIP-2, respectively. There was no significant difference between the concentrations of KC and MIP-2 as assessed by ELISA of BAL fluid after instillation of either native or polymeric AT, P = 0.14 and 0.22, respectively. There was no detectable KC or MIP-2 at 24 and 72 hours after instillation of native or polymeric α1-AT.
Chemotactic Effect of Native and Polymeric AT to Murine Neutrophils
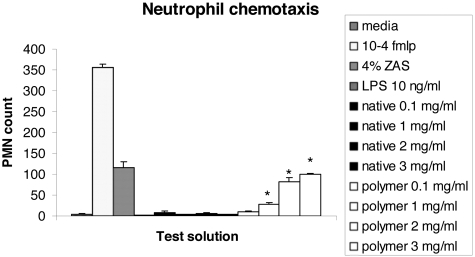
Thus polymer instillation in vivo produced a neutrophil influx that did not appear to be related to CXC chemokines. To investigate whether neutrophil recruitment could be a direct effect of polymers, in vitro neutrophil chemotaxis was assessed. Thioglycolate-induced peritoneal neutrophils were collected from C57BL/6J mice by peritoneal lavage and the chemotactic effect of polymers and native AT assessed. The native protein at a range of concentrations from 0.1 mg/ml to 3 mg/ml had no effect on neutrophil chemotaxis. In contrast, polymers of AT stimulated a significant dose-dependent chemotactic effect compared with the native protein, media, and LPS; P < 0.01 for all (Figure 6). At 3 mg/ml this effect was of similar magnitude to that of the positive control 4% (w/v) zymosan-activated serum. There was no effect on neutrophil chemotaxis with media and LPS controls.
Figure 6-4410.
Graph demonstrating the effect of native and polymeric AT on murine neutrophil chemotaxis compared with positive controls 10−4 formylated Met-Leu-Phe (fmlp) and 4% (w/v) zymosan-activated serum. *, P < 0.01 for polymeric AT compared with native AT. The data are the mean and SD of five wells for each potential chemoattractant repeated between three and six times.
Discussion
Chronic obstructive pulmonary disease due to emphysema and chronic bronchitis is one of the commonest reasons for ill health worldwide. The single most important factor in the development of emphysema is cigarette smoke, inhalation of which causes a chronic pulmonary inflammatory infiltrate of macrophages, neutrophils, and CD8+ cells that persists long after smoking cessation.43,44 A number of mechanisms have been implicated in the development of emphysema31–33,45 and although the precise mechanisms are unknown, most would accept that proteinases are central to the development of emphysema. There is strong support for this because deficiency of the anti-proteinase α1-AT is the only known genetic factor that causes accelerated emphysema in smokers.24,25
The development of emphysema in Z-AT homozygotes can occur as early as the fourth decade of life and is greatly accelerated by cigarette smoke.46 Oxidative inactivation of the P1 methionine residue by cigarette smoke or free radicals from leukocytes has been proposed to explain emphysema in cigarette smokers with normal concentrations of AT.1,47 However, although chronic cigarette smoke inhalation provides a common link, major differences in gross pathology exist between the centriacinar emphysema seen in individuals with normal (M) AT and the characteristic panacinar basal emphysema observed in those with Z-AT deficiency. This infers that there are mechanisms of tissue injury that are unique to emphysema associated with Z-AT.34 Two mechanisms have been reported previously. Firstly, severe deficiency of AT within the alveoli allows uncontrolled proteolytic attack and tissue destruction. This mechanism has formed the cornerstone of the anti-proteinase-proteinase hypothesis of tissue damage.30 Secondly, Z-AT in the native form is approximately fivefold less efficient at inhibiting neutrophil elastase than normal M-AT.41,48
Understanding the molecular mechanisms that underlie deficiency of Z-AT has allowed us to propose a novel pathway of tissue damage in individuals with Z-AT deficiency. We have previously shown that the polymeric conformer of AT is present in BAL from Z-AT homozygotes and that it is a chemoattractant for neutrophils in vitro.39,40 These findings have recently been confirmed by others.49 However, it is unknown where the polymers form and if they are chemotactic in vivo. We have demonstrated that polymers of Z α1-AT are present in the alveolar wall of Z-AT homozygotes with emphysema, which accounts for 20% of the total AT from lung homogenates. These polymers are likely to have derived from circulating monomeric AT from the plasma in addition may have derived from AT released by PMN degradation. These Z-AT individuals also have an excess of neutrophils in the alveolar wall compared with M-AT homozygotes. Furthermore, neutrophils and polymeric AT were co-localized in the alveolar wall. To investigate whether there was a direct relationship between polymers of Z-AT and the excess neutrophils, we instilled polymers of AT into the lungs of wild-type mice. This produced a significant increase in neutrophil influx into the lungs compared with instillation of the native protein. Examination of the time course demonstrated that the influx of neutrophils was closely linked to the presence of polymeric AT. The mechanism of neutrophil recruitment in this mouse model was likely to be because of a direct chemotactic effect rather than stimulation of IL-8 homologues or other CXC chemokines.
Two main factors give this latest data added significance. It is widely accepted that destruction of lung elastin in the interstitial space is critical to the development of emphysema, therefore it is likely that the local concentration of proteinase inhibitors is important in the development of emphysema. Thus, the close localization of polymers of AT with an excess of neutrophils at the site of disease in all 10 individuals with Z-AT-related emphysema is highly significant. Furthermore, the demonstration of a neutrophil influx after instillation of polymeric AT and the chemotactic ability of polymers to murine neutrophils is highly suggestive of a direct cause and effect.
These findings also support and help account for previous data indicating that Z-AT homozygotes have an accentuated and qualitatively different inflammatory process within the lungs from M-AT homozygotes. Previous studies have demonstrated that individuals with Z-AT deficiency have an excess of polymorphonuclear cells in BALF.36 This excess of neutrophils may in part be because of elevated proinflammatory cytokines such as interleukin-8 and leukotriene B4,37,38 and our data indicates that this may also be related to the presence of polymers of Z-AT within the lung. In this mouse model we did not find that polymeric AT mediated its chemotactic effect via IL-8 homologues MIP-2 or KC.
Although the ATZII antibody also recognizes both the α1-AT-elastase complex and polymeric AT, the positive staining seen in the lungs of Z-AT homozygotes and on ELISA was unlikely to be α1-AT-elastase complex in the because there was no staining within the alveolar wall of M-AT emphysematous lungs that would be expected to have higher concentrations of complex than Z-AT lungs, and there was no extracellular staining with neutrophil elastase antibody. Interestingly, there was positive staining with this antibody in the endothelium of larger vessels in both the M-AT and the Z-AT lungs. However, because it was not possible to abolish this staining by preincubation of the anti-polymer antibody with polymers, the endothelium did not demonstrate positivity to neutrophil elastase and M-AT itself has a very low tendency to polymerize compared with Z-AT.16,50 These observations indicate that the endothelial staining is unlikely to represent polymers of AT or α1-AT-neutrophil elastase complex, but may represent cross-reaction with another antigen exposed as part of the degradation and clearance of AT or AT-protease complexes other than the AT-neutrophil elastase complex. Previous reports have demonstrated the presence of α1-AT-elastase complex in the BAL from emphysematous individuals.32 Their absence in our tissue sections may reflect that fact that the complexes are present in the soluble form and are not bound to lung tissue.
The plasma concentration of M-AT is at least sevenfold higher than Z-AT in plasma. However, there was only a 2.5-fold increase in AT in M-AT lungs compared with Z-AT lungs. This observation allows us to speculate that the clearance of polymers of AT is impaired because they are bound within the alveolar wall. This enables them to act as a chronic stimulus for neutrophil influx into Z-AT lungs. This chronic stimulus will perpetuate the excess neutrophilic inflammation seen in this condition and contributes to the accelerated disease. Another contributing explanation for the relative excess of AT recovered from the Z-AT lungs may be as a result of the accentuated inflammatory response in these individuals. The production of AT as an acute phase protein rises in response to inflammation therefore in Z-AT individuals, the production of AT would be increased to a greater degree than in M-AT individuals.
The significance of these observations is twofold. Firstly, polymers of AT are inactive as proteinase inhibitors thereby further depleting protective levels of AT. Secondly, the switch from monomer to polymer converts the anti-inflammatory molecule into a proinflammatory one. This double hit compounds the effect of the plasma deficiency. The factors that cause the formation of polymers within the lung are not known. In vitro, their formation is accelerated by increasing concentration, temperature, and acidic environments.16,34 α1-AT is an acute phase protein whose synthesis is markedly increased at times of acute inflammation. Cigarette smoke and bacterial infection are factors that promote pulmonary inflammation, and cigarette smoke is also mildly acidic. Thus the formation of polymers may be accelerated by these proinflammatory stimuli. Polymers may therefore contribute to both the fulminant disease found in Z-AT homozygotes who smoke, and the increased neutrophilic inflammation seen in Z-AT homozygotes at times of pulmonary infection.51 We also speculate that Z polymer formation may play a role in chronic obstructive pulmonary disease in MZ heterozygotes.
In summary, these data establish a novel mechanism of disease in Z-AT homozygotes. In particular they illustrate how a structural transition resulting from a single amino acid substitution can dramatically alter a protein’s function: in this case converting an anti-inflammatory molecule into a proinflammatory stimulus. Although the factors that cause polymers to form and the exact contribution of polymers of AT to progression of the disease remain to be determined, these findings have implications for the therapy of Z-AT-related emphysema. The presence of polymers and their chemotactic properties in vivo indicate that replacement therapy alone may not be sufficient treatment for individuals with Z α1-AT-related emphysema. Indeed, this may provide an explanation for the progression of lung disease in Z α1-AT homozygotes after smoking cessation and despite adequate replacement therapy.
Acknowledgments
We thank Dr. Martin Goddard on behalf of the Papworth NHS Trust tissue bank for providing access to explanted lung tissue.
Footnotes
Address reprint requests to Ravi Mahadeva, Department of Medicine, Box 157, Level 5, Addenbrookes NHS Trust, Hills Rd., Cambridge CB2 2QQ, UK. E-mail: rm232@cam.ac.uk.
Supported by The Wellcome Trust, the National Institutes of Health, Papworth NHS Trust Research Funds, and the Medical Research Council (UK).
The authors have no conflicting financial interests.
Current address of J.P.: Adult CF Unit 6–045, St. Michael’s Hospital, 30 Bond St., Toronto M5B 1W8, Ontario, Canada.
References
- Johnson D, Travis J. Structural evidence for methionine at the reactive site of human α-1-proteinase inhibitor. J Biol Chem. 1978;253:7142–7144. [PubMed] [Google Scholar]
- Brantly M, Nukiwa T, Crystal RG. Molecular basis of alpha-1-antitrypsin deficiency. Am J Med. 1988;84(Suppl 6A):13–31. doi: 10.1016/0002-9343(88)90154-4. [DOI] [PubMed] [Google Scholar]
- Cichy J, Potempa J, Travis J. Biosynthesis of α1-proteinase inhibitor by human lung-derived epithelial cells. J Biol Chem. 1997;272:8250–8255. doi: 10.1074/jbc.272.13.8250. [DOI] [PubMed] [Google Scholar]
- Mornex JF, Chytil-Weir A, Martinet Y, Courtney M, LeCocq J, Crystal RG. Expression of the alpha-1-antitrypsin gene in mononuclear phagocytes of normal and alpha-1-antitrypsin-deficient individuals. J Clin Invest. 1986;77:1952–1961. doi: 10.1172/JCI112524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Bois RM, Bernandin JF, Paakko P, Hubbard R, Takahashi H, Ferrans V, Crystal RG. Human neutrophils express the alpha 1-antitrypsin gene and produce alpha 1-antitrypsin. Blood. 1991;77:2724–2730. [PubMed] [Google Scholar]
- Fagerhol MK, Cox DW. The pi polymorphism: genetic, biochemical, and clinical aspects of alpha-1 antitrypsin. Adv Hum Genet. 1981;11:1–62. [PubMed] [Google Scholar]
- Silverman GA, Bird PI, Carrell RW, Church FC, Coughlin PB, Gettins PG, Irving JA, Lomas DA, Luke CJ, Moyer RW, Pemberton PA, Remold-O’Donnell E, Salvesen GS, Travis J, Whisstock JC. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins Evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J Biol Chem. 2001;276:33293–33296. doi: 10.1074/jbc.R100016200. [DOI] [PubMed] [Google Scholar]
- Huber R, Carrell RW. Implications of the three-dimensional structure of α1-antitrypsin for structure and function of serpins. Biochemistry. 1989;28:8951–8966. doi: 10.1021/bi00449a001. [DOI] [PubMed] [Google Scholar]
- Potempa J, Korzus E, Travis J. The serpin superfamily of proteinase inhibitors: structure, function, and regulation. J Biol Chem. 1994;269:15957–15960. [PubMed] [Google Scholar]
- Wright HT, Scarsdale JN. Structural basis for serpin inhibitor activity. Proteins. 1995;22:210–225. doi: 10.1002/prot.340220303. [DOI] [PubMed] [Google Scholar]
- Loebermann H, Tokuoka R, Deisenhofer J, Huber R. Human α1-proteinase inhibitor Crystal structure analysis of two crystal modifications, molecular model and preliminary analysis of the implications for function. J Mol Biol. 1984;177:531–556. [PubMed] [Google Scholar]
- Stratikos E, Gettins PGW. Formation of the covalent serpin-proteinase complex involves translocation of the proteinase by more than 70Å and full insertion of the reactive centre loop into β-sheet A. Proc Natl Acad Sci USA. 1999;96:4808–4813. doi: 10.1073/pnas.96.9.4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilczynska M, Fa M, Karolin J, Ohlsson P-I, Johansson LB-A, Ny T. Structural insights into serpin-protease complexes reveal the inhibitory mechanism of serpins. Nat Struct Biol. 1997;4:354–357. doi: 10.1038/nsb0597-354. [DOI] [PubMed] [Google Scholar]
- Huntington JA, Read RJ, Carrell RW. Structure of a serpin-protease complex shows inhibition by deformation. Nature. 2000;407:923–926. doi: 10.1038/35038119. [DOI] [PubMed] [Google Scholar]
- Mahadeva R, Dafforn TR, Carrell RW, Lomas DA. Six-mer peptide selectively anneals to a pathogenic serpin conformation and blocks polymerisation: implications for the prevention of Z alpha-1 antitrypsin related cirrhosis. J Biol Chem. 2002;277:6771–6774. doi: 10.1074/jbc.C100722200. [DOI] [PubMed] [Google Scholar]
- Lomas DA, Evans DL, Finch JT, Carrell RW. The mechanism of Z α1-antitrypsin accumulation in the liver. Nature. 1992;357:605–607. doi: 10.1038/357605a0. [DOI] [PubMed] [Google Scholar]
- Sivasothy P, Dafforn TR, Gettins PG, Lomas DA. Pathogenic alpha 1-antitrypsin polymers are formed by reactive loop-beta-sheet A linkage. J Biol Chem. 2000;275:33663–33668. doi: 10.1074/jbc.M004054200. [DOI] [PubMed] [Google Scholar]
- Sharp HL, Bridges RA, Krivit W, Freier EF. Cirrhosis associated with alpha-1-antitrypsin deficiency: a previously unrecognised inherited disorder. J Lab Clin Med. 1969;73:934–939. [PubMed] [Google Scholar]
- Perlmutter DH. Liver injury in alpha-1 antitrypsin deficiency: an aggregated protein induces mitochondrial injury. J Clin Invest. 2002;110:1579–1583. doi: 10.1172/JCI16787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sveger T. Liver disease in alpha1-antitrypsin deficiency detected by screening of 200,000 infants. N Engl J Med. 1976;294:1316–1321. doi: 10.1056/NEJM197606102942404. [DOI] [PubMed] [Google Scholar]
- Eriksson S, Carlson J, Velez R. Risk of cirrhosis and primary liver cancer in alpha1-antitrypsin deficiency. N Engl J Med. 1986;314:736–739. doi: 10.1056/NEJM198603203141202. [DOI] [PubMed] [Google Scholar]
- Janciauskiene S, Dominaitiene R, Sternby NH, Piitulainen E, Eriksson S. Detection of circulating and endothelial cell polymers of Z and wild type alpha 1-antitrypsin by a monoclonal antibody. J Biol Chem. 2002;277:26540–26546. doi: 10.1074/jbc.M203832200. [DOI] [PubMed] [Google Scholar]
- Laurell C-B, Eriksson S. The electrophoretic α1-globulin pattern of serum in α1-antitrypsin deficiency. Scand J Clin Lab Invest. 1963;15:132–140. [Google Scholar]
- Lieberman J, Winter B, Sastre A. Alpha 1-antitrypsin pi-types in 965 COPD patients. Chest. 1986;89:370–373. doi: 10.1378/chest.89.3.370. [DOI] [PubMed] [Google Scholar]
- Silverman EK. Genetic epidemiology of COPD. Chest. 2002;121(Suppl 3):1S–6S. doi: 10.1378/chest.121.3_suppl.1s-a. [DOI] [PubMed] [Google Scholar]
- Snider GL, Lucey EC, Stone PJ. Animal models of emphysema. Am Rev Respir Dis. 1986;133:149–169. doi: 10.1164/arrd.1986.133.1.149. [DOI] [PubMed] [Google Scholar]
- Gross P, Pfitzer EA, Tolker E, Babyak MA, Kaschak M. Experimental emphysema Its production with papain in normal and silicotic rats. Arch Environ Health. 1965;11:50–58. doi: 10.1080/00039896.1965.10664169. [DOI] [PubMed] [Google Scholar]
- Janoff A, Sloan B, Weinbaum G, Damiano V, Sandhaus RA, Elias J, Kimbel P. Experimental emphysema induced with purified human neutrophil elastase: tissue localization of the instilled protease. Am Rev Respir Dis. 1977;115:461–478. doi: 10.1164/arrd.1977.115.3.461. [DOI] [PubMed] [Google Scholar]
- Eriksson S. Studies in α1-antitrypsin deficiency. Acta Med Scand. 1965;432:S1–S85. [PubMed] [Google Scholar]
- Gadek JE, Fells GA, Zimmerman RL, Rennard SI, Crystal RG. Antielastases of the human alveolar structures Implications for the protease-antiprotease theory of emphysema. J Clin Invest. 1981;68:889–898. doi: 10.1172/JCI110344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro SD. End-stage chronic obstructive pulmonary disease: the cigarette is burned out but inflammation rages on. Am J Respir Crit Care Med. 2001;164:339–340. doi: 10.1164/ajrccm.164.3.2105072c. [DOI] [PubMed] [Google Scholar]
- Stockley RA. Cellular and biochemical mechanisms in chronic obstructive pulmonary disease. Calverley P, Pride N, editors. Chapman and Hall,; London: Chronic Obstructive Pulmonary Disease. 1995:pp 93–133. [Google Scholar]
- Calverley PM, Walker P. Chronic obstructive pulmonary disease. Lancet. 2003;362:1053–1061. doi: 10.1016/s0140-6736(03)14416-9. [DOI] [PubMed] [Google Scholar]
- Lomas DA, Mahadeva R. Alpha1-antitrypsin polymerization and the serpinopathies: pathobiology and prospects for therapy. J Clin Invest. 2002;110:1585–1590. doi: 10.1172/JCI16782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantly ML, Paul LD, Miller BH, Falk RT, Wu M, Crystal RG. Clinical features and history of the destructive lung disease associated with alpha-1 antitrypsin deficiency of adults with pulmonary symptoms. Am Rev Respir Dis. 1988;138:327–366. doi: 10.1164/ajrccm/138.2.327. [DOI] [PubMed] [Google Scholar]
- Morrison HM, Kramps JA, Burnett D, Stockley RA. Lung lavage fluid from patients with α1-proteinase inhibitor deficiency or chronic obstructive bronchitis: anti-elastase function and cell profile. Clin Sci. 1987;72:373–381. doi: 10.1042/cs0720373. [DOI] [PubMed] [Google Scholar]
- Hubbard RC, Fells G, Gadek J, Pacholok S, Humes J, Crystal RG. Neutrophil accumulation in the lung in alpha 1-antitrypsin deficiency Spontaneous release of leukotriene B4 by alveolar macrophages. J Clin Invest. 1991;88:891–897. doi: 10.1172/JCI115391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolhouse IS, Bayley DL, Stockley RA. Sputum chemotactic activity in chronic obstructive pulmonary disease: effect of alpha-1 antitrypsin deficiency and the role of leukotriene B4 and interleukin 8. Thorax. 2002;57:709–714. doi: 10.1136/thorax.57.8.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott PR, Bilton D, Lomas DA. Lung polymers in Z α1-antitrypsin related emphysema. Am J Respir Cell Mol Biol. 1997;18:670–674. doi: 10.1165/ajrcmb.18.5.3065. [DOI] [PubMed] [Google Scholar]
- Parmar JS, Mahadeva R, Reed BJ, Farahi N, Cadwallader KA, Keogan MT, Bilton D, Chilvers ER, Lomas DA. Polymers of alpha(1)-antitrypsin are chemotactic for human neutrophils: a new paradigm for the pathogenesis of emphysema. Am J Respir Cell Mol Biol. 2002;26:723–730. doi: 10.1165/ajrcmb.26.6.4739. [DOI] [PubMed] [Google Scholar]
- Lomas DA, Evans DL, Stone SR, Chang W-SW, Carrell RW. Effect of the Z mutation on the physical and inhibitory properties of α1-antitrypsin. Biochemistry. 1993;32:500–508. doi: 10.1021/bi00053a014. [DOI] [PubMed] [Google Scholar]
- Owen CA, Campbell MA, Boukedes SS, Campbell EJ. Cytokines regulate membrane-bound leukocyte elastase on neutrophils: a novel mechanism for effector activity. Am J Physiol. 1997;272:L385–L393. doi: 10.1152/ajplung.1997.272.3.L385. [DOI] [PubMed] [Google Scholar]
- Hogg JC, Chu F, Utokaparch S, Woods RE, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Pare PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- Retamales I, Elliott WM, Meshi B, Coxson HO, Pare PD, Sciurba FC, Rogers RM, Hayashi S, Hogg JC. Amplification of inflammation in emphysema and its association with latent adenoviral infection. Am J Respir Crit Care Med. 2001;164:469–473. doi: 10.1164/ajrccm.164.3.2007149. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J. 2003;22:672–688. doi: 10.1183/09031936.03.00040703. [DOI] [PubMed] [Google Scholar]
- McElvaney NG, Stoller JK, Buist AS, Prakash UB, Brantly ML, Schluchter MD, Crystal RD. Baseline characteristics of enrollees in the National Heart, Lung and Blood Institute Registry of alpha 1-antitrypsin deficiency Alpha 1-Antitrypsin Deficiency Registry Study Group. Chest. 1997;111:394–403. doi: 10.1378/chest.111.2.394. [DOI] [PubMed] [Google Scholar]
- Carp H, Miller F, Hoidal JR, Janoff A. Potential mechanism of emphysema: α1-proteinase inhibitor recovered from lungs of cigarette smokers contains oxidised methionine and has decreased elastase inhibitory capacity. Proc Natl Acad Sci USA. 1982;79:2041–2045. doi: 10.1073/pnas.79.6.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogushi F, Fells GA, Hubbard RC, Straus SD, Crystal RG. Z-type α1-antitrypsin is less competent than M1-type α1-antitrypsin as an inhibitor of neutrophil elastase. J Clin Invest. 1987;80:1366–1374. doi: 10.1172/JCI113214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulgrew AT, Taggart CC, Lawless MW, Greene CM, Brantly ML, O’Neill SJ, McElvaney NG. Z alpha1-antitrypsin polymerizes in the lung and acts as a neutrophil chemoattractant. Chest. 2004;125:1952–1957. doi: 10.1378/chest.125.5.1952. [DOI] [PubMed] [Google Scholar]
- Dafforn TR, Mahadeva R, Elliott PR, Sivasothy P, Lomas DA. A kinetic mechanism for the polymerisation of α1-antitrypsin. J Biol Chem. 1999;274:9548–9555. doi: 10.1074/jbc.274.14.9548. [DOI] [PubMed] [Google Scholar]
- Hill AT, Campbell EJ, Bayley DL, Hill SL, Stockley RA. Evidence for excessive bronchial inflammation during an acute exacerbation of chronic obstructive pulmonary disease in patients with alpha(1)-antitrypsin deficiency (PiZ) Am J Respir Crit Care Med. 1999;160:1968–1975. doi: 10.1164/ajrccm.160.6.9904097. [DOI] [PubMed] [Google Scholar]