Abstract
Objective
Chronic use of methamphetamine (MA) has moderate effects on neurocognitive functions associated with frontal systems, including the executive aspects of verbal episodic memory. Extending this literature, the current study examined the effects of MA on visual episodic memory with the hypothesis that a profile of deficient strategic encoding and retrieval processes would be revealed for visuospatial information (i.e., simple geometric designs), including possible differential effects on source versus item recall.
Method
The sample comprised 114 MA-dependent (MA+) and 110 demographically-matched MA-nondependent comparison participants (MA−) who completed the Brief Visuospatial Memory Test – Revised (BVMT-R), which was scored for standard learning and memory indices, as well as novel item (i.e., figure) and source (i.e., location) memory indices.
Results
Results revealed a profile of impaired immediate and delayed free recall (p < .05) in the context of preserved learning slope, retention, and recognition discriminability in the MA+ group. The MA+ group also performed more poorly than MA− participants on Item visual memory (p < .05) but not Source visual memory (p > .05), and no group by task-type interaction was observed (p > .05). Item visual memory demonstrated significant associations with executive dysfunction, deficits in working memory, and shorter length of abstinence from MA use (p < 0.05).
Conclusions
These visual memory findings are commensurate with studies reporting deficient strategic verbal encoding and retrieval in MA users that are posited to reflect the vulnerability of frontostriatal circuits to the neurotoxic effects of MA. Potential clinical implications of these visual memory deficits are discussed.
Keywords: Methamphetamine, neuropsychological assessment, encoding, episodic memory, frontal lobe
INTRODUCTION
In recent decades, abuse of methamphetamine (MA) has risen in prevalence in the United States (Substance Abuse and Mental Health Services Administration, 2006) and globally (e.g., New Zealand; Degenhardt et al., 2010; Wilkins et al, 2006). Acute effects include increased energy and alertness, subjective increased physical and mental capacity, euphoria, and enhanced productivity (Cretzmeyer et al., 2003), as well as sleeplessness and increase in sexual behaviors during binge use (i.e., repeated administration over hours or days; Semple et al., 2003). Significant consequences related to MA intoxication include violent behavior (e.g., Von Mayrhauser et al., 2002) and automobile accidents (e.g., Logan, 2006). Chronic use results in several health complications, including hypertension (e.g., Chin et al., 2006) and stroke (Yen et al, 1994), as well as increased rates of comorbid infections such as HIV and hepatitis C virus (HCV) (Gonzales et al., 2006; Shoptaw et al., 2003). Psychiatric disorders also frequently co-occur with chronic MA abuse during both intoxication and withdrawal phases, including psychosis (McKetin et al., 2006) depression and increased risk for suicidal ideation (Kalechstein et al., 2000; Zweben et al., 2004), and bipolar disorder (Shoptaw et al., 2003). Long-term use often results in considerable disruption in daily functioning, including interpersonal difficulties (Cretzmeyer et al., 2003), impulsivity (Semple et al., 2004, 2005), and unemployment (Baberg et al., 1996).
As a psychostimulant, MA has considerable effects on the central nervous system (CNS). After readily crossing the blood-brain barrier (Barr et al., 2006), MA promotes release of dopamine, norepinephrine, and serotonin (Kokoshka et al. 1998). Although the exact neurotoxic pathways of MA are not fully understood, potential mechanisms of neural injury include oxidative stress, hyperthermia, and apoptosis. The neurotoxic effects of long-term MA use are most potent in the dopamine-rich fronto-striato-thalamocortical circuits (Cadet et al. 2005), including the striatum (Volkow et al., 2001a) and prefrontal cortex (Ernst et al., 2000), but MA-related neuropathology has also been observed in the medial temporal lobe and hippocampal formation (Tobias et al., 2010), as well as the parietal lobe (Jernigan et al., 2005). Emergent evidence also indicates that these MA-related effects on the CNS are associated with neurocognitive dysfunction, with upwards of 40% of MA-dependent individuals demonstrating global neuropsychological impairment (Rippeth et al., 2004). A recent meta-analysis by Scott and colleagues (2007) demonstrated that chronic MA use was associated with a medium overall effect size across multiple cognitive ability areas, with most significant deficits shown in episodic memory and executive functions. A recent study by Henry, Minassian, and Perry (2010) illustrated the potential clinical importance of MA-related neurocognitive dysfunction by demonstrating significant impairment on an objective measure of daily functioning ability (e.g., financial and medication management). It is therefore important to understand the specific elements of neurocognitive performance that are affected by chronic MA dependence so that these impairments can be more readily detected and studied in association with functional outcomes.
Episodic memory is a particularly critical neurocognitive ability because of its prevalence and severity of impairment in MA (Scott et al., 2007) and its importance for numerous daily activities (e.g., treatment adherence). The meta-analysis on MA-related neurocognitive effects (Scott et al., 2007) revealed a slightly larger effect size for learning as compared to delayed recall across studies, which was hypothesized to reflect deficient executive control of encoding and retrieval secondary to MA-related toxicity in the prefronto-striatal pathways (McCann et al., 1998; Volkow et al., 2001a). Importantly, executive dysfunction was another prominent neurocognitive feature of MA dependence revealed by the meta-analysis, likely characterized by poor set shifting and response inhibition (Scott et al., 2007). Accordingly, the pattern of findings within the domain of episodic memory is consistent with deficiency of higher-level (i.e., executive) processes that is typically observed in frontal systems dysfunction. That is, deficient list learning and delayed free recall have been observed in several studies (e.g., Kalechstein et al., 2003, Simon et al., 2004), and Woods and colleagues (2005) extended these findings by specifically examining the component processes of verbal episodic memory in MA dependent individuals. Utilizing a conceptual model developed by Moscovitch (1992, 1994), their study examined the verbal memory profile of MA users by delineating two components subserving explicit memory that are separable based on their relative roles and underlying neural correlates. Specifically, the associative/cue-dependent component that is dependent on the temporolimibic system to efficiently create lasting and accessible memory stores, and the strategic component that is reliant on frontal systems to initiate and effectively utilize organizational strategies to enhance encoding and retrieval. Their study revealed that MA-dependent individuals evidenced dysfunction of the strategic component processes as evidenced by impaired overall learning (i.e., poor performance on immediate recall trials) and delayed free recall, increased tendency to perseverate on previously generated items from the list (possibly reflecting poor cognitive flexibility), and deficient use of semantic clustering, which is a strategy for imposing organizational structure based on the elements within the list (i.e., categories in which the target words belong) that must be self-initiated (i.e., no instructions regarding semantic clustering are given). These deficits were in contrast to normal performance on associative/cue-dependent processes, including retention, suggesting that there was no evidence of rapid forgetting, and recognition discrimination, or accuracy in differentiating target words from foil words on a yes/no recognition trial in which recall demands are minimized.
Although the verbal memory profile has been clearly characterized, there has been little investigation of the profile of visual memory deficits in MA dependence. Visual aspects of memory warrant investigation given that neuroimaging studies have demonstrated abnormal findings within the parietal cortex in MA users, including reduced regional cerebral blood flow (Chang et al., 2002) and alterations in volume (Jernigan et al., 2005). This region, in combination with parieto-striato-frontal circuits, is involved with spatial cognition (Lawrence et al., 2000) and therefore visual memory could be impacted by disruption in its function. Furthermore, visual memory deficits may result in dysfunction in performing instrumental activities of daily living, including those that are known to be affected in MA dependent individuals, such as driving and employment.
It may therefore be informative to characterize visual memory performance in MA dependence by examining component processes of visual memory, including strategic and associative/cue-dependent processes that have been shown to be dissociable on verbal memory tasks in MA users (Woods et al., 2005). Moreover, another aspect of the Moscovitch model posits that the encoding and retrieval strategies are initiated and implemented by the frontal systems not only in response to task demands but also for placing the item information in proper temporal-spatial context (Moscovitch and Winocur, 2002), and as such this process may play a role in visual source memory, in which units of visual information are linked to contextual features (e.g., location) to construct an intact visual “episode.” A single dissociation exists between item and source memory such that an individual may recall the item information but not the source information (e.g., Glisky et al., 1995). For example, an individual may remember a simple visual design but not the location in which the design had been presented in an array (i.e., source memory deficit), which has previously been demonstrated among HIV-seropositive individuals (Morgan et al., 2009), a group that has also been shown to have deficient strategic control of encoding and retrieval (Woods et al., 2005). While item memory has been strongly linked to the medial temporal lobes, source memory (in this case, memory for location) may be especially reliant upon frontal systems functioning because it likely requires self-initiated, strategic encoding and retrieval strategies for binding the item and contextual features to promote successful memory performance (Mitchell and Johnson, 2009; Shimamura, 1995). In fact, studies have demonstrated the single item-source dissociation in several populations with frontal systems dysfunction (Glisky et al., 1995; Pirogovsky et al., 2007). Furthermore, that item and source memory are reliant upon overlapping, but separable neural substrates has been reported by several functional MRI (fMRI) studies that revealed selective altered BOLD activity in the prefrontal cortex, but not the medial temporal lobe, during source memory tasks (Dobbins et al., 2002; Slotnick et al., 2003), including tasks involving memory for spatial context (Hayes et al., 2004).
The aim of the current study was to characterize the visual episodic memory profile of individuals with chronic MA dependence. Given that self-initiated, strategic processes are dependent upon frontal systems and therefore may be preferentially affected by MA, it was hypothesized that chronic MA users would demonstrate poorer learning and free recall of visual designs in the context of intact retention and recognition discrimination. Furthermore, we hypothesized that interactive effects would be evident such that a disproportionately greater effect of MA dependence would be observed for source memory (location recall) versus item memory (figure recall). To further examine these findings in the context of the cognitive mechanisms at work, the associations between deficient visual memory deficits and measures of executive functions and working memory were evaluated. We also examined potential relationships between the cognitive variables of interest and MA use parameters to further characterize the effect of MA dependence on visual episodic memory.
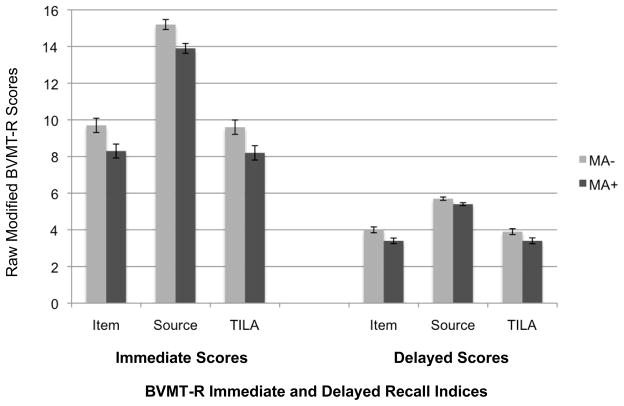
Figure 1.
A bar chart displaying BVMT-R Item Immediate and Delay, Source Immediate and Delay, and TILA Immediate and Delay scores in the methamphetamine dependent (MA+) and comparison (MA−) groups. Error bars represent standard error of the mean. All group differences were significant, p < .05
MATERIALS AND METHODS
Participants
The sample comprised 114 individuals in the MA-dependent group (MA+) and 110 participants in a comparison group who have never met criteria for MA dependence (MA−). These groups represent a subset of a larger sample (including MA+ and MA− individuals) of participants in an ongoing study funded by the National Institute on Drug Abuse (NIDA) for which participants were recruited from the San Diego community using a variety of outreach measures by a Participant Accrual and Retention team (e.g., advertisements). For determination of eligibility for each of the study groups, all participants were administered the Structured Clinical Interview for DSM-IV (SCID; First et al., 1996) for diagnosis of psychiatric disorders, including substance use disorders, according to the Diagnostic and Statistical Manual-IV (DSM-IV; American Psychiatric Association, 1994) criteria. Interviewers included staff psychologists, postdoctoral psychology fellows, and advanced clinical psychology doctoral students who were trained to criterion standards. Characteristics of MA use, including frequency, duration, and quantity of use, were collected using a semistructured timeline follow-back interview (i.e., chronologically identifying epochs of substance use to obtain detailed information about patterns of use over time).
Recent alcohol or drug abuse (i.e., within the last year) or dependence (i.e., within the past five years) represented exclusion criteria for both groups (with the exception of MA abuse or dependence in the MA+ group) in the larger, ongoing NIDA study. Specifically, individuals meeting DSM-IV criteria for the following were excluded: alcohol dependence within the last year or a remote (i.e., > 5 years) but significant history of alcohol dependence; drug dependence within 5 years of the evaluation and/or drug abuse within the year prior to the evaluation (with the exception of MA for the MA+ group); remote (i.e., > 5 years) but significant history of drug dependence (with the exception of MA for the MA+ group). Exceptions to these exclusion criteria include histories of alcohol or marijuana abuse and marijuana dependence, which are frequently comorbid in MA users and therefore individuals with such histories were not excluded from either study group in order to recruit a representative MA sample and an appropriate non-MA-using comparison group. Participants met inclusion criteria for the MA+ group if they had met criteria for MA dependence in their lifetime, as well as met criteria for MA abuse or dependence within the last 18 months. At least 10 days of abstinence prior to testing was required for inclusion (by self-report), and a urine toxicology screen was conducted for all participants to confirm recent abstinence from all illicit substances with the exclusion of marijuana. For the present study, in order to better evaluate the effect of MA dependence on visual memory performance, the MA− comparison group was specifically recruited to have comparable prevalence of risk factors for MA abuse and dependence (e.g., there was no significant difference in the rate of Major Depressive disorder, or MDD, between the groups) and other important cofactors (e.g., rate of HCV infection).
Given the emphasis of this study on the neurocognitive effects of MA dependence, individuals with histories of HIV infection, neurological diseases (e.g., seizure disorders, closed head injuries with loss of consciousness greater than 30 minutes, and central nervous system neoplasms or opportunistic infections) or severe psychiatric illnesses (e.g., schizophrenia), all of which may also affect cognitive functioning, were excluded. As shown in Table 1, the groups were comparable with regard to demographics (i.e., age, education, sex, ethnicity) and estimated premorbid verbal intelligence (all p > .05).
Table 1.
Demographic and Psychiatric Characteristics of the Study Participants
| Variable | MA− (n = 110) | MA+ (n = 114) | p |
|---|---|---|---|
| Demographic Characteristics | |||
| Age (years) | 37.6 (12.4) | 37.3 (9.3) | 0.83 |
| Education (years) | 12.4 (1.9) | 11.9 (2.3) | 0.11 |
| Sex (% men) | 63.6 | 70.2 | 0.30 |
| Ethnicity (% Caucasian) | 69.1 | 76.3 | 0.26 |
| Estimated verbal IQ a | 99.9 (10.5) | 97.5 (9.5) | 0.07 |
| Hepatitis C Infection (% seropositive) | 21 (20.4%) | 36 (31.6%) | 0.06 |
| Psychiatric Characteristics b | |||
| Major Depressive Disorder (%) | |||
| Current | 7.3 | 6.1 | 0.79 |
| Lifetime | 31.8 | 30.7 | 0.89 |
| Bipolar Disorder(%) | |||
| Current | 0.0 | 4.4 | 0.06 |
| Lifetime | 0.9 | 6.1 | 0.07 |
| ADHD | |||
| Current | 0.9 | 10.0 | 0.005 |
| Lifetime | 2.8 | 15.5 | 0.002 |
| ASPD | 0.0 | 23.7 | <0.001 |
| Methamphetamine Use Characteristics c | |||
| Last use (days since last use) | -- | 91 (45, 167) | -- |
| Cumulative duration of use (days of use) | -- | 3960 (2130, 5826) | -- |
| Cumulative quantity of use d | -- | 2908.0 (1180.0, 5833.0) | -- |
| Duration of use in last 30 days (days) | -- | 3.0 (2.0, 8.0) | -- |
| Quantity used in last 30 days d | -- | 3.0 (0.3, 6.0) | -- |
| Quantity used in last 12 months d | -- | 136.8 (18.0, 540.0) | -- |
| Other Substance Use Disorders e | |||
| Canabis dependence | 8.2 | 19.3 | 0.02 |
| Opioid dependence | 5.5 | 4.4 | 0.77 |
| Cocaine dependence | 6.4 | 18.4 | 0.02 |
| Alcohol dependence | 15.5 | 41.2 | <0.001 |
| Other substance dependence f | 1.8 | 1.8 | 0.97 |
| POMS Total Mood Disturbance g | 51.9 (35.6) | 64.4 (39.8) | 0.014 |
Note.
Based on the WRAT-3 Reading Standard Score;
p-values based on Fisher’s Exact Test;
data presented as medians and IQRs;
quantity of MA used presented in grams;
lifetime diagnoses;
other substance dependence = hallucinogens and sedatives; and
POMS = Profile of Mood States.
Materials and Procedure
Written, informed consent was obtained from all participants who then completed comprehensive neuropsychological, psychiatric, and medical research assessments that were administered as part of a larger, ongoing investigation.
Visual Memory
The Brief Visuospatial Memory Test – Revised (BVMT-R; Troyer et al., 2008) was administered according to standardized procedures described in the test manual. Three learning trials involved presentation of a 2 × 3 array of 6 simple visual designs for 10 seconds each time, followed by an immediate free recall trial in which the participants were given a blank sheet of paper and instructed to draw the figures as accurately as possible and in their correct location. A delayed recall trial was administered approximately 25 minutes after the third trial was completed, at which time participants were asked to draw the figures from memory. The delayed recall trial was followed by a recognition trial, in which the participants were shown a series of 12 figures including both target and foil figures, and were asked to indicate whether or not each figure had been included in the original array. Lastly, the array was again presented to the participants, who were then asked to copy the figures.
Standardized scoring for the BVMT-R yields immediate learning trial scores that are summed across the three trials to create a total learning score, as well as a delayed recall score, recognition discriminability, and a copy trial score. According to the Benedict (1997) standardized scoring rules, each drawn figure is scored for accuracy (1 point) and placement in the correct location (1 point), with a maximum of 2 points per figure. A figure may receive a point for correct location without receiving an accuracy point. Notably, the accuracy and placement points are collapsed into a single, summed score per trial (maximum of 12 points) and the total learning score is summed across three immediate learning trials (Total Leaning: maximum of 36 points). Given that this does not allow for separate examination of item and location scores, Troyer and colleagues (2008) developed a scoring scheme whereby the points for accuracy and location were scored separately. The current study employed a similar modified system of scoring the standard BVMT-R administration such that drawn figures were scored according to BVMT-R manual criteria (for full-credit points) and the accuracy and location points were independently summed for each immediate learning trial (maximum score = 6) and across the three trials (maximum score = 18) to yield two of the primary outcome variables for the current study: Item Immediate (based on accuracy points summed across three learning trials) and Source Immediate (based on location points summed across three learning trials). Delay trials were also scored separately (i.e., Item Delay and Source Delay). Another set of scores was calculated by awarding location points only for those figures that received full credit for accuracy and were drawn in the correct location, which was labeled Total Item-Location Accuracy Immediate (TILA Immediate) because it reflects both accurate item and location recall for each figure per trial (i.e., source memory performance in the context of accurate item performance). A similar variable was created for delayed memory, namely Total Item-Location Accuracy Delay (TILA Delay). The difference between the Item Immediate and TILA scores yielded location error scores (i.e., Errors of Location Immediate and Errors of Location Delay). Given that the TILA Immediate and Delay variables restrict the scoring of location points to those figures that received accuracy (i.e., item) points, the discrepancy between the item and total scores reflects the number of figures that were drawn accurately but in the incorrect location.
Neurobehavioral Battery
In addition to the item and source memory tasks described above, participants were administered several other standardized neuropsychological tests, including: Stroop Color-Word Interference Test (Golden, 1978), Halstead Category Test (Reitan and Wolfson, 1993), Paced Auditory Serial Addition Test (PASAT; Gronwall, 1977), Trail Making Test (TMT; Army Individual Test Battery, 1994), Wisconsin Card Sorting Test – 64 Card Version (WCST-64; Kongs et al., 2000), Controlled Oral Word Association Test (COWAT; Benton et al., 1983), and Category Fluency (Animals; Gladsjo et al., 1999). As stated above, psychiatric assessment was conducted using the SCID (First et al., 1996) administered by trained clinicians and advanced trainees to establish lifetime and current (i.e., endorsing clinical symptoms within 1 month of evaluation) diagnoses of psychiatric (e.g., mood) and substance use disorders according to DSM-IV criteria, including MA use disorders. MA use characteristics were obtained using a semistructured timeline follow-back interview in order to collect information regarding frequency, duration, and quantity of use throughout the various epochs in the participants’ total history of use, and although this information is necessarily self-reported, the structure imposed by the trained interviewers was aimed at collecting as accurate and detailed information regarding substance use characteristics as possible. Participants also completed the Profile of Mood States (POMS; McNair et al., 1981), a self-report measure of current (i.e, past seven days) mood states. The POMS Total Mood Disturbance score was used in all analyses, with higher scores indicating greater general affective distress.
RESULTS
Profile of Item Memory
We examined the profile of visual memory indices to evaluate the hypothesis that pattern would be consistent with deficient strategic memory processes (Woods et al., 2005). Table 2 displays the visual memory profile scores and demonstrates that, concordant with our hypothesis, no significant differences were found for associative/cue-dependent scores, including Learning Slope (p = .13, Cohen’s d = 0.18), Item Percent Retained (which is Delayed Recall performance relative to Trial 3 Item total; p = .2, Cohen’s d = .19), Item Recognition Discriminability (p = .36, Cohen’s d = 0.13), or the Item Copy Trial (p = .58, Cohen’s d = .07). However, the MA+ group was significantly worse than the MA− sample on scores that may reflect a strategic deficit, including Trial 1 (p = .001, Cohen’s d = 0.43), Total Learning (p = .002, Cohen’s d = 0.43), Delayed Free Recall (p = .02, Cohen’s d = 0.32) and the Item Retrieval Index, which is the difference between Item Recognition Discriminability and Item Delayed Free Recall (p = .01, Cohen’s d = .36).
Table 2.
Group Differences on BVMT-R Outcome Variables
| Variable | MA− (n = 110) | MA + (n = 114) | pa | db |
|---|---|---|---|---|
| Item Memory | ||||
| Trial 1 | 6.3 (2.7) | 5.2 (2.3) | .001 | 0.43 |
| Total Learning (Trials 1–3) | 25.2 (6.5) | 22.5 (6.1) | .002 | 0.43 |
| Learning Slope | 1.8 (1.1) | 2.0 (1.1) | .13 | 0.18 |
| Delayed Free Recall | 9.6 (2.1) | 8.9 (2.3) | .02 | 0.32 |
| Percent Retained | 99.3 (33.7) | 93.0 (38.5) | .20 | 0.19 |
| Recognition Discriminability | 5.7 (0.6) | 5.6 (0.6) | .63 | 0.17 |
| Retrieval Index | 1.7 (1.4) | 2.2 (1.5) | .01 | 0.36 |
| Copy | 11.5 (0.8) | 11.5 (0.8) | .96 | 0 |
Note.
p-value based on one-way ANOVA;
Cohen’s d effect size estimate.
BVMT-R scores based on the modified scoring system for the present study are presented in Figure 1. The MA+ and MA− groups differed on Item Immediate (p = .01, Cohen’s d = 0.31), Item Delay (p = .006, Cohen’s d = 0.35), Source Immediate (p = .0007, Cohen’s d = 0.48), Source Delay (p = 0.03, Cohen’s d = 0.38), TILA Immediate (p = .01, Cohen’s d = 0.31), and TILA Delay (p = .005, Cohen’s d = 0.29). Mixed model ANOVAs revealed significant main effects of group [F (1, 222) = 10.1, p = .002, partial eta squared = 0.04] and task type [Item Immediate vs. Source Immediate; F (1, 222) = 760.1, p < .0001, partial eta squared = 0.77], but no significant group by task-type interaction effects [F (1, 222) = 0.01, p = .91, partial eta squared = 0.00]. Notably, the TILA Immediate score was created as a measure of location information that was correctly associated with item information, but the restriction of location scoring to only those figures with item points resulted in a correlation of 0.99 between TILA Immediate with Item Immediate (p < .0001). We next examined the Errors of Location variables because they represent the discrepancy between item memory and total memory performance (i.e., figures that received Item Immediate points but not TILA Immediate points due to incorrect figure placement). For both immediate and delay trials, there was restriction of range observed for the error variables in both groups (i.e., Errors of Location Immediate, p = .52, Cohen’s d = 0; Errors of Location Delay, p = .44, Cohen’s d = 0). Such restricted range in these variables suggests that the collinearity between Item Immediate and TILA Immediate is due to the low prevalence of location errors, or correctly drawn figures placed in the incorrect location. That is, in most cases when an item was drawn correctly, it was drawn in the correct location. In fact, the prevalence of one or more location errors was quite low in both the MA+ (8.8%) and MA− (6.3%) groups. Given that the ceiling effect for the source memory portion of the BVMT-R task resulted in collinearity between the Item and TILA scores and a low prevalence of location errors (observed as a floor effect for the error variable), between-group analyses using these variables were not performed.
Post-hoc Evaluation of Potential Confounds
Follow-up multiple linear regression analyses confirmed that the MA effect remained significant (i.e., MA group status remained a significant predictor) when important psychiatric (i.e., lifetime diagnoses of Bipolar Disorder and Attention Deficit Hyperactivity Disorder [ADHD]; Antisocial Personality Disorder [ASPD] diagnosis; lifetime diagnoses of Substance Dependence for the following: cannabis, cocaine, and alcohol; self-reported affective distress [POMS Total Mood Disturbance]) and medical cofactors (i.e., HCV infection) upon which the MA+ and MA− groups differed were considered simultaneously as predictors of Item Immediate and Delay (p < .05); the MA effect was evident at a trend level for the Retrieval Index (p = .068) in a similar analysis.
Next, planned correlational analyses were conducted to examine potential associations between Item Immediate and several measures of executive functions. These analyses revealed significant associations in both the MA+ and MA− groups (p < .05, with the exception of letter fluency in the MA− group), and are summarized in Table 3. The associations were observed in the expected directions and were characterized by generally moderate effect sizes.
Table 3.
Correlationsa Between BVMT-R Item Immediate Total and Standard Neuropsychological Tests of Executive Functions and Working Memory
| Variable | MA− (n = 110) | MA+ (n = 114) |
|---|---|---|
| Stroop Color-Word Incongruence | 0.40*** | 0.32** |
| Category Fluency (Animals) | 0.29** | 0.37*** |
| Letter Fluency (COWAT) | 0.06 | 0.29** |
| Halstead Category Total Errors | −0.48*** | −0.43*** |
| PASAT 200 | 0.41*** | 0.22* |
| WAIS-III Letter-Number Sequencing | 0.32** | 0.26** |
| Trail Making Test Part B (time) | −0.44*** | −0.42*** |
| WCST-64 Perseverative Responses | −0.38*** | −0.30** |
Note.
Spearman’s rho:
p < 0.0001,
p < 0.01,
p < .05; COWAT = Controlled Oral Word Association Test; PASAT = Paced Auditory Serial Addition Task; WAIS-III = Wechsler Adult Intelligence Scale (3rd ed.); WCST-64 = Wisconsin Card Sorting Test – 64 Card Version.
Finally, correlational analyses were conducted in the MA+ group to investigate the association between MA use variables and both Item Immediate and Total Learning performance. Notably, Item Immediate was significantly and positively correlated with Last Use of MA (greater value indicates longer time elapsed since last use), Spearman’s rho = 0.23, p = .01, as was Total Learning, Spearman’s rho = .20, p = .04. All other MA use characteristics were not significantly associated with either Item Immediate or Total Learning (p > .10).
DISCUSSION
Given the global prevalence of MA dependence (Degenhardt et al., 2010) and its effect on everyday functioning (Henry et al., 2010), it is important to understand the profile of neurocognitive deficits related to chronic MA use. The present study characterized visual memory performance among chronic MA users by demonstrating significant effects of MA dependence on strategic control of encoding and retrieval as compared to associative/cue-dependent processes, in accordance with a cognitive paradigm integrating frontal systems and memory functions (i.e., Moscovitch, 1992, 1994). Specifically, significantly poorer performance of the MA-dependent group relative to the comparison group was observed on measures of learning (i.e., initial learning and total learning across multiple trials) and delayed free recall, but there was no evidence of rapid forgetting as indexed either through retention or recognition discriminability. Furthermore, the groups differed significantly on the Item Retrieval Index of the BVMT-R, which provides a direction comparison (i.e., a difference score) between recognition performance (lower strategic demand) and free recall (greater strategic demand) such that a higher score is indicative of a retrieval deficit rather than a purer deficit in encoding, which would manifest as impaired recognition memory performance. These data are concordant with Woods and colleagues (2005), who reported that on a verbal learning and memory task, MA-dependent individuals evidenced deficiencies in the use of strategic, but not automatic, aspects of verbal learning and memory. Accordingly, these findings indicate that MA-dependent individuals have greater difficulty with higher-level control of visual memory processes, such as use of strategies to enhance encoding and retrieval of novel visual information. The results of this study thereby expand our understanding of the effects of chronic MA-use on episodic memory, which had previously been largely based on evidence from verbal paradigms (Scott et al., 2007).
These specific findings regarding visual memory raise important questions about the clinical significance of these effects due to potential implications for everyday functioning. Indeed, a visual memory deficit may have several potential negative effects on a range of instrumental activities of daily living. Automobile accidents have been shown to be common among MA users (Logan, 1996), and visual memory deficits have been linked to driving ability in Parkinson’s disease (Uc et al., 2009) and multiple sclerosis (Lincoln and Radford, 2008). Therefore, it is possible that the demonstrated pattern of visual memory deficits among MA dependent individuals may have a role in increase risk for driving difficulties, including accidents. A related ability that may also be impacted by visual memory deficits is route-finding, difficulty with which could interfere with an individual’s ability to independently navigate while driving, as well as other modes of transportation (e.g., following a complex route using public transportation). Failure to successfully navigate to a variety of destinations could result in several other types of functional difficulties downstream, such as finding a new doctor’s office, attending social engagements, or job interviews. Visual memory abilities are also required for many types of vocational activities, ranging from a more direct impact, as in the case of truck drivers and construction workers, to secondary influences that still interfere with job performance (e.g., efficient use of computers). Furthermore, poor visual memory may also increase difficulty with medication management, and failure to comply with a prescribed medication regimen has numerous potential health consequences, including poor outcomes for substance dependence treatment involving medication.
The present study also evaluated the hypothesis that a greater effect of lifetime MA dependence would be observed for an index of visual source memory (i.e., location) than visual item memory (i.e., figure) based on the purported role self-initiated, strategic processes in successful source memory performance. Although significant differences were observed between the MA+ and MA− groups for the Source Immediate score (i.e., location points awarded regardless of item accuracy), there was no meaningful interaction effect for this criterion in the context of item memory (as shown in Figure 1). The lack of a group by task-type (Item Immediate versus Source Immediate) interaction, in addition to low prevalence of location errors in both groups, suggests that the group-level difference on the Source Immediate score was driven by the significant MA effect on item memory (i.e., Item Immediate). That is, the MA+ group recalled fewer figures than the MA− group across the learning trials (reflected in the Item Immediate score), but the low rates of Errors of Location in both groups suggests that figures that were recalled tended to be placed in the correct location in the array.
These findings might seem to suggest that source memory is not impaired in MA dependence, but further study with different methodology is needed to fully evaluate this effect. Specifically, the scoring modification of the BMVT-R provided an approximation of item and source memory performance, but given that immediate item memory was evaluated with a recall task, the challenging nature of the task may have affected the findings. In most cases, those studies that have demonstrated a dissociation between item and source memory in frontal systems populations have used a recognition paradigm. Relatedly, the Item and TILA indices were highly collinear, suggesting that when an individual was able to correctly recall a figure, he/she was also able to recall the correct location of the figure in the array on this task. Furthermore, the structured item array (i.e., presentation of figures in a 2 × 3 grid) may have rendered the source (i.e., location) information too easy, further obscuring the interpretation. According to Chapman and Chapman (1978), the relatively large variation in difficulty between these two tasks makes it difficult to assess a potential differential deficit. Consequently, we may have observed a greater MA-related effect on source memory indices if the figures were presented in a random scatter on the array, which would reduce the ceiling effect by placing greater organizational demand on the strategic aspects of visual learning and memory, thereby increasing the difficulty of this task. A paradigm such as this might also yield stronger evidence of a source deficit because participants are instructed to attend to both the item and source information (i.e., the figure and its location in the array), which addresses the consistent critique that demonstrations of the single dissociation between item and source memory were based on differential task types (intentional versus incidental) and levels of difficulty.
Further supporting the frontal systems hypothesis suggested by the overall profile of deficient strategic control of visual memory in MA users, significant associations (median effect size = 0.31, Interquartile Range (IQR) = 0.26, 0.40) were observed in the expected directions between item memory performance and measures of executive functions and working memory in the MA+ group. Specifically, significant correlations were evident between Item Immediate and errors on a test of logical analysis and new concept formation (Halstead Category Test), timed complex sequencing (Trailmaking Part B), rule-guided word generation (verbal fluency), prepotent response inhibition (Stroop Color-Word), speeded working memory (PASAT) and cognitive flexibility (WCST-64 perseverations). These significant associations provide convergent validity for the hypothesis that Item Immediate deficits were driven by frontal systems dysfunction, which was based on evidence in the literature demonstrating the preferential impact of chronic MA use on frontal cortex and frontostriatal circuitry via neurotransmitter (Cadet et al., 2005), metabolic (Ernst et al., 2000; cf. Taylor et al., 2007), and structural (Chang et al., 2005; Jernigan et al., 2005) alterations. A similar pattern was observed in the MA− group. However, it should be noted that we do not have evidence of divergent validity. Furthermore frontal systems are purported to work in concert with the hippocampus with regard to memory functioning and some evidence for a MA effect on hippocampal formation integrity (including white matter pathways) has been presented (Thompson et al., 2004; Tobias et al., 2010). Similarly, the posterior parietal cortex (PPC) has a role in visual processing and executive functions, and findings of MA-related structural changes (Jernigan et al., 2005), alterations in regional cerebral blood flow (Berman et al., 2002), and altered glucose metabolism (Hwang et al., 2006) in the PPC have been reported. Therefore the potential contribution of medial temporal or PPC dysfunction in visual item memory cannot be excluded.
Given that no neuroimaging or biomarker data have been presented in relation to these findings, a limitation of the current study is that interpretations regarding regional brain involvement (i.e., frontostriatal circuits) are necessarily inferential. Furthermore, the small effect observed in a relatively large sample raises that possibility that the high comorbidity of psychiatric and other substance use disorders in dependent MA users limits conclusions regarding a direct association between chronic MA use and item memory impairment. However, Item Immediate was significantly correlated with time since last MA use, which indicates that an independent MA effect was present. Given the presence of this signal, the present study used a non-MA using comparison group that had comparable prevalence of MA abuse risk factors (e.g. HCV infection, Major Depressive Disorder) in an effort to further temper limitations to interpretation of our results due to comorbidity. For those factors in which the MA-dependent group and the comparison group were not comparable (e.g., Bipolar Disorder, Substance Dependence) we statistically examined their potential effect using multiple linear regression analyses, which revealed that MA group status remained a significant predictor of those BVMT-R item memory measures that differed in the between-groups analysis (including Item Immediate and Delay, and the Retrieval Index at a trend level) when considered simultaneously with the other factors. Moreover, the groups performed similarly on the BVMT-R Copy trial, which suggests that our findings were not confounded by group differences in visuoconstructional ability. Thus, although we cannot entirely rule out the influence of these factors, there does appear to be strong evidence of a MA-related effect given this relatively conservative evaluation of potential confounds.
Importantly, post-hoc analyses of the association between item memory performance and MA-use indices potentially shed additional light on the effect of recency of use and possible recovery of function. Although there was no relationship between MA-use variables such as duration and quantity of MA use and the Item Immediate score, a statistically (and possibly clinically) significant association was observed for last use of MA, which indicates that greater duration of abstinence (i.e., last use was more remote) was associated with better item memory performance. Although it has been well documented that extended abstinence is associated with some degree of recovery of neural functioning (e.g., dopamine terminal functioning, metabolism, rCBF, and reduction in reactive microgliosis; (Hwang et al., 2006; Sekine et al., 2008; Volkow et al., 2001b; Wang et al., 2004), there has been less consistent evidence that this recovery extends to improved neuropsychological functioning, including episodic memory (Wang et al., 2004). The findings from the current study provide evidence that some aspects of the episodic memory deficit observed in MA-dependent individuals may improve with time; specifically, the retrieval deficit on a visual item memory index was reduced in those individuals with a greater duration of abstinence. Although cross-sectional in nature, this finding is consistent with the results of a longitudinal study of neurocognitive functioning among MA dependent individuals that demonstrated improved global neurocognitive performance after one year of sustained abstinence, such that the performance of the abstinent groups was comparable to controls whereas the MA-using group showed impaired performance (Iudicello et al., 2010).
Evidence regarding the effects of recency of use on neuropsychological functioning is of considerable importance as it may have implications for the delivery of treatment. Given that cognitive impairment has been linked to poorer outcomes in substance abuse treatment (Fals-Stewart, 1993), a better understanding of the relationship between potential recovery of neurocognitive functioning and duration of abstinence from MA may influence the timing of treatment administration in order to maximize positive outcomes. Furthermore, recent evidence shows that MA dependence is associated with decreased everyday functioning, possibly related to executive dysfunction (Henry et al., 2010), further highlighting the importance of understanding the impact of MA use on cognitive functioning and facilitating appropriate treatment. As stated previously, impaired visual memory may have negative implications for important daily activities such as driving, route finding, employment, and medication management, and therefore these outcomes represent important future directions for research on MA-dependent individuals.
Acknowledgments
The Translational Methamphetamine AIDS Research Center (TMARC) is supported by Center award P50DA026306 from the National Institute on Drug Abuse (NIDA) and is affiliated with the University of California, San Diego (UCSD) and the Sanford-Burnham Medical Research Institute (SBMRI). The TMARC is comprised of: Director – Igor Grant, M.D.; Co-Directors – Ronald J. Ellis, M.D., Ph.D., Cristian L. Achim, M.D., Ph.D., and Scott L. Letendre, M.D.; Center Manager – Steven Paul Woods, Psy.D.; Assistant Center Manager – Aaron M. Carr, B.A.; Clinical Assessment and Laboratory (CAL) Core: Scott L. Letendre, M.D. (Core Director), Ronald J. Ellis, M.D., Ph.D., Rachel Schrier, Ph.D.; Neuropsychiatric (NP) Core: Robert K. Heaton, Ph.D. (Core Director), J. Hampton Atkinson, M.D., Mariana Cherner, Ph.D., Thomas D. Marcotte, Ph.D.; Neuroimaging (NI) Core: Gregory Brown, Ph.D. (Core Director), Terry Jernigan, Ph.D., Anders Dale, Ph.D., Thomas Liu, Ph.D., Miriam Scadeng, Ph.D., Christine Fennema-Notestine, Ph.D., Sarah L. Archibald, M.A.; Neurosciences and Animal Models (NAM) Core: Cristian L. Achim, M.D., Ph.D. (Core Director), Eliezer Masliah, M.D., Stuart Lipton, M.D., Ph.D.; Administrative Coordinating Core (ACC) – Data Management and Information Systems (DMIS) Unit: Anthony C. Gamst, Ph.D. (Unit Leader), Clint Cushman (Unit Manager); ACC – Statistics Unit: Ian Abramson, Ph.D. (Unit Leader), Florin Vaida, Ph.D., Reena Deutsch, Ph.D., Anya Umlauf, M.S.; ACC – Participant Unit: J. Hampton Atkinson, M.D. (Unit Leader), Rodney von Jaeger, M.P.H. (Unit Manager); Project 1: Arpi Minassian, Ph.D. (Project Director), William Perry, Ph.D., Mark Geyer, Ph.D., Brook Henry, Ph.D.; Project 2: Amanda B. Grethe, Ph.D. (Project Director), Martin Paulus, M.D., Ronald J. Ellis, M.D., Ph.D.; Project 3: Sheldon Morris, M.D., M.P.H. (Project Director), David M. Smith, M.D., M.A.S., Igor Grant, M.D.; Project 4: Svetlana Semenova, Ph.D. (Project Director), Athina Markou, Ph.D.; Project 5: Marcus Kaul, Ph.D. (Project Director).
This research was also supported by National Institute of Health grants DA12065, MH62512, and T32 AA013525. The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States Government.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, D.C: Author; 1994. [Google Scholar]
- 2.Army Individual Test Battery. Manual of directions and scoring. Washington, D.C: War Department, Adjutant General’s Office; 1994. [Google Scholar]
- 3.Baberg HT, Nelesen RA, Dimsdale JE. Amphetamine use: return of an old scourge in a consultation psychiatry setting. American Journal of Psychiatry. 1996;153:789–793. doi: 10.1176/ajp.153.6.789. [DOI] [PubMed] [Google Scholar]
- 4.Barr AM, Panenka WJ, MacEwan GW, et al. The need for speed: An update on methamphetamine addiction. Journal of Psychiatry & Neuroscience. 2006;31:301–313. [PMC free article] [PubMed] [Google Scholar]
- 5.Benedict RHB. Brief Visuospatial Memory Test-Revised. Lutz, FL: Psychological Assessment Resources; 1997. [Google Scholar]
- 6.Benton AL, Hamsher K, Sivan AB. Multilingual Aphasia Examination. 3. Iowa City, IA: AJA Associates; 1983. [Google Scholar]
- 7.Berman SM, Voytek B, Mandelkern MA, et al. Changes in cerebral glucose metabolism during early abstinence from chronic methamphetamine abuse. Molecular Psychiatry. 2002;13:897–908. doi: 10.1038/sj.mp.4002107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cadet JL, Jayanthi S, Deng X. Methamphetamine-induced neuronal apoptosis involves the activation of multiple death pathways. Neurotoxicity Research. 2005;8:199–206. doi: 10.1007/BF03033973. [DOI] [PubMed] [Google Scholar]
- 9.Chang L, Ernst T, Speck O, et al. Perfusion MRI and computerized cognitive test abnormalities in abstinent methamphetamine users. Journal of Psychiatry Reasearch. 2002;114:65–79. doi: 10.1016/s0925-4927(02)00004-5. [DOI] [PubMed] [Google Scholar]
- 10.Chang L, Cloak C, Patterson K, Grob C, Miller EN, Ernst T. Enlarged striatum in abstinent methamphetamine abusers: A possible compensatory response. Biological Psychiatry. 2005;57:967–974. doi: 10.1016/j.biopsych.2005.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman LJ, Chapman JP. The measurement of differential deficit. Journal of Psychiatric Research. 1978;14:303–311. doi: 10.1016/0022-3956(78)90034-1. [DOI] [PubMed] [Google Scholar]
- 12.Chin KM, Channick RN, Rubin LJ. Is methamphetamine use associated with idiopathic pulmonary arterial hypertension? Chest. 2006;130:1657–1663. doi: 10.1378/chest.130.6.1657. [DOI] [PubMed] [Google Scholar]
- 13.Cretzmeyer M, Sarrazin MV, Huber DL, Block RI, Hall JA. Treatment of methamphetamine abuse: Research findings and clinical directions. Journal of Substance Abuse Treatment. 2003;24:267–277. doi: 10.1016/s0740-5472(03)00028-x. [DOI] [PubMed] [Google Scholar]
- 14.Degenhardt L, Dierker L, Chiu WT, et al. Evaluating the drug use “gateway” theory using cross-national data: consistency and associations of the order of initiation of drug use among participants in the WHO World Mental Health Surveys. Drug and Alcohol Dependence. 2010;108:84–97. doi: 10.1016/j.drugalcdep.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobbins IG, Foley H, Schacter DL, et al. Executive control during episodic retrieval: multiple prefrontal processes subserve source memory. Neuron. 2002;35:989–996. doi: 10.1016/s0896-6273(02)00858-9. [DOI] [PubMed] [Google Scholar]
- 16.Ernst T, Chang L, Leonido-Yee M, Speck O. Evidence for long-term neurotoxicity associated with methamphetamine abuse: A 1H MRS study. Neurology. 2000;54:1344–1349. doi: 10.1212/wnl.54.6.1344. [DOI] [PubMed] [Google Scholar]
- 17.Fals-Stewart W. Neurocognitive defects and their impact on substance abuse treatment. Journal of Addictions & Offender Counseling. 1993;13:46–57. [Google Scholar]
- 18.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders. New York: Biometrics Research Department; 1996. [Google Scholar]
- 19.Gladsjo JA, Schuman CC, Evans JD, Peavy GM, Miller SW, Heaton RK. Norms for letter and category fluency: Demographic corrections for age, education, and ethnicity. Assessment. 1999;6:147–178. doi: 10.1177/107319119900600204. [DOI] [PubMed] [Google Scholar]
- 20.Glisky EL, Polster MR, Routhieaux BC. Double dissociation between item and source memory. Neuropsychology. 1995;9:229–235. [Google Scholar]
- 21.Golden CJ. Stroop Color and Word Test. Chicago: Stoelting; 1978. [Google Scholar]
- 22.Gonzales R, Marinelli-Casey P, Shoptaw S, Ang A, Rawson RA. Hepatitis C virus infection among methamphetamine-dependent individuals in outpatient treatment. Journal of Substance Abuse Treatment. 2006;31:195–202. doi: 10.1016/j.jsat.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Gronwall DMA. Paced auditory serial-addition task: A measure of recovery from concussion. Percepetual & Motor Skills. 1977;44:367–375. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- 24.Hayes SM, Ryan L, Schnyer DM, Nadel L. An fMRI study of episodic memory: Retrieval of object, spatial, and temporal information. Behavorial Neurosciences. 2004;118:885–896. doi: 10.1037/0735-7044.118.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henry BL, Minassian A, Perry W. Effect of methamphetamine dependence on everyday functional ability. Addictive Behaviors. 2010;35:593–98. doi: 10.1016/j.addbeh.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang J, Lyoo IK, Kim SJ, et al. Decreased cerebral blood flow of the right anterior cingulate cortex in long-term and short-term abstinent methamphetamine users. Drug and Alcohol Dependence. 2006;82:177–181. doi: 10.1016/j.drugalcdep.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Iudicello JE, Woods SP, Vigil O, et al. Longer term improvement in neurocognitive functioning and affective distress among methamphetamine users who achieve stable abstinence. Journal of Clinical and Experimental Neuropsychology. 2010;32:704–18. doi: 10.1080/13803390903512637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jernigan TL, Gamst AC, Archibald SL, et al. Effects of methamphetamine dependence and HIV infection on cerebral morphology. American Journal of Psychiatry. 2005;162:1461–1472. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- 29.Kalechstein AD, Newton TF, Longshore D, Anglin MD, van Gorp WG, Gawin FH. Psychiatric comorbidity of methamphetamine dependence in a forensic sample. The Journal of Neuropsychiatry and Clinical Neurosciences. 2000;12:480–484. doi: 10.1176/jnp.12.4.480. [DOI] [PubMed] [Google Scholar]
- 30.Kalechstein AD, Newton TF, Green M. Methamphetamine dependence is associated with neurocognitive impairment in the initial phases of abstinence. Journal of Neuropsychiatry and Clinical Neurosciences. 2003;15:215–220. doi: 10.1176/jnp.15.2.215. [DOI] [PubMed] [Google Scholar]
- 31.Kokoshka JM, Metzger RR, Wilkins, Gibb JW, Hanson GR, Flackenstein AE. Methamphetamine treatment rapidly inhibits serotonin, but not glutamate, transporters in rat brains. Brain Research. 1998;799:78–83. doi: 10.1016/s0006-8993(98)00472-7. [DOI] [PubMed] [Google Scholar]
- 32.Kongs SK, Thompson LL, Iverson GL, Heaton RK. Wisconsin Card Sorting Test-64 Card Computerized Version. Odessa, FL: Psychological Assessment Resources; 2000. [Google Scholar]
- 33.Lawrence AD, Watkins LH, Sahakian BJ, Hodges JR, Robbins TW. Visual object and visuospatial cognition in Huntington’s disease: implications for information processing in corticostriatal circuits. Brain. 2000;123(Pt 7):1349–1364. doi: 10.1093/brain/123.7.1349. [DOI] [PubMed] [Google Scholar]
- 34.Lincoln NB, Radford KA. Cognitive abilities as predictors of safety to drive in people with multiple sclerosis. Multiple Sclerosis Journal. 2008;14:123–128. doi: 10.1177/1352458507080467. [DOI] [PubMed] [Google Scholar]
- 35.Logan BK. Methamphetamine and driving impairment. Journal of Forensic Science. 1996;41:457–464. [PubMed] [Google Scholar]
- 36.McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: Evidence from positron emission tomography studies with [11C]WIN-35,428. The Journal of Neuroscience. 1998;18:8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKetin R, McLaren J, Lubman DI, Hides L. The prevalence of psychotic symptoms among methamphetamine users. Addiction. 2006;101:1473–1478. doi: 10.1111/j.1360-0443.2006.01496.x. [DOI] [PubMed] [Google Scholar]
- 38.McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. San Diego: Educational and Industrial Testing Service; 1981. [Google Scholar]
- 39.Mitchell KJ, Johnson M. Source monitoring 15 years later: What have we learned from fMRI about the neural mechanisms of source memory? Psychological Bulletin. 2009;135:638–677. doi: 10.1037/a0015849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morgan EE, Woods SP, Weber E, et al. HIV-associated episodic memory impairment: evidence of a possible differential deficit in source memory for complex visual stimuli. Journal of Neuropsychiatry and Clinical Neurosciences. 2009;21:189–198. doi: 10.1176/appi.neuropsych.21.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moscovitch M. Memory and working-with-memory: A component process model based on modules and central systems. Journal of Cognitive Neuroscience. 1992;4:257–267. doi: 10.1162/jocn.1992.4.3.257. [DOI] [PubMed] [Google Scholar]
- 42.Moscovitch M. Memory and working-with-memory: Evaluation of a component process model and comparisons with other models. In: Schacter DL, Tulving E, editors. Memory Systems. Cambridge, MA: MIT/Bradford Press; 1994. pp. 269–310. [Google Scholar]
- 43.Moscovitch M, Winocur G. The frontal cortex and working with memory. In: Stuss Donald T, Knight Robert T., editors. Principles of Frontal Lobe Function. Oxford: Oxford University Press; 2002. pp. 188–209. [Google Scholar]
- 44.Pirogovsky E, Gilbert PE, Jacobson M, et al. Impairments in source memory for olfactory and visual stimuli in preclinical and clinical stages of Huntington’s disease. Journal of Clinical and Experimental Neuropsychology. 2007;29:95–404. doi: 10.1080/13803390600726829. [DOI] [PubMed] [Google Scholar]
- 45.Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and clinical interpretation. Tucson, AZ: Neuropsychology Press; 1993. [Google Scholar]
- 46.Rippeth JD, Heaton RK, Carey CL, et al. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. Journal of the International Neuropsychological Society. 2004;10:1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- 47.Scott JC, Woods SP, Matt GE, et al. Neurocognitive effects of methamphetamine: A critical review and meta-analysis. Neuropsychology Review. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- 48.Sekine Y, Ouchi Y, Sugihara G, et al. Methamphetamine causes microglial activation in the brains of human abusers. The Journal of Neuroscience. 2008;28:5756–5761. doi: 10.1523/JNEUROSCI.1179-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Semple SJ, Patterson TL, Grant I. Binge use of methamphetamine among HIV-positive men who have sex with men: Pilot data and HIV prevention implications. AIDS Education and Prevention. 2003;15:133–147. doi: 10.1521/aeap.15.3.133.23835. [DOI] [PubMed] [Google Scholar]
- 50.Semple SJ, Patterson TL, Grant I. A comparison of injection and non-injection methamphetamine-using HIV positive men who have sex with men. Drug and Alcohol Dependence. 2004;76:203–212. doi: 10.1016/j.drugalcdep.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 51.Semple SJ, Zians J, Grant I, Patterson TL. Impulsivity and methamphetamine use. Journal of Substance Abuse Treatment. 2005;29:85–93. doi: 10.1016/j.jsat.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 52.Shimamura AP. Memory and the prefrontal cortex. Annals of the New York Academy of Sciences. 1995;769:151–59. doi: 10.1111/j.1749-6632.1995.tb38136.x. [DOI] [PubMed] [Google Scholar]
- 53.Shoptaw S, Peck J, Reback CJ, Rotheram-Fuller E. Psychiatric and substance dependence comorbidities, sexually transmitted diseases, and risk behaviors among methamphetamine-dependent gay and bisexual men seeking outpatient drug abuse treatment. Journal of Psychoactive Drugs. 2003;35:161–168. doi: 10.1080/02791072.2003.10400511. [DOI] [PubMed] [Google Scholar]
- 54.Simon SL, Dacey J, Glynn S, Rawson R, Ling W. The effect of relapse on cognition in abstinent methamphetamine abusers. Journal of Substance Abuse Treatment. 2004;27:59–66. doi: 10.1016/j.jsat.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 55.Slotnick SD, Moo LR, Segal JB, et al. Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Brain Research. 2003;17:75–82. doi: 10.1016/s0926-6410(03)00082-x. [DOI] [PubMed] [Google Scholar]
- 56.Substance Abuse and Mental Health Services Administration. NSDUH Series H-30, DHHS publication no. SMA 06-4194. Rockville, MD: Office of Applied Studies; 2006. Results from the 2005 national survey on drug use and health: National findings. [Google Scholar]
- 57.Taylor M, Schweinsburg B, Alhassoon O, et al. Effects of human immunodeficiency virus and methamphetamine on cerebral metabolites measured with magnetic resonance spectroscopy. Journal for Neurovirology. 2007;13:150–159. doi: 10.1080/13550280701194230. [DOI] [PubMed] [Google Scholar]
- 58.Thompson PM, Hayashi KM, Simon SL, et al. Structural abnormalities in the brains of human subjects who use methamphetamine. The Journal of Neuroscience. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tobias MC, O’Neill J, Hudkins M, Bartzokis G, Dean AC, London ED. White-matter abnormalities in brain during early abstinence from methamphetamine abuse. Journal of Psychopharmacology. 2010;209:13–24. doi: 10.1007/s00213-009-1761-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Troyer AK, Murphy KJ, Anderson ND, Haymen-Abello BA, Craik FIM, Moscovitch M. Item and associative memory in amnestic mild cognitive cognitive impairment: Performance on standardized memory tests. Neuropsychology. 2008;22:10–16. doi: 10.1037/0894-4105.22.1.10. [DOI] [PubMed] [Google Scholar]
- 61.Uc EY, Rizzo M, Johnson AM, Dastrup E, Anderson SW, Dawson JD. Road safety in drivers with Parkinson disease. Neurology. 2009;73:2112–2119. doi: 10.1212/WNL.0b013e3181c67b77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Volkow ND, Chang L, Wang GJ, et al. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. American Journal of Psychiatry. 2001a;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- 63.Volkow ND, Chang L, Wang GJ, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: Association with metabolism in the orbitofrontal cortex. American Journal of Psychiatry. 2001b;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 64.Von Mayrhauser C, Brecht ML, Anglin MD. Use ecology and drug use motivations of methamphetamine users admitted to substance abuse treatment facilities in Los Angeles: An emerging profile. Journal of Addictive Diseases. 2002;21:45–60. doi: 10.1300/j069v21n01_05. [DOI] [PubMed] [Google Scholar]
- 65.Wang GJ, Volkow ND, Chang L, et al. Partial recovery of brain metabolism in methamphetamine abusers after protracted abstinence. American Journal of Psychiatry. 2004;16:242–248. doi: 10.1176/appi.ajp.161.2.242. [DOI] [PubMed] [Google Scholar]
- 66.Wilkins C, Sweetsur P, Casswell S. Recent population trends in amphetamine use in New Zealand: comparisons of findings from national household drug surveying in 1998, 2001, and 2003. The New Zealand Medical Journal. 2006;119:U2285. [PubMed] [Google Scholar]
- 67.Woods SP, Rippeth JD, Conover E, et al. Deficient strategic control of verbal encoding and retrieval in individuals with methamphetamine dependence. Neuropsychology. 2005;19:35–43. doi: 10.1037/0894-4105.19.1.35. [DOI] [PubMed] [Google Scholar]
- 68.Yen DJ, Wang SJ, Ju TH, et al. Stroke associated with methamphetamine inhalation. European Neurology. 1994;34:16–22. doi: 10.1159/000117002. [DOI] [PubMed] [Google Scholar]
- 69.Zweben JE, Cohen JB, Christian D, et al. Psychiatric symptoms in methamphetamine users. American Journal on Addictions. 2004;13:181–190. doi: 10.1080/10550490490436055. [DOI] [PubMed] [Google Scholar]