Abstract
Background:
Colorectal cancer (CRC) is the third most common malignancy in Iran. Limited data are available on knowledge and barriers in regard to CRC and screening tests in Iran. The aim of the study was to characterize knowledge, practice, and barriers toward CRC and its screening tests among an Iranian at-risk population.
Methods:
This cross-sectional study was conducted with participation of 200 individuals of both genders aged 50 years or older in a teaching hospital in Tehran, Iran. Data were collected via face-to-face interviews. A questionnaire containing demographics; knowledge about CRC and screening tests; screening practice; and reasons for not being screened was administered. The reliability alpha for knowledge items was 0.52.
Results:
The age of the participants ranged from 50 to 83 years (mean 60.13). Overall, 11% of the respondents reported prior screening by either fecal occult blood test (6.5%) or colonoscopy (4.5%). The majority of individuals had poor knowledge although respondents with prior screening obtained slightly higher score in comparison with nonparticipants in screening (26.74 vs. 23.24; P<0.05). Four commonly cited reasons for not having CRC tests were “doctor did not recommend the test,” “did not think it was needed,” “never think of the test,” and “no symptoms/problems” which were reported by 29%, 26%, 20%, and 17% of the participants, respectively.
Conclusion:
It is necessary to design appropriate educational interventions to increase the general population's knowledge about CRC and screening before implementing preventive programs in Iran.
Keywords: Colorectal cancer, prevention, screening tests
INTRODUCTION
Colorectal cancer (CRC) is the third most commonly diagnosed malignancy and the fourth leading cause of death worldwide.[1–3] During the past few decades, there have been considerable changes in the CRC incidence in Asian countries.[4]
CRC is the third most common cancer after stomach and breast cancer in both genders in Iran. Although CRC is more prevalent in population older than 50 years, in Iran it threatens younger cases and the onset age has decreased with an increasing prevalence near the western rates.[2,5,6] Considering the importance of the problem, the early detection and effective treatment of CRC during the initial stages would affect the health of the population and this can be achieved greatly by screening tests. According to the US Preventive Services Task Force and the American Cancer Society recommendations, every man and woman aged 50 years and older should be screened for CRC using one of the following screening tests: annual fecal occult blood test (FOBT), sigmoidoscopy every 5 years, barium enema every 5 years, or colonoscopy every 10 years.[7–10]
Despite evidence that CRC morbidity and mortality can be reduced through early detection and treatment, adherence to CRC guidelines and screening rates remains relatively low even in developed countries in regard to their national guideline recommendations.[11,12]
A growing body of evidence has explored the knowledge, attitudes, practices, and barriers to CRC screening among at risk population. Studies have shown that the general population's knowledge about CRC is an important factor affecting at-risk population's screening beliefs and behavior. Indeed, increased knowledge about CRC and screening encourages people to undergo screening tests. However, there are many barriers that might prevent people from performing CRC screening, including not being recommended by physician for screening, embarrassment, fear of developing cancer, poor knowledge about the screening tests, high costs, time restrictions, and transportation problems.[13–18]
To our knowledge, there is no national guideline on CRC screening in Iran and the Iranian Ministry of Health needs to develop national guidelines and recommendations regarding CRC screening to implement this screening. Since limited data are available on knowledge, awareness and barriers in regard to CRC and screening tests among at-risk populations in Iran, this study was conducted to build a database about Iranian's knowledge and practice regarding CRC and its screening. The objectives of the study were to characterize knowledge, practice, and barriers associated with CRC and its screening tests among Iranian population.
METHODS
This was a cross-sectional study carried out in Tehran, Iran. A convenience sample of 200 individuals of both genders was recruited from January 2011 to March 2011. Study setting was a multispecialty outpatient clinic in a teaching hospital affiliated to Tehran University of Medical Sciences. This medical center was chosen because it is a referral center and people from different socioeconomic backgrounds attend the center.
The study participants were selected through sequential sampling method among patients’ relatives and caregivers. The inclusion criteria were age 50 years or older and being physically and mentally competent to answer the questions. Respondents with colitis, Crohn's disease, personal history of CRC or polyps were ineligible for the study.
Data were collected via face-to-face interviews by two trained interviewers. An instrument containing questions about age, gender, marital status, formal education, employment status, family history of CRC or polyps, and health insurance was administered. Ten true–false knowledge statements reflected the participants’ knowledge about CRC and screening tests, including: “CRC often starts with no symptoms,” “blood in the stool is a symptom for CRC,” “general abdominal pain that does not go away can be a CRC symptom,” “unexplained weight loss is not a symptom for CRC,” “a change in bowel habits is a symptom for CRC,” “both men and women ≥50 get CRC,” “people with family history of CRC are at higher risk,” “all colon polyps are cancerous,” “screening is recommended for men and women ≥50,” and “screening tests cannot prevent CRC”. Content validity for revised knowledge items was confirmed by national experts, and the reliability alpha was 0.52.
Items: Knowledge items were given a score value of 1 if a respondent correctly selected “true” or “false” and 0 if the respondent answered incorrect or was “unsure.” To make the total score more informative, it was converted to 0–100 scales; therefore, the possible range of scores was 0–100.
To prepare a simple frame of reference, a brief description about FOBT and colonoscopy was read aloud to the participants. After the description was given, they were asked about their personal screening experience with the corresponding test; a response of yes required follow-up questions to ascertain when they had their most recent test. Respondents who had never had CRC screening tests were asked about the most important reason for not being screened (barriers to CRC screening), and read a list options from which to choose. The instrument took about 15 minutes to complete. Individuals were classified as high risk if they reported having a blood relative with CRC.
Collected data were analyzed using SPSS version 13.00 for Windows. Continuous variables were computed in the form of mean and standard deviation (±SD). Categorical variables were presented as relative and absolute frequency. Chi-square and t-tests were performed to assess statistical significance. Logistic regression was applied to assess factors associated with prior screening for CRC. A P value of less then 0.05 was considered statistically significant.
Ethical concerns
The study was approved by the Digestive Diseases Research Center institutional review board. Administrative approval was also granted for conducting the study at the Shariati Hospital. Informed consent was obtained from participants after providing adequate information about the significance and aim of the study. Participants were assured that their participation was voluntary and their responses would be treated with confidentiality.
RESULTS
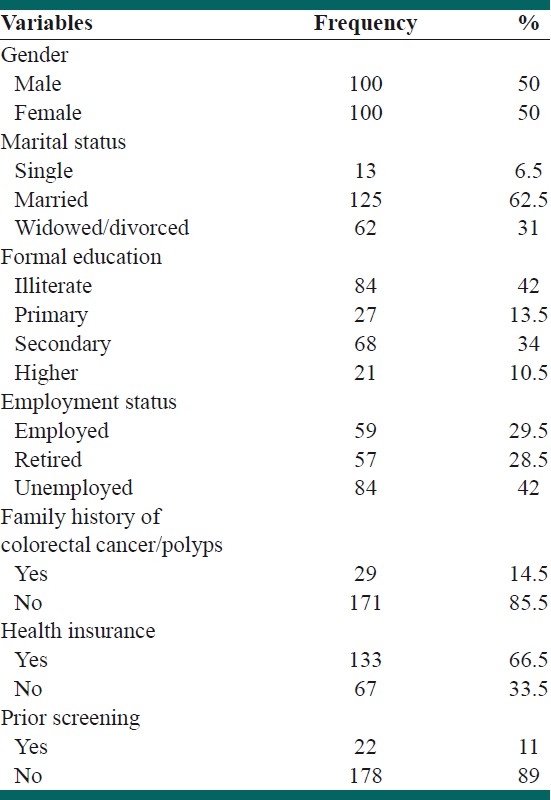
The sample included 100 females and 100 males. The age of the participants ranged from 50 to 83 years (mean 60.13). Socio-demographic characteristics of the study participants are shown in Table 1. Most respondents were married (62.5%), unemployed (42%), and had secondary or higher education (44.5%). The majority of participants reported having health insurance. Twenty-nine of individuals (14.5%) were identified as high risk, who reported a positive history of CRC among their first-degree relatives. Overall, 89% of respondents had never been tested for CRC, whereas 11% reported undergoing screening tests [Table 1]. Respondents with positive screening history had been screened with either FOBT (6.5%) or a colonoscopy (4.5%).
Table 1.
Demographic characteristics of the study participants (N=200)
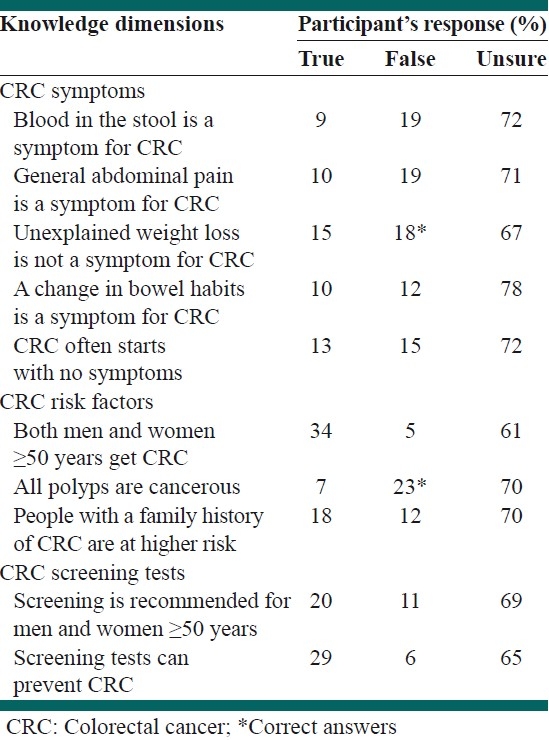
Lack of knowledge on CRC and screening tests emerged from analysis of knowledge items. In other words, the majority of respondents had poor knowledge and did not respond to the items correctly (false/unsure) as shown in Table 2.
Table 2.
Knowledge of colorectal cancer and its screening tests (N=200)
Four commonly cited reasons for not having CRC tests were “doctor did not recommend the test,” “did not think it was needed,” “never think of the test,” “no symptoms/problems,” which were reported by 29%, 26%, 20%, and 17% participants, respectively. Results indicated that “fear of developing CRC” and “the cost of tests” were the least important barriers to receiving screening tests [Table 3].
Table 3.
Reasons cited for not having colorectal cancer screening tests (N=178)

Univariate analysis of data indicated that variables such as age, gender, marital status, health insurance, education, and employment status had no significant association with prior screening. There was no statistically significant association between a family history of CRC and prior screening, indicating that having a positive family history of CRC did not increase the screening rates among high-risk group.
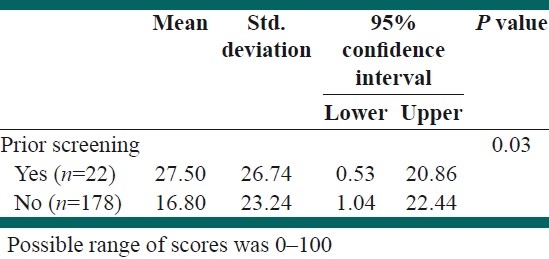
Knowledge score toward CRC and screening between screened and unscreened respondents was compared, and the results indicated a statistically significant difference between them [Table 4]. In other words, respondents, who reported having screening in the past, obtained slightly higher score regarding knowledge in comparison with nonparticipants in screening (26.74 vs. 23.24; P=0.03).
Table 4.
Distribution of knowledge score toward colorectal cancer and screening tests by prior screening
DISCUSSION
Our findings revealed that the majority of individuals, despite being eligible, had never been screened. Since there is no current CRC screening program in Iran, it was a reasonable and expected finding. However, even among the developed countries, screening rate still is relatively less, and despite some progress in the last few years, nearly 50% of eligible people are still not having appropriate screening tests for CRC.[19]
Several studies have consistently demonstrated an increased risk of CRC in individuals with a family history of CRC, and also one study in Iran reported that such positive family history increases the risk of CRC about 4.8-fold.[20,21] In the present study, having a positive family history of CRC did not increase the screening rates among high-risk people. This finding reflects that respondents were not aware of the importance of family history as a risk factor for CRC. To our knowledge, limited information is available on the CRC screening behaviors associated with family history in Iran. Only one other study in Shiraz has explored some barriers of performing the screening tests among employees aged 40 years or older.[22]
One of the notable results in this study was the poor awareness and knowledge about CRC and screening tests among respondents. Indeed, the mean score of knowledge significantly associated with undergoing previous screening. The findings are congruent with those of Shiraz study that cited lack of awareness as the most common barrier for FOBT.[22] Moreover, much greater knowledge deficits about CRC and screening have been documented through a number of international studies, confirming limited knowledge and awareness as major barriers for CRC screening.[3,17,23–25] These results indicate that Iranians are not well informed about CRC and screening, therefore they may have poor basic information about CRC and a limited capacity to understand the educational materials and certain features of CRC screening. Indeed, limited knowledge may be an important factor in explaining low rates of CRC screening.
Despite the importance of early detection, a considerable proportion (29%) of the participants who had never undergone previous screening reported that they were never informed about CRC screening by their doctor. In other words, the lack of physician recommendations was the most commonly reported barrier in obtaining CRC screening tests. This factor has been identified as the main barrier for CRC screening in Shiraz study as well.[22] The association between physician recommendation for CRC tests and screening rate has been documented in other investigations.[26–31] Since individuals with limited literacy skills are more likely to identify their physician as the main source of health information, the lack of doctor recommendation can be a particularly challenging issue.[27]
This finding may reflect that in Iran, the emphasis of physicians is on curative rather than preventive approaches. To fill this gap, nurses working in medical centers and health providers in healthcare centers can also persuade and educate people regarding the benefits of CRC screening. However, this result illuminates the important role of the physicians in increasing CRC screening rate and taking such factor into account will be helpful in implementing CRC screening in Iran in the near future.
Other mentioned barriers to receive CRC screening in the present study were “did not think it was needed,” “never think of the test,” and “no symptoms/problems”. These barriers again reflected poor knowledge skills about CRC and screening tests among participants, suggesting that there are also other knowledge-related barriers to colorectal screening. Moreover, studies have shown that people with limited knowledge skills often have problem in obtaining appropriate information from the available sources.[3,27]
Other barriers such as fear of the detection of cancer and cost of tests have been reported less often in our study. Even though our study did not highlight them as major barriers, they are important factors and have been cited in other studies.[17,32–34]
Study limitations
Although, the healthy individuals studied were target and more likely to be representative for average risk people, the small sample size from one outpatient clinic, limits the generalizability of the results. However the findings of this study provide knowledge of the barriers to CRC screening that exist among at-risk population.
CONCLUSION
Our study, for the first time, investigated the knowledge and awareness of Iranians toward CRC and screening tests. In this study, participants mostly did not participate in CRC screening programs due to several barriers. Whereas each population is unique, understanding these barriers can provide a foundation before implementing screening programs in Iran. Also the results of the study would help the health policy makers design appropriate educational interventions to increase the general population's knowledge about CRC and its screening tests. The Iranian Ministry of Health should play a major role in developing national guidelines toward CRC prevention and implementing its screening at least among high-risk groups.
ACKNOWLEDGMENTS
Our special thanks to Negahban Z. and Movahhed E. for their helpful cooperation.
Footnotes
Source of Support: This survey was funded by Tehran University of Medical Sciences.
Conflict of Interest: None declared.
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 10. Lyon, France: International Agency for Research on Cancer; 2010. [Last accessed on 2011 July 22]. GLOBOCAN 2008 v1.2. Available from: http://globocan.iarc.fr . [Google Scholar]
- 3.Shokar N, Vernon S, Weller S. Cancer and colorectal cancer: Knowledge, beliefs, and screening preferences of a diverse patient population. Fam Med. 2005;37:341–7. [PubMed] [Google Scholar]
- 4.Tu SP, Taylor V, Yasui Y, Chun A, Yip MP, Acorda E, et al. Promoting Culturally Appropriate Colorectal Cancer Screening through a Health Educator a Randomized Controlled Trail. Cancer. 2006;107:959–66. doi: 10.1002/cncr.22091. [DOI] [PubMed] [Google Scholar]
- 5.Kolahdoozan S, Sadjadi A, Radmard AR, Khademi H. Five common cancers in Iran. Arch Iran Med. 2010;13:143–6. [PubMed] [Google Scholar]
- 6.Salari AA, Dehghan HR. Evaluation and treatment of colorectal cancer in Shahid Rahnemoon and Afshar hospitals, Yazd-Iran. J Shahid Sadoughi Univ Med Sci Health Serv. 2007;15:20–5. [Google Scholar]
- 7.US Preventive Services Task Force. Screening for colorectal cancer: Recommendation and rationale. Ann Intern Med. 2002;137:129–31. doi: 10.7326/0003-4819-137-2-200207160-00014. [DOI] [PubMed] [Google Scholar]
- 8.Levin B, Lieberman DA, McFarland, Smith RA, Brooks D, Andrews KS, et al. Screening and Surveillance for the Early Detection of Colorectal Cancer and Adenomatous Polyps, 2008: A Joint Guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130–60. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 9.Smith RA, Cokkinides V, Brawley OW. Cancer screening in the United States, 2008: A review of current American Cancer Society guidelines and cancer screening issues. 2008. CA Cancer J Clin. 2008;58:161–79. doi: 10.3322/CA.2007.0017. [DOI] [PubMed] [Google Scholar]
- 10.Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, et al. Colorectal cancer screening and surveillance: Clinical guidelines and rationale-Update based on new evidence. Gastroenterology. 2003;124:544–60. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 11.Walsh J, Kaplan C, Nguyen B, Gildengorin G, McPhee S, Perez-Stable E. Barriers to colorectal cancer screening in Latino and Vietnamese-Americans. Compared with non-Latino white Americans. J Gen Intern Med. 2004;19:156–66. doi: 10.1111/j.1525-1497.2004.30263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cancer Facts and Figures 2011. Atlanta, GA: American Cancer Society; 2011. American Cancer Society. [Google Scholar]
- 13.Straus WL, Mansley EC, Gold KF, Wang Q, Reddy P, Pashos CL. Colorectal Cancer Screening Attitudes and Practices in the General Population: A Risk-adjusted Survey. J Public Health Manag Pract. 2005;11:244–51. doi: 10.1097/00124784-200505000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Holmes-Rovner M, Williams GA, Hoppough S, Quillan L, Butler R, Given CW. Colorectal cancer screening barriers in persons with low income. Cancer Pract. 2002;10:240–7. doi: 10.1046/j.1523-5394.2002.105003.x. [DOI] [PubMed] [Google Scholar]
- 15.James AS, Campbell MK, Hudson MA. Perceived barriers and benefits to colon cancer screening among African Americans in North Carolina: How does perception relate to screening behavior? Cancer Epidemiol Biomarkers Prev. 2002;11:529–34. [PubMed] [Google Scholar]
- 16.Tang TS, Solomon LJ, McCracken LM. Barriers to fecal occult blood testing and sigmoidoscopy among older Chinese-American women. Cancer Prev. 2001;9:277–82. doi: 10.1046/j.1523-5394.2001.96008.x. [DOI] [PubMed] [Google Scholar]
- 17.Omran S, Ismail AA. Knowledge and Beliefs of Jordanians toward Colorectal Cancer Screening. Cancer Nurs. 2010;33:141–8. doi: 10.1097/NCC.0b013e3181b823f3. [DOI] [PubMed] [Google Scholar]
- 18.Klabunde C, Schenck A, Davis W. Barriers to colorectal cancer among Medicare consumers. Am J Prev Med. 2006;30:313–9. doi: 10.1016/j.amepre.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Shapiro JA, Seeff LC, Thompson TD, Nadel MR, Klabunde CN, Vernon SW. Colorectal Cancer Test Use from the 2005 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2008;17:1623–30. doi: 10.1158/1055-9965.EPI-07-2838. [DOI] [PubMed] [Google Scholar]
- 20.Rawl SM, Champion VL, Scott LL, Zhou H, Monahan P, Ding Y, et al. A randomized trial of two print interventions to increase colon cancer screening among first-degree relatives. Patient Educ Couns. 2008;71:215–27. doi: 10.1016/j.pec.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Safaee A, Moghimi-Dehkordi B, Pourhoseingholi MA, Vahedi M, Maserat E, Ghiasi S, et al. Risk of Colorectal Cancer in Relatives: A Case Control Study. Indian J Cancer. 2010;47:27–30. doi: 10.4103/0019-509X.58855. [DOI] [PubMed] [Google Scholar]
- 22.Roozitalab M, Moatari M, Gholamzadeh S, SaberiFiroozi M, Zare N. The effect of health belief on participation of the official administrative personnel in colorectal cancer screening programs in Shiraz University of Medical Sciences: 2004. Govaresh, Iran Assoc Gastroenterol Hepatol. 2008;13:19–24. [Google Scholar]
- 23.Greiner A, Born W, Nollen N, Ahluwalia J. Knowledge and perception of colorectal cancer screening among urban African Americans. J Gen Intern Med. 2005;20:977–83. doi: 10.1111/j.1525-1497.2005.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guessous I, Dash C, Lapin P, Doroshenk M, Smith RA, Klabunde CN. Colorectal cancer screening barriers and facilitators in older persons. Prev Med. 2010;50:3–10. doi: 10.1016/j.ypmed.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Harewood GC, Murray F, Patchett S, Garcia L, Leong WL, Lim YT, et al. Assessment of colorectal cancer knowledge and patient attitudes towards screening: Is Ireland ready to embrace colon cancer screening? Iran J Med Sci. 2009;178:7–12. doi: 10.1007/s11845-008-0163-x. [DOI] [PubMed] [Google Scholar]
- 26.James AS, Campbell MK, Hudson MA. Perceived barriers and benefits to colon cancer screening among African Americans in North Carolina: How does perception relate to screening behavior? Cancer Epidemiol. 2002;11:529–34. [PubMed] [Google Scholar]
- 27.Dolan NC, Ferreira MR, Davis TC, Fitzgibbon ML, Rademaker A, Liu D, et al. Colorectal Cancer Screening Knowledge, Attitudes, and Beliefs among Veterans: Does Literacy Make a Difference? J Clin Oncol. 2004;22:2617–22. doi: 10.1200/JCO.2004.10.149. [DOI] [PubMed] [Google Scholar]
- 28.Katz ML, James AS, Pignone MP, Hudson MA, Jackson E, Oates V, et al. Colorectal cancer screening among African American church members: A qualitative and quantitative study of patient-provider. BMC Public Health. 2004;4:62. doi: 10.1186/1471-2458-4-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jonathan P. Embarrassment is a major barrier to colon cancer prevention, especially among women. Gastroenterology. 2006;130:1364–5. doi: 10.1053/j.gastro.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 30.Wee CC, McCarthy EP, Phillips RS. Factors associated with colon cancer screening: The role of patient factors and physician counseling. Prev Med. 2005;41:23–9. doi: 10.1016/j.ypmed.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Jagot C. The importance of improving awareness of colorectal cancer. Nurs Times. 2004;100:30–1. [PubMed] [Google Scholar]
- 32.Rawl S, Menon U, Champion VL, May FE, Loehrer P, Hunter C, et al. Do benefits and barriers differ by stage of adoption for colorectal cancer screening? Health Educ Res. 2005;20:137–48. doi: 10.1093/her/cyg110. [DOI] [PubMed] [Google Scholar]
- 33.Hannon P, Harris JR, Martin D, VanEenwyk J, Bowen D. Colorectal cancer screening in Washington State: Predictors of current screening and explanations for no screening. Prev Chronic Dis. 2005;12:1006–13. [Google Scholar]
- 34.Green AR, Peters-Lewis A, Percac-Lima S, Betancourt JR, Richter JM, Janairo MP, et al. Barriers to Screening Colonoscopy for Low-income Latino and White Patients in an Urban Community Health Center. J Gen Intern Med. 2008;23:834–40. doi: 10.1007/s11606-008-0572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


