Abstract
Background:
Considering the role of maternal thyroid stimulating hormone (TSH) receptor blocking antibody (TRAb) in the etiology of congenital hypothyroidism (CH), this study aimed to determine TRAb among patients with CH in Isfahan, Iran.
Methods:
In this case–control study, patients with CH and their mothers were compared with a group of healthy neonates and their mothers. Venous blood samples were obtained for measurement of TRAb using enzyme-linked immunosorbent assay (ELISA) method among mothers and their neonates. TSH of mothers was also determined.
Results:
The case group consisted of 65 patients with CH and their mothers; controls were 148 healthy neonates and their mothers. The prevalence of positive TRAb in patients with CH and their mothers was higher than in the control group (81.5% vs. 1.3% in mothers and 80% vs. 0% in neonates, respectively, P<0.05). The relationship between the TRAb and occurrence of CH was significant (P<0.05), whereas the corresponding figure was not significant for TRAb and the level of maternal and neonatal TSH in case and control groups (P>0.05).
Conclusion:
It seems that autoimmunity has an important role in the etiology of CH. Further studies are necessary to determine other autoantibodies in CH patients.
Keywords: Congenital hypothyroidism, etiology, thyroid stimulating hormone receptor blocking, autoimmunity
INTRODUCTION
Congenital hypothyroidism (CH) is the most common cause of preventable mental retardation and occurs in 1 in 2000–4000 newborns. It can be either permanent or transient.[1,2] Newborns with permanent form of CH have a defect in thyroid gland location and structure (development and hormone synthesis) which is represented as thyroid dysgenesis (ectopia or agenesis) or dyshormonogenesis. These infants require lifelong thyroid hormone replacement therapy. Though in newborns with transient form of CH the replacement therapy is not lifelong, treatment during the first years of life is crucial, and the patients are indistinguishable at birth from those with permanent CH. The causes of transient CH are maternal thyrotropin receptor blocking antibodies, exposure to maternal antithyroid medications, iodine deficiency, and iodine excess.[3–5]
Several studies have investigated the role of genetic, environmental and autoimmune factors in the etiology of CH.[6–8] Though the roles of autoimmune factors in the pathogenesis of CH have been supported in many studies, the findings are controversial because some evidences have reported their role in both transient and permanent forms of CH, but others have reported their role only in the transient form. Bogner and colleagues showed that cellular cytotoxicity induced by maternal autoantibodies have an important role in the pathogenesis of CH.[9–13]
The roles of many maternal autoantibodies such as TPOAb, TgAb and TSH receptor blocking Ab (TRAb) have been investigated in the etiology of CH. According to these researches, TRAb has more diagnostic value in the diagnosis of CH, especially its transient form, than the other studied autoantibodies. Evidences suggest that transplacental transfer of maternal TRAb both in prenatal and postnatal period causes delay in infants’ thyroid gland development. Though studies in this field reported transient form of CH due to the above-mentioned autoantibodies, permanent cases of CH due to these antibodies have been reported too.[14–18]
Thyroid autoimmune diseases are a common problem in women, and they may be asymptomatic. They may be undiagnosed during and after pregnancy and the autoantibodies may be transferred to fetus during pregnancy and result in hypothyroidism.[19] Thus, considering the high prevalence of CH in our community as well as its transient form and the importance of etiologic studies in this field,[20,21] this study was planned to investigate the role of TRAb in the etiology of this common disorder in Isfahan.
METHODS
In this case–control study, the newborns who were referred to Isfahan Endocrine and Metabolism Research Center (EMRC) for CH screening during 2010–2011 were enrolled.
According to the CH screening program, 3–7-day-old neonates with thyroid stimulating hormone (TSH)>10 mIU/l (filter paper) were recalled and re-examined and those with abnormal T4 and TSH levels (TSH>10 mIU/l and T4<6.5 μg/dl) in their second measurement (during 7–14 days of life) were diagnosed as CH patients and received treatment and regular follow-up. Levothyroxin was prescribed for hypothyroid neonates at a dose of 10–15 mg/kg/day as soon as the diagnosis was confirmed. Neonates with CH were followed up according to the CH screening guideline for appropriate treatment regarding the level of TSH,T4, height, weight and other supplementary tests.[14]
Neonates diagnosed with CH during the study period and their mothers were selected as the case group. Those with abnormal screening results but normal thyroid function test in the second measurement and their mothers were selected as the control group. They were selected by convenience method. The number of neonates and mothers studied in the control group was three times higher than that in the case group.
Written consent was obtained from the parents of CH patients. The protocol was approved by the Institutional Review Board and Medical Ethics Committee of Isfahan University of Medical Sciences.
Baseline characteristics and screening results of participants were obtained from their screening questionnaire. Complementary information of CH patients and their parents was obtained from their registered profiles in EMRC.
The level of TRAb was measured in mothers and their neonates. Level of TSH also was measured in mothers. Level of TSH in neonates was obtained from their profiles. Peripheral blood samples were obtained from the study population for TRAb and TSH measurements. Participants with positive TRAb were determined.
Considering that the circulatory level of TRAb in newborn decreases gradually after 28th day of life and it is detectible till 3 months after birth,[16] participants who were referred after 4 weeks of birth were excluded from the study. In addition, those who were not cooperative or had chronic disorder were excluded.
The case and control groups were compared regarding positive TRAb and the level of TSH, and the role of TRAb in CH was determined.
Laboratory measurement
Serum T4 and TSH concentrations were measured by radioimmunoassay (RIA) and immunoradiometric assay (IRMA), respectively, using Kavoshyar (Tehran, Iran) kits. Thyroid function tests were performed using Berthold-LB2111 unit Gamma Counter equipment.
TRAb was measured by enzyme-linked immunosorbent assay (ELISA) method using RSR TSH Receptor Autoantibody 3rd Generation ELISA kit (United Kingdom). TRA≥0.4 units/l was considered as positive.
Statistical analysis
Obtained data were analyzed using the SPSS statistical software ver. 13 (SPSS Inc., Chicago, IL, USA). Descriptive data were expressed as mean±standard deviation (SD). Quantitative data of the two groups and the relation between maternal and neonatal TRAb levels was compared using the Student's t-test or paired t-test and Fisher tests. P value<0.05 was considered statistically significant.
RESULTS
Sixty-five neonates with CH and their mothers were studied in the case group and 148 neonates with normal screening results and their mothers were studied in the control group. Demographic and screening findings of the two studied groups are presented in Table 1.
Table 1.
Demographic and screening findings of the two studied groups
The prevalence of positive TRAb in neonates and mothers in the case and control groups is presented in Figure 1.
Figure 1.
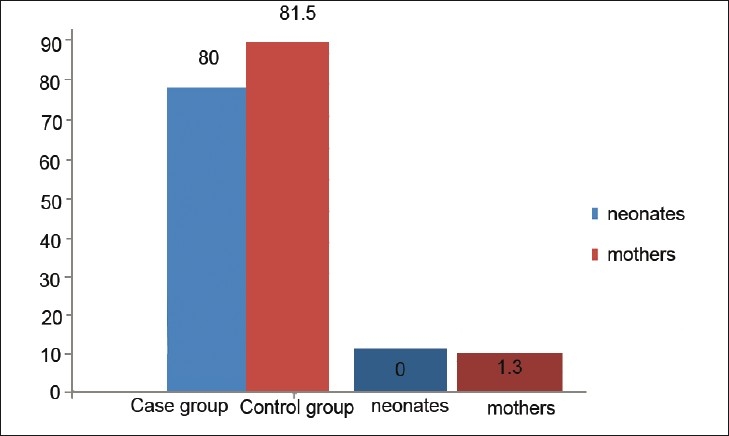
The prevalence of positive thyroid stimulating hormone (TSH) receptor blocking Ab in neonates and mothers in the case and control groups (P<0.05 for both neonates and mothers)
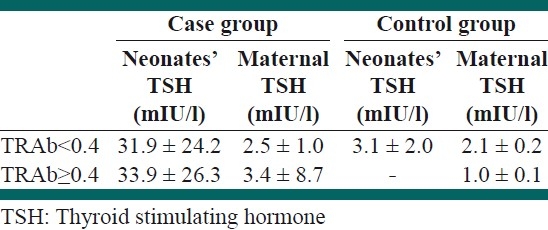
Mean of TSH in neonates and mothers in the case and control groups according to positive and negative TRAb is presented in Table 2. There was no significant relationship between TRAb and the level of both maternal and neonatal TSH in the case and control groups (P>0.05).
Table 2.
Mean of TSH in neonates and mothers in the case and control groups according to positive and negative TRAb
DISCUSSION
Implementation of CH screening program has been greatly facilitated its early detection and treatment which result in normal psychomotor development of the infants affected with CH. Though this program in our region was successful in achieving the mentioned goals, it seems that considering the high prevalence of CH, etiological studies are necessary for an appropriate screening program. So, in this study, the prevalence of positive TRAb in neonates with and without CH and their mothers was determined. The prevalence of positive TRAb in neonates with CH and their mothers was significantly higher than in the control group and there was significant correlation between TRAb and CH in our studied population.
Several studies have investigated the role of autoimmunity in the etiology of CH by focusing on different autoantibodies, of which TRAb seems to be more specific. So, we evaluated the role of TRAb in the etiology of CH.[16]
Ordookhani and colleagues in their study regarding the etiologic factors of transient CH in Tehran have not reported any significant relation between TRAb antibody and transient CH. They concluded that iodine excess is considered as the most important factor in this field.[22]
In a case report in the UK, Evans et al. have reported a case of neonate with CH with positive TRAb. The cause of CH was maternal transplacental passage of TRAb. Though the level of TRAb was decreased in the neonate's serum to normal range within 3–4 months after birth, the thyroid function did not return to normal till 16 months of age. They concluded that maternal TRAb antibody during pre-and postnatal period could delay the development of thyroid gland and result in transient CH. Thyroid replacement therapy is necessary for these neonates and it should be continued until the normal thyroid function is resumed even when the autoantibody is not detectable in serum.[17]
In another report from Greece, Mengreli and colleagues studied 173 neonates with CH (157 permanent and 16 transient forms of CH) out of 508,358 screened ones. The prevalence of positive TRAb among all the studied neonates with CH was 5.8% and it was 31.2% and 2.9% in transient and permanent forms, respectively. The prevalence of TRAb was significantly higher in the transient form of CH compared with the permanent form and control healthy neonates (1.9%). According to their findings, transient CH caused by maternal–fetal transfer of TRAb is considered a rare condition with a prevalence of 2.7% of all cases with CH. But its diagnosis, i.e. detection of transient CH cases due to maternal-fetal transfer of TRAb, is an important issue in CH screening. However, detecting these cases is useful for genetic counseling and preventing the occurrence of transient CH, especially in subsequent offspring, and consequently neurodevelopmental abnormality of the fetus.[16]
In contrast, in the study of Ginsberg et al. in 15 neonates with diagnosed CH, only one of their mothers had positive TRAb and they concluded that transplacental transfer of TRAb had no significant role in the development of CH.[23]
In a recent study conducted in Wales, Evans et al. have reported that from seven neonates with transient CH, TRAb was positive in the mothers of all patients. They indicated that identifying neonates with CH due to maternal TRAb is important for optimizing CH screening programs.[24]
The reported prevalence of positive TRAb was different in different studies. In a study, during the screening of over 1 million babies, the prevalence of transient CH due to this autoantibody was reported to be 2% and it was detected in the serum of all mothers of transient CH cases.[25] The prevalence of positive TRAb was 5.5% in neonates with CH and 7.1% in their mothers in a study conducted in Italy.[26] It was positive in 8.2% of infants with CH and 6.5% in their mothers in a survey conducted in Germany.[13]
In the current study, the prevalence of positive TRAb was 80% and 81.5% in neonates with CH and their mothers, respectively. It seems the prevalence of CH due to TRAb is significantly higher in our studied population in comparison with others. The results obtained could be explained as follows. Considering that TRAb which is found in infants’ circulation gradually clears by 3–4 months of age, we studied neonates who were primarily diagnosed with CH and it included both transient and permanent forms, whereas the permanency of CH would be determined in 3–4 years of age. But considering the high rate of transient CH in one study among this population (40.2%),[21] the high rate of positive TRAb may be due to the high rate of cases with transient CH.
On the other hand, a recent study in Isfahan among this population indicated that iodine excess is considered as one of the possible factors for the high prevalence of CH in our community.[27] Iodine excess has an important role in inducing thyroid autoimmunity,[28] so it seems that it is one of the responsible factors for our findings. However, the different laboratory methods and ethnic variation should also be considered.
In this study, there was no significant relationship between TRAb and neonatal TSH level and maternal TSH level. Our results are similar to the results of Mengreli et al.[16] In a recent study conducted in mice, Postiglione et al. showed that TSH or a functional TSH-R is not an essential factor for the development of a normal thyroid gland during prenatal period in utero.[29] However, further studies are needed in this field.
To conclude, the findings of this study indicate the role of TRAb in the etiology of CH in this population. It emphasizes the importance of screening of thyroid autoimmune disorders among mothers during pregnancy or CH screening program for preventing some autoimmune cases of CH. But for more conclusive results, it is recommended to evaluate the role of this autoantibody and also other autoantibodies in permanent and transient cases separately.
Footnotes
Source of Support: This study was conducted as a Medical specialty thesis funded by Isfahan University of Medical Sciences, Isfahan, Iran.
Conflict of Interest: None declared.
REFERENCES
- 1.Rastogi MV, LaFranchi SH. Congenital hypothyroidism. Orphanet J Rare Dis. 2010;5:17. doi: 10.1186/1750-1172-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grüters A, Krude H. Update on the management of congenital hypothyroidism. Horm Res. 2007;68(Suppl 5):107–11. doi: 10.1159/000110591. [DOI] [PubMed] [Google Scholar]
- 3.Büyükgebiz A. Newborn screening for congenital hypothyroidism. J Pediatr Endocrinol Metab. 2006;19:1291–8. doi: 10.1515/jpem.2006.19.11.1291. [DOI] [PubMed] [Google Scholar]
- 4.Parks JS, Lin M, Grosse SD, Hinton CF, Drummond-Borg M, Borgfeld L, et al. The impact of transient hypothyroidism on the increasing rate of congenital hypothyroidism in the United States. Pediatrics. 2010;125(Suppl 2):S54–63. doi: 10.1542/peds.2009-1975F. [DOI] [PubMed] [Google Scholar]
- 5.Eugster EA, LeMay D, Zerin JM, Pescovitch OH. Definitive diagnosis in children with congenital hypothyroidism. J Pediatr. 2004;144:643–7. doi: 10.1016/j.jpeds.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 6.Park SM, Chatterjee VK. Genetics of congenital hypothyroidism. J Med Genet. 2005;42:379–89. doi: 10.1136/jmg.2004.024158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medda E, Olivieri A, Stazi MA, Grandolfo ME, Fazzini C, Baserga M, et al. Risk factors for congenital hypothyroidism: Results of a population case-control study (1997-2003) Eur J Endocrinol. 2005;153:765–73. doi: 10.1530/eje.1.02048. [DOI] [PubMed] [Google Scholar]
- 8.Carlé A, Laurberg P, Knudsen N, Perrild H, Ovesen L, Rasmussen LB, et al. Thyroid peroxidase and thyroglobulin auto-antibodies in patients with newly diagnosed overt hypothyroidism. Autoimmunity. 2006;39:497–503. doi: 10.1080/08916930600907913. [DOI] [PubMed] [Google Scholar]
- 9.Klein RZ, Mitchell ML. Hypothyroidism in infants and children. In: braverman LE, Utiger RD, editors. The thyroid. 8th ed. Philadelphia: Lippincott Williams and Wilkins; 2000. pp. 973–88. [Google Scholar]
- 10.Fisher DA. Disorders of the thyroid in the newborn and infant. In: Sperling MA, editor. Pediatric Endocrinology. 1st ed. Philadelphia: W. B. Saunders Company; 1996. pp. 51–70. [Google Scholar]
- 11.Srtakosch CR, Wenzel BE, Row VV, Vople R. Immunology of autoimmune thyroid disease. N Engl J Med. 1982;307:1499–507. doi: 10.1056/NEJM198212093072407. [DOI] [PubMed] [Google Scholar]
- 12.Van dergoag RD, Drexhange HA, Dussault JH. Role of Maternal immunglubins blocking TSH induced thyroid growth in sporadic forms of congenital hypothyroidism. Lancet. 1985;2:246–50. doi: 10.1016/s0140-6736(85)91028-1. [DOI] [PubMed] [Google Scholar]
- 13.Bogner U, Gruters A, Sigle B, Helge H, Dcheusener H. Cytotoxic antibodies in congenital hypothyroidism. (14-18).J Clin Endocrinal Metab. 1989;68:671–5. doi: 10.1210/jcem-68-3-671. [DOI] [PubMed] [Google Scholar]
- 14.Iafranch S. Congenital hypothyroidism etiologies, diagnosis and management. Thyroid. 1999;9:735–48. doi: 10.1089/thy.1999.9.735. [DOI] [PubMed] [Google Scholar]
- 15.Schwingshandi J, Donaghue K, Luttsell B, Cowelk , Ward P, Silink M. Transient congenital hypothyroidism due to maternal thyrotropin binding inhibiting immunoglublobin. J Paediatr Child Health. 1993;22:315–8. doi: 10.1111/j.1440-1754.1993.tb00521.x. [DOI] [PubMed] [Google Scholar]
- 16.Mengreli C, Maniati-Christidi M, Kanaka-Gantenbein C, Girginoudis P, Vagenakis AG, Dacou-Voutetakis C. Transient congenital hypothyroidism due to maternal autoimmune thyroid disease. Hormones (Athens) 2003;2:113–9. doi: 10.14310/horm.2002.1190. [DOI] [PubMed] [Google Scholar]
- 17.Evans C, Jordan NJ, Owens G, Bradley D, Ludgate M, John R. Potent thyrotrophin receptor-blocking antibodies: A cause of transient congenital hypothyroidism and delayed thyroid development. Eur J Endocrinol. 2004;150:265–8. doi: 10.1530/eje.0.1500265. [DOI] [PubMed] [Google Scholar]
- 18.Karlsson FA, Dahlberg PA, Ritzen EM. Thyroid blocking antibodies in thyroiditis. Acta Medica Scandinavica. 1984;215:461–6. doi: 10.1111/j.0954-6820.1984.tb17679.x. [DOI] [PubMed] [Google Scholar]
- 19.Radetti G, Zavallone A, Gentili L, Beck-Peccoz P, Bona G. Foetal and neonatal thyroid disorders. Minerva Pediatr. 2002;54:383–400. [PubMed] [Google Scholar]
- 20.Hashemipour M, Amini M, Iranpour R, Sadri GH, Javaheri N, Haghighi S, et al. Prevalence of congenital hypothyroidism in Isfahan, Iran: Results of a survey on 20,000 neonates. Horm Res. 2004;62:79–83. doi: 10.1159/000079392. [DOI] [PubMed] [Google Scholar]
- 21.Hashemipour M, Hovsepian S, Kelishadi R, Iranpour R, Hadian R, Haghighi S, et al. Permanent and transient congenital hypothyroidism in Isfahan-Iran. J Med Screen. 2009;16:11–6. doi: 10.1258/jms.2009.008090. [DOI] [PubMed] [Google Scholar]
- 22.Ordookhani A, Pearce EN, Mirmiran P, Azizi F, Braverman LE. Transient congenital hypothyroidism in an iodine-replete area is not related to parental consanguinity, mode of delivery, goitrogens, iodine exposure, or thyrotropin receptor autoantibodies. J Endocrinol Invest. 2008;31:29–34. doi: 10.1007/BF03345563. [DOI] [PubMed] [Google Scholar]
- 23.Ginsberg J, Walfish PG, Rafter DJ, von Westarp C, Ehrlich RM. Thyrotrophin blocking antibodies in the sera of mothers with congenitally hypothyroid infants. Clin Endocrinol (Oxf) 1986;25:189–94. doi: 10.1111/j.1365-2265.1986.tb01681.x. [DOI] [PubMed] [Google Scholar]
- 24.Evans C, Gregory JW, Barton J, Bidder C, Gibbs J, Pryce R, et al. Transient congenital hypothyroidism due to thyroid-stimulating hormone receptor blocking antibodies: A case series. Ann Clin Biochem. 2011;48:386–90. doi: 10.1258/acb.2011.011007. [DOI] [PubMed] [Google Scholar]
- 25.Brown RS, Bellisario RL, Botero D, Fournier L, Abrams CA, Cowger ML, et al. Incidence of transient congenital hypothyroidism due to maternal thyrotropin receptor-blocking antibodies in over one million babies. J Clin Endocrinol Metab. 1996;81:1147–51. doi: 10.1210/jcem.81.3.8772590. [DOI] [PubMed] [Google Scholar]
- 26.Bona G, Chiovato L, Campra D, Paniccia P, Zaffaroni M, Costa L, et al. Thyroid autommunity: Really an important cause of sporadic congenital hypothyroidism? Panminerva Med. 1991;33:145–51. [PubMed] [Google Scholar]
- 27.Hashemipour M, Nasri P, Hovsepian S, Hadian R, Heidari K, Attar HM, et al. Urine and milk iodine concentrations in healthy and congenitally hypothyroid neonates and their mothers. Endokrynol Pol. 2010;61:371–6. [PubMed] [Google Scholar]
- 28.Wémeau JL. Hypothyroidism related to excess iodine. Presse Med. 2002;31:1670–5. [PubMed] [Google Scholar]
- 29.Postiglione MP, Parlato R, Rodriguez-Mallon A, Rosica A, Mithobaokar P, Maresca M, et al. Role of the thyroid-stimulating hormone receptor signalling in development and differentiation of the thyroid gland. PNAS. 2002;99:15462–7. doi: 10.1073/pnas.242328999. [DOI] [PMC free article] [PubMed] [Google Scholar]


