Abstract
White matter lesions (WMLs) are commonly observed in stroke patients with small vessel disease (SVD) and are thought to result from a progressive, irreversible disease process following arteriolosclerosis. In this study, we report a case of partial disappearance of WMLs 1 year after a lacunar stroke in a 69-year-old man with evidence of SVD. We also discuss possible mechanisms associated with this observation.
Keywords: Brain white matter hyperintensities, leukoaraiosis, stroke
INTRODUCTION
White matter lesions (WMLs) are commonly observed as hyperintensities on T2 and fluid attenuated inversion recovery (FLAIR) magnetic resonance imaging (MRI) sequences in stroke patients with small vessel disease (SVD)[1] and may reflect the ischemic components of vascular brain injury.[2] Although the precise disease mechanism has yet to be determined, age- and vascular risk factor-related WMLs are thought to be symptomatic of a progressive, irreversible process that follows on from arteriolosclerosis.[3]
In this study, we report a case in which WMLs had partly disappeared 1 year after a lacunar stroke in a patient with evidence of SVD and receiving optimal secondary prevention. We also discuss the potential mechanistic implications of this finding.
CASE REPORT
A 69-year-old, right-handed man suddenly developed right-side weakness. There was no significant medical or medication history and his only vascular risk factors were arterial hypertension and obesity. The patient was referred to our acute stroke unit 3 days after symptom onset.
In a neurological examination, the patient was found to be alert. There was no lower facial or arm paresis but a motor examination revealed decreased muscle tone in his right leg and slight dysarthria. The results of a sensory examination were normal. The National Institutes of Health Stroke Scale (NIHSS) score was 2 out of 42.[4] The patient's blood pressure was 180/100 mmHg in both arms and the pulse was regular. There were no cardiac, cervical or femoral bruits and no signs of heart failure. The patient weighed 97 kg (body mass index: 33.6 kg/m2).
Initial laboratory test results (including a complete blood count, electrolyte profile, blood glucose, and kidney status) were normal. Blood gases were not measured. The chest X-ray and electrocardiogram were normal. A computed tomography (CT) scan of the head revealed old, silent lacunar infarcts and several areas of periventricular white matter (WM) hypodensity (i.e. leukoaraiosis). Brain MRI was performed 5 days after symptom onset and showed an acute, small infarct in the posterior limb of the left internal capsule [Figure 1]. Other findings included old, silent lacunar infarcts, WMLs, and cerebral microbleeds in the left thalamus (on gradient echo-imaging) that were suggestive of SVD [Figure 1]. Brain magnetic resonance angiography (MRA) did not reveal any abnormalities. Carotid ultrasonography revealed the presence of carotid plaques but no stenosis. The transthoracic echocardiography result was normal but transoesophageal echocardiography was not performed.
Figure 1.
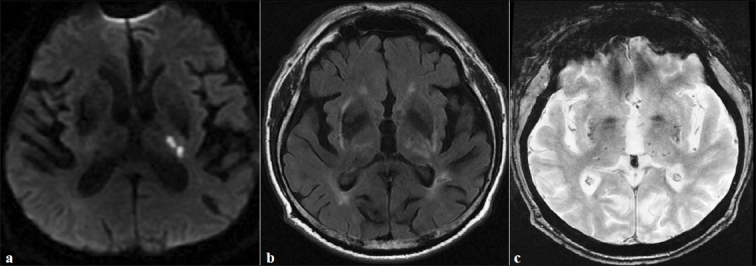
(a) Diffusion-weighted MRI showing limited infarct in the left posterior arm of the internal capsule. (b) Axial FLAIR imaging showing white matter lesions and lacunar infarcts. (c) Axial gradient-echo MRI sequence showing multiple microbleeds (small foci of hypointensity) located in the basal ganglia
The Mini Mental Status Examination (MMSE) and the Montreal Cognitive Assessment (MoCA) were administered 4 days after admission and scored as 24 out of 30 and 17 out of 30, respectively. Since the patient presented motor weakness in the right leg when walking (NIHSS score: 0 out of 42), he was transferred to a rehabilitation centre. The patient had a favorable outcome after intensive physiotherapy and was discharged 2 weeks after admission.
Six months after the stroke, the patient had made a full recovery; the NIHSS score was 0 out of 42 and the modified Rankin scale score was 2 (the patient had not driven since his stroke). We also observed an improvement in the patient's global cognitive function, since the MMSE and MoCA scores were 27 out of 30 and 24 out of 30, respectively. The patient was taking aspirin (300 mg/day), atorvastatin (40 mg/day), an ACE inhibitor (perindopril, 5 mg/day), and a non-thiazide diuretic (indapamide, 1.5 mg/day). Furthermore, he had lost 25 pounds (12 kg; weight: 85 kg; body mass index: 29.4 kg/m2) and engaged in regular physical activity (walking 1 h a day) after recovery from his stroke.
Imaging analysis
MRI data were acquired on a 3T clinical system (HDX, General Electric Medical System, Milwaukee, WI, USA) 5 days after symptom onset and then 6 months later. The examination protocol included conventional sagittal, T1-weighted images, axial T1- and T2-weighted images, FLAIR and T2*-weighted gradient echo-imaging (GRE) sequences. Circle of Willis magnetic resonance angiography was performed using a three-dimensional (3D) time-of-flight sequence. The axial FLAIR parameters were as follows: TR/TE: 9000/120 ms; flip angle: 90°; radial field of view: 240 × 240 mm; reconstruction matrices: 256 × 256; 28 slices (5 mm) with a 1 mm gap.
The FLAIR MRI volumes were first registered using a 3D rigid body algorithm. WML segmentation was performed on registered FLAIR acquisitions using MIPAV software.[5] The WML volumes on MRI were evaluated by an experienced neurologist who was blinded to the patient's clinical data. The WMLs were defined on a slice-by-slice basis using MIPAV's level set algorithm, which represents the edge of a structure via active contours without prior knowledge of its shape.[6] The WML volumes were calculated by multiplying the (slice thickness + gap) term by the total lesion area. There was a marked difference in the WML volume when comparing the immediate post-stroke images and the 6-month follow-up (9184 mm3 vs. 4527 mm3, respectively) [Figure 2].
Figure 2.
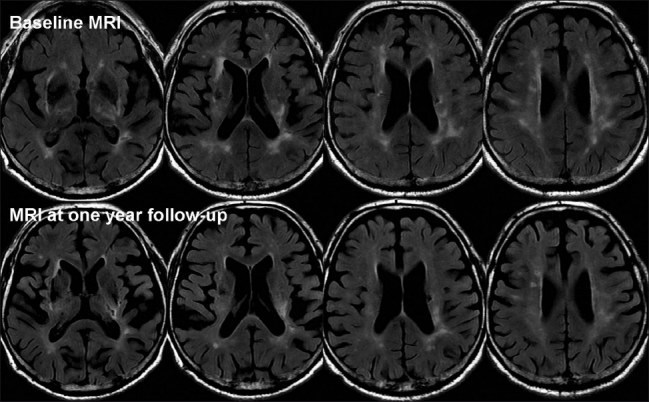
Axial FLAIR MRI at baseline and after 6 months of follow-up, showing partial regression of white matter lesions in the centrum ovale
DISCUSSION
In this case report on a stroke patient with evidence of SVD, we observed the partial disappearance of WMLs located in the centrum ovale 6 months after symptom onset.
In radiological terms, WMLs are defined as confluent hyperintensities on T2-weighted and FLAIR sequences. They may affect one or both sides of the brain and are mainly located in the hemispheric WM, although they can occur in the pons.[7] These lesions are mostly thought to reflect an underlying ischemic (type 1) form of SVD (also referred to as arteriolosclerosis).[1] The pathophysiological characteristics of this age- and vascular risk factor-related SVD include structural changes affecting the small intraparenchymal cerebral arteries and arterioles, such as loss of smooth muscle cells from the tunica media, deposits of fibro-hyaline material, narrowing of the lumen, and thickening of the vessel wall.[1] It has been suggested that these conditions might alter cerebral blood flow regulation and thus contribute to the development of WMLs.
Two other cases of partial WML regression following ischemic stroke have been reported. First, Moriya et al. reported on a 78-year-old woman who developed an acute cerebral infarct in the right occipital lobe.[8] Brain MRI performed 1 year later showed attenuation of the WMLs in the centrum ovale. The authors speculated that attenuation of brain WMLs was due to transient blood–brain barrier (BBB) disturbance during the stroke acute phase, with leakage of cerebral fluid into the WM and subsequent recovery. More recently, Yamada et al. reported the case of a 75-year-old man in whom partial reversal of WMLs was observed 1 week after right carotid artery stenting.[9] The authors concluded that cerebral blood flow impairment could explain their findings. This hypothesis was supported by the initial MRA results (severe right ICA stenosis with flow compromise in the middle cerebral artery). Although the attenuated and non-attenuated WMLs had apparently similar diffusion coefficients, the attenuated lesions tended to have higher axial diffusivity that was suggestive of reduced axonal flow.
In the two previous case reports and our present observation, the WML attenuation only concerned the centrum ovale. This may be due to a specific pattern of arterial vascularisation. In fact, anastomoses between the brain surface vessels originating from the pial network (supplying the cerebral hemispheric WM) and those branching off the subependymal arteries (supplying the periventricular WM) are generally rare or absent.[10] Hence, this region is particularly susceptible to injury through systemic or focal decreases in cerebral blood flow. Arteriolosclerosis might cause a decrease in blood flow that damages the WM. This condition is strongly associated with aging, diabetes, and (in particular) hypertension.[2] Considering these histological particularities, vessel lumen restriction is thought to lead to a state of chronic WM hypoperfusion, degeneration of myelinated fibres, loss of oligodendrocytes, and axonal damage.[1] This ischemic mechanism may induce progressive, irreversible damage. Although this may be particularly the case for confluent WMLs (consistent with more advance SVD), punctuate and early confluent WMLs may constitute an intermediate (and thus reversible) state of tissue damage.[11]
Alternative hypotheses include endothelial dysfunction. Indeed, circulating blood markers of endothelial activation are particularly elevated in patients with leukoaraiosis.[12] This type of endothelial dysfunction may (i) predispose individuals to decrease in cerebral blood flow and impaired self-regulatory responses and/or (ii) increase the permeability of the BBB: both these scenarios would result in damage to the brain parenchyma.[3] Evidence in favor of the latter hypothesis was reported recently.[13] Arterial hypertension has been particularly incriminated in capillary permeability dysfunction.[14] Furthermore, in a substudy of the Perindopril Protection Against Recurrent Stroke Study,[15] Dufouil et al. showed that an active blood pressure-lowering regimen stopped or delayed the progression of WMLs in stroke patients—especially in those with severe WMLs at the baseline.[16] Finally, one can speculate that aspirin (by decreasing vascular inflammation and endothelial dysfunction) or the statin (with its numerous protective effects, such as lipoprotein alterations, improved endothelial function, decreased vascular inflammation, and an antioxidant effect) could have helped to mitigate WMLs in our patient.[2]
In conclusion, this case emphasizes that even though the underlying disease mechanism remains uncertain, WMLs can be attenuated after ischemic stroke. However, in addition to the pathophysiological information, our observations suggest that optimal secondary stroke prevention may not only prevent the progression of WMLs in ischemic stroke patients but could also partly reverse the process.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Pantoni L. Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 2.Ovbiagale B, Saver JL. Cerebral white matter hyperintensities on MRI: Current concepts and therapeutic implications. Cerebrovasc Dis. 2006;22:83–90. doi: 10.1159/000093235. [DOI] [PubMed] [Google Scholar]
- 3.Wardlaw JM, Sandercock PA, Dennis MS, Starr J. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke. 2003;34:806–12. doi: 10.1161/01.STR.0000058480.77236.B3. [DOI] [PubMed] [Google Scholar]
- 4.Kasner SE, Chalela JA, Luciano JM, Cucchiara BL, Raps EC, McGarvey ML, et al. Reliability and validity of estimating the NIH stroke scale score from medical records. Stroke. 1999;30:1534–7. doi: 10.1161/01.str.30.8.1534. [DOI] [PubMed] [Google Scholar]
- 5.McAuliffe MJ, Lalonde FM, McGarry D, Gandler W, Csaky K, Trus BL. Medical Image Processing, Analysis and Visualization in Clinical Research. 14th IEEE Symposium on Computer-Based Medical Systems. 2001 [Google Scholar]
- 6.Li C, Huang R, Ding Z, Gatenby C, Metaxas DN, Gore JC. A level set method for image segmentation in the presence of intensity inhomogeneities with application to MRI. IEEE Trans Image Process. 2010;19:3243–54. doi: 10.1109/TIP.2011.2146190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pullicino P, Ostrow P, Miller L, Snyder W, Munschauer F. Pontine ischemic rarefaction. Ann Neurol. 1995;37:460–6. doi: 10.1002/ana.410370408. [DOI] [PubMed] [Google Scholar]
- 8.Moriya Y, Kozaki K, Nagai K, Toka K. Attenuation of brain white matter hyperintensities after cerebral infarction. AJNR Am J Neuroradiol. 2009;30:E43. doi: 10.3174/ajnr.A1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamada K, Sakai K, Owada K, Mineura K, Nishimura T. Cerebral white matter lesions may be partially reversible in patients with carotid artery stenosis. Am J Neuroradiol. 2010;31:1350–2. doi: 10.3174/ajnr.A1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Reuck J. The human periventricular arterial blood supply and the anatomy of cerebral infarctions. Eur Neurol. 1971;5:321–34. doi: 10.1159/000114088. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt R, Fazekas F, Kleinert G, Offenbacher H, Gindl K, Payer F, et al. Magnetic resonance imaging signal hyperintensities in the deep and subcortical white matter.A comparative study between stroke patients and normal volunteers. Arch Neurol. 1992;49:825–7. doi: 10.1001/archneur.1992.00530320049011. [DOI] [PubMed] [Google Scholar]
- 12.Hassan A, Hunt BJ, O’Sullivan M, Parmar K, Bamford JM, Briley D, et al. Markers of endothelial dysfunction in lacunar infarction and ischaemic leukoaraiosis. Brain. 2003;126:424–32. doi: 10.1093/brain/awg040. [DOI] [PubMed] [Google Scholar]
- 13.Topakian R, Barrick TR, Howe FA, Markus HS. Blood-brain barrier permeability is increased in normal-appearing white matter in patients with lacunar stroke and leucoaraiosis. J Neurol Neurosurg Psychiatry. 2010;81:192–7. doi: 10.1136/jnnp.2009.172072. [DOI] [PubMed] [Google Scholar]
- 14.Nag S. Cerebral changes in chronic hypertension: Combined permeability and immunohistochemical studies. Acta Neuropathol (Berl) 1984;62:178–84. doi: 10.1007/BF00691850. [DOI] [PubMed] [Google Scholar]
- 15.PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033–41. doi: 10.1016/S0140-6736(01)06178-5. [DOI] [PubMed] [Google Scholar]
- 16.Dufouil C, Chalmers J, Coskun O, Besançon V, Bousser MG, Guillon P, et al. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: The PROGRESS (Perindopril protection against recurrent stroke study) Magnetic resonance imaging substudy. Circulation. 2005;112:1644–50. doi: 10.1161/CIRCULATIONAHA.104.501163. [DOI] [PubMed] [Google Scholar]


