Abstract
Background:
Sleep apnea is associated with increased risk of diabetes mellitus. However, no studies have compared sleep apnea symptoms in diabetic patients and their first degree relatives. The purpose of our study was to investigate high risk for sleep apnea syndrome, in diabetics and their first degree relatives for prevention of diabetes in family.
Methods:
As a part of a cohort study, all of diabetic and their first degree relatives who came for glucose control in diabetes clinic were invited to take part in the survey. Two thousand, four hundred and sixty-two individuals (82% of invited) agreed to fill out the Berlin and Epworth sleep questionnaire. Participants consisted of 2462 subjects of 15–70 years of age, both males and females with diabetes and family history of type 2 diabetes mellitus. A total of 1234 participants had diabetes and 11,231 were relatives of diabetic patients. High risk for sleep apnea regarding Berlin questionnaire and Epworth sleepiness scale, diabetic and relative were analyzed.
Results:
Prevalences of high risk for sleep apnea were higher among diabetics than relatives (P-value<0.001). In a multiple regression analysis, “age, body mass index, education, high blood pressure” were risk factor for sleep apnea symptoms while isolated blood glucose level was not by Berlin questionnaire. By Epworth sleep scale only education level was a risk factor for sleep apnea symptoms while isolated blood glucose level was not risk factor.
Conclusions:
Sleep apnea symptoms may not have significant difference between diabetics and their relatives. We need more study on sleep apnea in the family of diabetic patients. We hope that more studies on mentioned field may help prevention of diabetes in their family.
Keywords: Diabetes, first degree relatives, Iran, sleep apnea
INTRODUCTION
Many medical publications have indicated that habitual snoring and sleep apnea is associated with increased risks of obesity and some obesity-related disorders including hypertension, dyslipidemia, hyperglycemia, insulin resistance and type 2 diabetes.[1] Some recent studies have suggested an independent association between obstructive sleep apnea (OSA), and the mentioned components of metabolic syndrome, particularly insulin resistance and diabetes.[2] Thus, it seems that OSA and the risk factors of type 2 diabetes are correlated in an unknown pathway, but the evidence supporting a role for OSA itself in the development of type 2 diabetes is scarce.[3]
It has been proposed that one of the additional ways that OSA could contribute to morbidity and or mortality of its sufferers is through initiation or accentuation of type 2 diabetes.[4] There are several biologically plausible path-ways to explain this correlation, including the effects of slow wave sleep deprivation and intermittent hypoxia.[4]
Several risk factors have been hypothesized in developing sleep apnea syndrome such as obesity, age, sex, hypertension, heart failure, stroke and diabetes. However, little is known regarding the existence and influence of family history of diabetes (with varying degrees of blood glucose level) and sleep apnea.
The objectives of the present study were to examine the rates of those being at high risk for sleep apnea in diabetics and their relatives.
METHODS
Ethical committee of institution reviewed and approved the protocol and the methods. All participants signed in a written consent of agreement to take part in the study.
All diabetic patients registered at the Isfahan Endocrine and Metabolism Research Centre who had been under regular follow-up for at least the past 12 months, and their first degree relatives were invited to take part in the study.
The study population comprised the 2465 subjects (82% of invited) who accepted to take part in the study.
Weight was measured to the nearest kilogram. Height was determined to the nearest centimeter. Body mass index (BMI) was calculated as the weight (kg) divided by the square of height (meters squared).
Participants verbally reported their blood pressure.
Two venous whole blood samples were drawn; one after 8–10 h of fasting and the second, 2-hours after ingestion of 75-g glucose (2-h PG).[5]
Hemoglobin A1C was measured for all subjects.
The participants were given the study questionnaires to feel it in with the assistance of the medical nurses that had been oriented for the study proposals.
The questionnaire harbored three parts. The first part included questions about education, smoking, marriage status, fasting blood sugar, HbA1c and sleep duration.
The second part was Berlin questionnaire that is well-known worldwide and has been adopted and used in previous studies in Iran.[6]
The Berlin Questionnaire is a 10-item questionnaire, divided in three categories according to symptoms: Snoring, sleepiness or chronic fatigue and presence of hypertension or obesity defined as BMI above 30 kg/m2. A subject is considered to have a high risk for OSA if he is positive in any two of the categories.[6]
The third part consisted of the Epworth sleepiness Scale (ESS) Sleep Test questionnaire, which added detailed questions about sleepiness during various conditions.[7] ESS is a validated eight-item self-rating scale. Scores range from 0 to 24, where a score greater than 10 indicates Excessive Daytime Sleepiness.[7]
Analysis
Statistical analyses were performed using Statistical package for the social sciences, (SPSS version 14). The numerical measurement findings are presented as the means±SD. χ2 test was used to test for differences between two groups. Multiple logistic regression analysis was performed for evaluations of variables and the results are expressed as odds ratios (OR) with 95% confidence intervals (CI).
RESULTS
Descriptive statistics for all the predictor variables modeled are listed in Table 1 among diabetics and relatives. Sex, marital status, educational status and age were statistically significant. But there were no significant difference for smoking and BMI.
Table 1.
Distribution of the characteristics, anthropometric and risk of sleep apnea
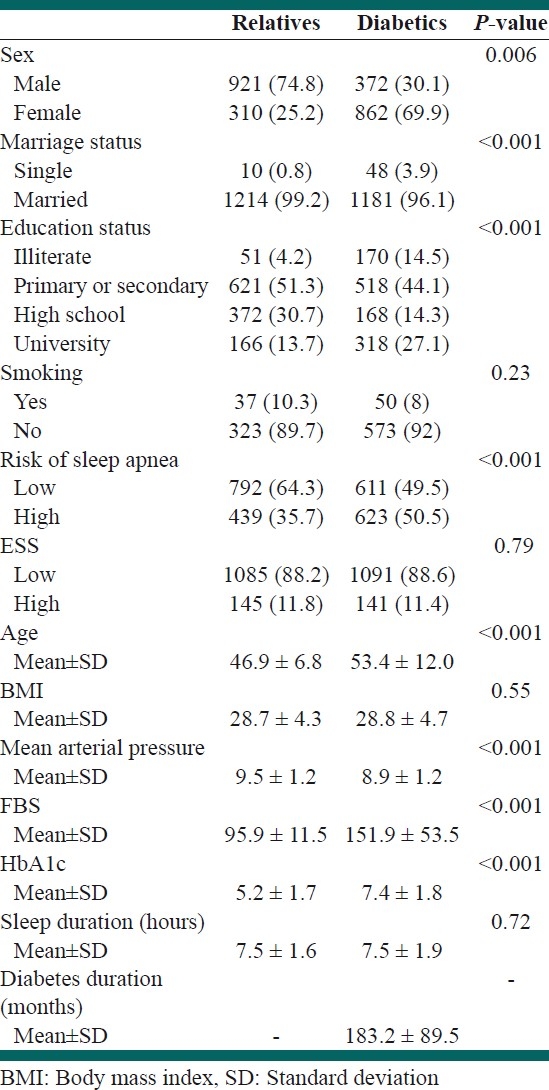
The mean age was higher in the diabetic group (53.4 ±12 years) than in their relatives (46.9 ± 6.8 years).
Diabetics were predominantly women (69.9%). Nearly most of both groups were non-smoker.
Over half of diabetic people (50.5%) had high risk for sleep apnea by Berlin questionnaire. But the majority (88.6%) were nonsleepy by Epworth sleep questionnaire.
Sleep duration was around 7.5 hours in both groups.
Comparisons of characteristics with high risk of sleep apnea by Berlin questionnaire in both groups are presented in Table 2.
Table 2.
Comparison of baseline characteristics with Berlin's risk of apnea
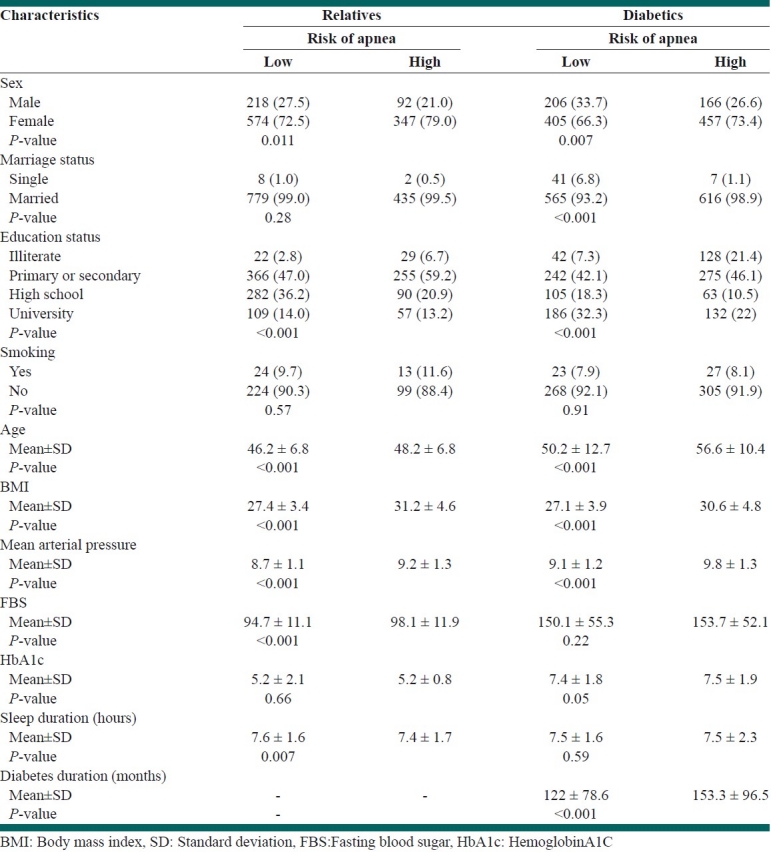
Significant difference for age, sex, BMI, education, blood pressure, HbA1c, sleep duration were reported in diabetics for high risk of sleep apnea by Berlin questionnaire [Table 2].
Significant difference for age, sex, BMI, education, blood pressure and FBS were reported in relatives for high risk of sleep apnea by Berlin questionnaire [Table 2].
Comparison of characteristics with high risk of sleep apnea by Epworth sleep questionnaire in both groups is presented in Table 3.
Table 3.
Comparison of baseline characteristics with Epworth sleepiness scale
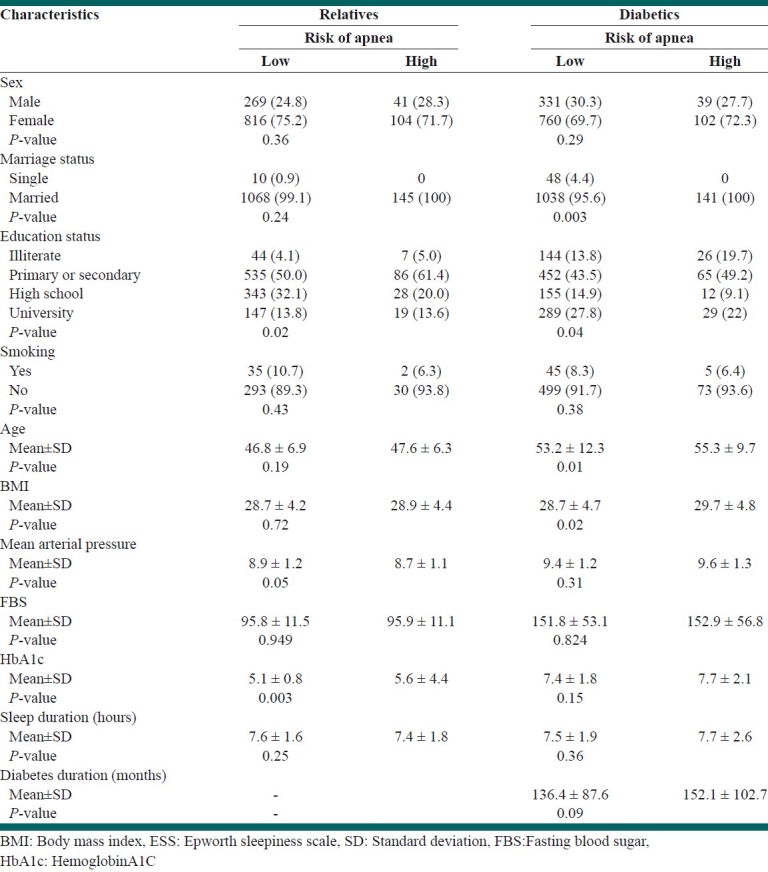
Education and HbA1c had significant difference in relatives [Table 3].
Significant difference for age, marital status and education were reported in diabetics [Table 3].
Multiple logistic regressions [Table 4] was performed to identify which factors were independently related to high risk for sleep apnea by Berlin questionnaire and Epworth sleep questionnaire.
Table 4.
Predictors of risk of apnea from Berlin and ESS questionnaires using multiple logistic regression
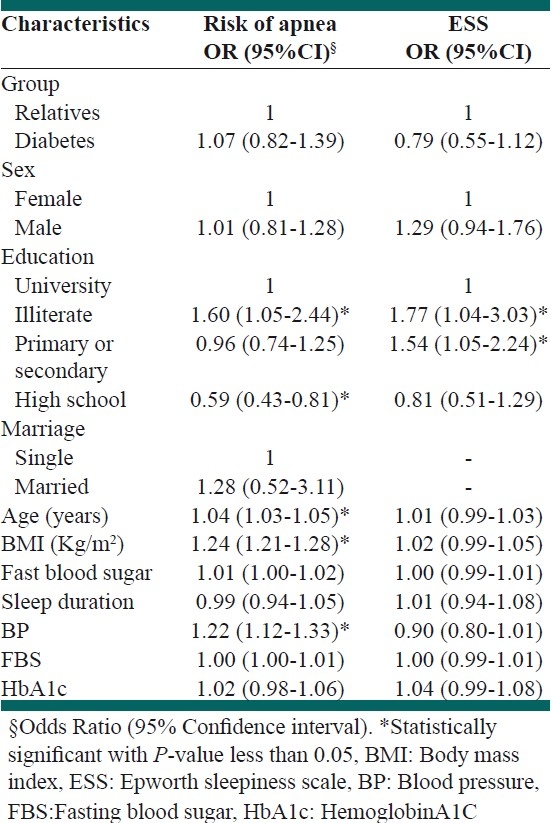
The association of high risk for sleep apnea by Berlin questionnaire and Epworth sleep questionnaire with fasting blood sugar and HbA1c remained nonsignificant [Table 4].
However, age, BMI, education and high blood pressure remained as significant independent predictor by Berlin sleep questionnaire [Table 4].
With Epworth sleep questionnaire only education remained as significant independent predictor [Table 4].
DISCUSSION
To our knowledge, this is the first epidemiological study in diabetics and their first degree relatives designed to analyze high risk for sleep apnea symptoms in both groups in Iran.
The present study showed that diabetics and their first degree relatives present with high prevalence sleep apnea symptoms by Berlin questionnaire regardless of blood glucose level.
In our previous study, prevalence of sleep apnea symptoms with Berlin questionnaire was found to be 5%.[6] So individu-als with diabetes and subjects with family history of diabetes are more likely to report sleep apnea symptoms than normal population. Our study showed that high risk for sleep apnea by Berlin sleep questionnaire in diabetics also were significantly higher than in their relatives. But sleepiness score was not different in both groups based on ESS questionnaire.
Blood glucose level was not determinant of sleep apnea symptoms between two groups.
One of the major limitations of this study is the fact that it is a questionnaire-based study. We also did not use polysomnography for sleep apnea syndrome diagnosis. Second limitation of this study was that we did not have control group.
Despite some methodological limitations, these findings suggesting that both diabetics and their families may predispose to the sleep apnea syndrome.
The underlying mechanisms of short sleep duration, sleep-related breathing disorders, including snoring; with altered glucose metabolism and diabetes are likely to be multifactorial.[8]
Some previous studies have shown that sleep loss adversely affects glucose metabolism and increases the risk of type 2 diabetes.[9,10]
As we know there are no previous studies concerning the prevalence of sleep apnea in nondiabetic relatives, and there is no suggestion of these subjects.
The finding of our study raises the question of whether first degree relatives of diabetics share the same factors as diabetics in sleep apnea syndrome.
Few published previous studies reported improved insulin sensitivity with continuous positive airway pressure among patients with diabetes mellitus type 2 and among nondiabetic sleep apnea patients.[11,12]
Some studies have suggested sleep fragmentation and intermittent hypoxia increase sympathetic activity, which may lead to disorders of glucose metabolism.[13] Alternatively other studies have reported that insulin resistance and chronic hypoxemia may lead to development of sleep apnea syndrome.[14]
So we have several possible explanations for these findings: Might reflect pre-existing undiagnosed basic mechanism underlying sleep apnea.
In conclusion, our results support that sleep apnea symptoms are not significant different between diabetics and their relatives. We need more study on sleep apnea in family of diabetic patient which must be confirmed by polysomnography.
Further research is needed to determine the sleep apnea in diabetic relatives for the prevention of diabetes in clinical and public health settings.
Footnotes
Source of Support: Grant funded by Isfahan University of Medical Sciences, Isfahan, Iran.
Conflict of Interest: None declared.
REFERENCES
- 1.Levy P, Bonsignore MR, Eckel J. Sleep, sleep-disordered breathing and metabolic consequences. Eur Respir J. 2009;34:243–60. doi: 10.1183/09031936.00166808. [DOI] [PubMed] [Google Scholar]
- 2.Tasali E, Mokhlesi B, Van Cauter E. Obstructive sleep apnea and type 2 diabetes: Interacting epidemics. Chest. 2008;133:496–506. doi: 10.1378/chest.07-0828. [DOI] [PubMed] [Google Scholar]
- 3.Bopparaju S, Surani1 S. Sleep and Diabetes. Int J Endocrinol. 2010;2010:759509. doi: 10.1155/2010/759509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105:1044–9. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaccaro O, Riccardi G. Changing the definition of impaired fasting glucose: Impact on the classification of individuals and risk definition. Diabetes Care. 2005;28:1786–8. doi: 10.2337/diacare.28.7.1786. [DOI] [PubMed] [Google Scholar]
- 6.Amra B, Farajzadegan Z, Golshan M, Fietze I, Penzel T. Prevalence of sleep apnea-related symptoms in a Persian population. Sleep Breath. 2011;15:425–9. doi: 10.1007/s11325-010-0353-4. [DOI] [PubMed] [Google Scholar]
- 7.Amra B, Dorali R, Mortazavi S, Golshan M, Farajzadegan Z, Fietze I, et al. Sleep apnea symptoms and accident risk factors in Persian commercial vehicle drivers. Sleep Breath. 2011 doi: 10.1007/s11325-010-0473-x. [In press] [DOI] [PubMed] [Google Scholar]
- 8.Arnardottir ES, Mackiewicz M, Gislason T, Teff KL, Pack AL. Molecular signatures of obstructive sleep apnea in adults: A review and perspective. Sleep. 2009;32:447–70. doi: 10.1093/sleep/32.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaput JP, Despres JP, Bouchard C, Tremblay A. Association of sleep duration with type 2 diabetes and impaired glucose tolerance. Diabetologia. 2007;50:2298–304. doi: 10.1007/s00125-007-0786-x. [DOI] [PubMed] [Google Scholar]
- 10.Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, et al. Sleep duration as arisk factor for diabetes incidence in a large U.S. sample. Sleep. 2007;30:1667–73. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harsch IA, Schahin SP, Radespiel-Troger M, Weintz O, Jahreiss H, Fuchs FS, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2004;169:156–62. doi: 10.1164/rccm.200302-206OC. [DOI] [PubMed] [Google Scholar]
- 12.Harsch IA, Schahin SP, Bruckner K, Radespiel-Troger M, Fuchs FS, Hahn EG, et al. The effect of continuous positive airway pressure treatment on insulin sensitivity in patients with obstructive sleep apnoea syndrome and type 2 diabetes. Respiration. 2004;71:252–9. doi: 10.1159/000077423. [DOI] [PubMed] [Google Scholar]
- 13.Peltier AC, Consens FB, Sheikh K, Wang L, Song Y, Russell JW. Autonomic dysfunction in obstructive sleep apnea is associated with impaired glucose regulation. Sleep Med. 2007;8:149–55. doi: 10.1016/j.sleep.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Vgontzas AN, Legro RS, Bixler EO, Grayev A, Kales A, Chrousos GP. Polycystic ovary syndrome is associated with obstructive sleep apnea and daytime sleepiness: Role of insulin resistance. J Clin Endocrinol Metab. 2001;86:517–20. doi: 10.1210/jcem.86.2.7185. [DOI] [PubMed] [Google Scholar]


