Abstract
Purpose of review
Obesity is a widespread condition associated with a variety of mechanical, metabolic, and physiologic changes that affect both health outcomes and delivery of care. Nutrition support is a key element of management during critical illness known to improve outcomes favorably, but is likewise complicated in the presence of obesity. This review serves to discuss the challenges unique to management of critically ill obese patients and an evidence-based approach to nutrition support in this patient population.
Recent findings
High-protein, hypocaloric feeding has emerged as a nutrition support strategy capable of reducing hyperglycemia and protein catabolism, while promoting favorable changes in body composition and fluid mobilization. Recent data have shown a protective effect of mild-moderate obesity (BMI 30–39.9 kg/m2), with improved morbidity and mortality outcomes in this subgroup. Therefore, it is unclear whether hypocaloric feeding represents an inferior approach in this subgroup in which weight maintenance may be preferable.
Summary
There are many obstacles that limit provision of nutrition support in the obese ICU patient. Calculating energy needs accurately is extremely problematic due to a lack of reliable prediction equations and a wide variability in body composition among the obese patients. Further research is needed to determine a better approach to estimating energy needs in this population, in addition to validating hypocaloric feeding as the standard approach to nutrition support in the obese patients.
Keywords: critical illness, hypocaloric feeding, nutrition support, obesity
Introduction
Obesity is a worldwide public health issue with extensive medical, social, and economic consequences [1,2]. Obesity, which is defined by the presence of excess adiposity, negatively impacts health and increases an individual’s risk for developing a variety of medical conditions, including cardiovascular disease, certain cancers, and diabetes mellitus [3–7]. Over the past three decades, the prevalence of obesity has doubled in the USA [8]. At present, an astounding two-thirds of the US population is overweight and about one-third, or roughly 100 million Americans, are obese [8,9]. And although the most recent data published in the 2005–2006 update of the National Health and Nutrition Examination Survey (NHANES) suggest that obesity rates have stabilized, others project that the obesity ‘epidemic’ will only continue to worsen, with as many as 75% of Americans potentially being overweight in the year 2015 [10,11]. Physicians will undoubtedly encounter obese persons in clinical practice and must, therefore, be able to identify and address care needs specific to this patient population.
Obesity presents the intensive care unit (ICU) team with a unique set of challenges. Not only does the greater frequency of comorbid diseases in this population lead to increased complexity of care, but the physical aspect of severe obesity makes routine elements of nursing care and diagnostic/therapeutic interventions extremely demanding. Nutrition support is a key component in managing critically ill patients, with early and aggressive feeding interventions shown to improve outcomes favorably. Like other aspects of care, feeding also becomes complicated in the presence of obesity. Calculating daily caloric needs remains controversial in this population, and other issues, such as difficulty obtaining central venous access, frequently limit the provision of adequate nutrition support. Hypocaloric feeding shows great promise as a mechanism for blunting hyperglycemia while promoting favorable changes in body composition, though this approach has yet to be fully validated. This article will discuss the nutritional assessment and management of the critically ill obese patient, highlighting some of the challenges in caring for this population.
Defining obesity
Obesity is a chronic condition characterized by the presence of excess body fat. Overweight and obesity definitions in adults are presently based on BMI, a ratio of weight (kilograms) to height (square meters). BMI generally corresponds well to body fat, though there are obvious limitations in using this method with patients at extremes of body composition. The National Heart, Lung and Blood Institute (NHLBI) defines a healthy BMI range as 18.5–24.9 kg/m2, overweight as BMI of 25–29.9 kg/m2, and obesity as a BMI of more than 30 kg/m2 [12]. Obesity is further subdivided into classes based on associated health risk: mild (class I) obesity is BMI30–34.9 kg/m2, moderate (class II) obesity isBMI35–39.9 kg/m2, and severe/morbid (class III) obesity is BMI of more than 40 kg/m2.
Medical comorbidities of obesity and intensive care unit
Obesity is associated with an increased risk for developing a number of chronic medical conditions (see below) and, therefore, obese individuals are probably at higher risk for hospitalization and ICU admission than the nonobese. One might also expect obesity to be associated with poorer critical care outcomes, given comorbid disease affects the ability to cope with metabolic and physiologic stress, but this relationship remains unclear. Published data are inconsistent, with some studies demonstrating increased morbidity and mortality in obese ICU patients [13–15,16•] and others showing no effect or improved outcomes [17,18,19•,20••,21]. Select medical conditions associated with obesity are as follows [3–7]:
-
Cardiovascular
Congestive heart failure, hypertension, myocardial infarction, dyslipidemia
-
Respiratory
Hypoventilation (Pickwickian) syndrome, obstructive sleep apnea, asthma, respiratory failure
-
Gastrointestinal
Gastroesophageal reflux (GERD) nonalcoholic fatty liver disease (NAFLD), steatohepatosis (NASH), gastroparesis, gallstones, biliary tract disease, pancreatitis, hernias
-
Endocrine
Diabetes mellitus (type II), metabolic syndrome, polycystic ovarian syndrome, hypothyroidism, infertility
-
Neurologic/Psychologic
Stroke, depression, idiopathic intracranial hypertension, disordered eating
-
Hematologic
Deep vein thrombosis, hypercoagulable state, chronic venous stasis
-
Musculoskeletal
Degenerative joint disease, chronic back pain
-
Immune system/infection
Pressure ulcers, skin-fold infections, poor wound healing, proinflammatory state
-
Increased cancer risk
Kidney, esophagus, pancreas, colon, breast, ovary, endometrial, and prostate.
Two recent large-scale meta-analyses both concluded that BMI was not an independent risk factor of mortality outcomes in the ICU, though it was associated with longer ICU stays and increased length of ventilator dependence [18,19•]. These reviews are largely based on retrospective analyses, however, and may not represent a true causal relationship. In a rare prospective study, Frat et al. [20••] compared 82 severely obese (mean BMI, 42 ± 6 kg/m2) and 124 nonobese (mean BMI, 24 ± 4 kg/m2) mechanically ventilated patients and found that obese patients had higher rates of intubation-related complications, but otherwise no significant differences in mortality, length of ICU stay, duration of mechanical ventilation, or infection rates.
Several studies have also suggested that modest obesity may have a protective effect during critical illness. Akkinusi et al. [19•] found that obesity overall was not associated with ICU mortality [relative risk (RR) = 1.0; 95% confidence interval (CI) 0.86–1.16; P = 0.97], but the mild-moderately obese subgroup actually had improved survival outcomes (RR = 0.86; 95% CI 0.81–0.91; P < 0.001). Yaegashi et al. [13] found that critically ill patients with severe obesity had increased rates of mortality, nursing home admission, ICU complications, and length of stay relative to mild-moderately obese patients. These data support the hypothesis of an ‘obesity paradox’, in which a modestly increased BMI actually confers a survival advantage to the individual, probably because the benefit of excess adipose tissue (which acts as a reservoir for energy, hormones, and anti-inflammatory mediators) exceeds the negative physiologic consequences of obesity [22]. This dictum does not hold true once BMI is more than 40 kg/m2, but has interesting implications when considering overall goals of nutrition support in patients with lesser degrees of obesity.
Metabolic response to critical illness and obesity
Regardless of the inciting cause of injury or illness, there is a common hypermetabolic, inflammatory response to physiologic stress, directed at promoting acute survival, which affects macronutrient (protein, lipid, and carbohydrate) utilization throughout the body [23,24,25•,26,27]. Obesity is a proinflammatory state and probably lowers the threshold at which these mechanisms become overwhelmed or exaggerated during critical illness.
Carbohydrate metabolism and hyperglycemia
Stress-induced hyperglycemia is a frequent complication of critical illness and the end-product of increased counter regulatory hormone production (glucagon, glucocorticoids, and catecholamines) and inflammatory cytokine release, leading to accelerated hepatic gluconeogenesis, lipolysis, and peripheral insulin resistance [23,24,25•]. Hyperglycemia during critical illness is associated with poorer outcomes in both diabetic and nondiabetic individuals alike [27,28]. Given the increased prevalence of diabetes and insulin resistance among the obese patients, it is especially important to include glycemic control into the plan for nutrition support. Care should be taken to avoid iatrogenic hyperglycemia from overfeeding, as administration of excess glucose (calorie) loads can lead to increased lipogenesis, hepatic steatosis, and increased CO2 production, which in turn increases the work of breathing [23,25•]. Insulin infusion is the preferable method to achieve normoglycemia in the ICU setting, especially as insulin absorption may vary in obese patients with substantial amounts of subcutaneous adipose tissue [29•]. Alternatively, regular insulin can also be added directly to total parenteral nutrition (TPN) solution once requirements are stable.
Fatty acid oxidation and protein utilization
At fasting baseline, obese persons have increased blood levels of hormones and substrate, including amino acids and free fatty acids (FFAs). Elevations in FFA usually signify insulin resistance, which causes increased lipolysis, impaired skeletal muscle FFA oxidation, and reduced suppression of plasma FFA by insulin [30]. Despite having a relative abundance of serum FFAs and triglyceride-rich adipose stores, it appears the obese individuals are ineffective at mobilizing or using these energy sources during critical illness [26,30,31]. Although their study has yet to be repeated, Jeevanandam et al. [26] showed major differences in utilization of endogenous fuel sources between starved obese and nonobese trauma patients; lean patients relied largely on fatty acid oxidation for energy [about 61% of resting energy expenditure (REE)], whereas obese patients derived most energy from catabolism of lean mass (only 39% of energy from FFA).
Muscle protein catabolism is a hallmark feature of critical illness, regardless of BMI, with studies showing losses of up to 10–20% of skeletal muscle after 1 week in the ICU [32,33]. Obese persons have increased amounts of fat-free mass (FFM) over their height-matched lean counterparts, but are more likely to use this muscle mass as fuel during critical illness when fasted, only accelerating the rate of protein losses [26]. FFM (protein) catabolism typically persists despite the provision of nutrition support, though administration of either greater total calories or protein calories has been shown to mitigate its rate and improve nitrogen balance. Hypocaloric, high-protein nutrition is a preferable approach in obese patients, as it can promote endogenous fat oxidation and shift obese patients away from utilization of FFM as the predominant fuel source, while simultaneously inducing favorable changes in body composition [34,35]. Avoidance of overfeeding is also critical because excess caloric load is associated with increased protein turnover and fat storage [36].
Initial evaluation of patient
A focused assessment prior to initiation of feeding allows care providers to identify nutritional risk factors and comorbidities that may affect goals, route, and formulation of nutrition support plan.
History
There is often a misconception that obese persons are well nourished or ‘over’ nourished based on the excess caloric intake typically required to maintain an obese phenotype. However, BMI does not correlate well with nutritional status and a thorough dietary and weight history can help identify any pre-existing nutritional risk factors. Providers should elicit whether there has been any significant gain or loss of weight and whether this change was intentional, as this may not be obvious in an obese person. Risk factors for enteral failure should be reviewed, including changes in gastrointestinal function, prior abdominal or bariatric surgeries, and/or mechanical limitations to eating. These key elements should reveal the baseline nutritional status, level of risk for refeeding syndrome, and whether weight maintenance or weight loss is preferable when determining the best approach to nutrition support in the obese ICU patient.
Physical examination
Physical examination is often limited in the severely obese individuals, but should at least include cardiopulmonary assessment, abdominal examination, determination of volume status, and identification of muscle wasting indicative of chronic protein-calorie malnutrition. Additionally, whole-body skin surface, including skin folds and surgical sites, should be examined for integrity and presence of wounds. This may require additional staff members or specialized lifting equipment to perform, but is imperative, as obese patients are at increased risk for developing skin infections and wound complications.
Accurate determination of weight and height is also essential before calculating energy needs. Data reported by family members or patients are often unreliable, especially among the obese individuals and should be verified by staff upon admission. Weights should be monitored daily, typically via level, calibrated bed scale in the ICU. This becomes problematic in extremely obese patients who may exceed the limits of bed scale equipment or require specialized, oversized hospital beds that depend on institution-specific availability. Monitoring 24-h fluid balance (intake-output) can help identify major fluid shifts in patients wherein measured weights are unreliable or unavailable and allows for adjustment of nutrition support in response to fluctuating volume/caloric needs.
Assessment of energy requirements
Determining REE is an integral part of the nutrition assessment, allowing the clinician to minimize negative outcomes associated with underfeeding and overfeeding. Unfortunately, calculation of daily energy requirements from REE for hospitalized, obese patients remains challenging, as there is no consensus as to which prediction equation for REE is most accurate in this patient population [37–43]. Common equations for estimation of energy expenditure are as follows:
-
Hamwi equation for ideal body weight (IBW)
Male (lbs): 106 + 6 (Height in inches − 60)
Female (lbs): 100 + 5 (Height in inches − 60)
-
Adjusted body weight (ABW):
[(actual body weight − IBW) × (0.25 to 0.5)] + IBW
-
Weir equation for REE:
From metabolic cart: kcal/d = 1.44 × [3.9(O2 consumption) + 1.1(CO2 production)]
-
Harris–Benedict equation (HBE) [37]
Men: kcal/d = 66.47 + 13.75 (w) + 5 (h) − 6.75 (a)
Women: kcal/d = 655.1 + 9.56 (w) + 1.85 (h) − 4.68 (a)
Adjusted: kcal/d = HBE × (injury factor) × (activity factor)
-
Mifflin–St. Jeor (MSJ) [38]
Men: kcal/d = 10 (w) + 6.25 (h) −5 (a) + 5
Women: kcal/d = 10 (w) + 6.25 (h) −5 (a) − 161
-
Ventilator-dependant: kcal/d = 1784 + 5 (w) − 11 (a) + 244 (if men) + 239 (if trauma) + 804 (if burn)
Spontaneously breathing: kcal/d = 629 − 11 (a) + 25 (w) − 609 (if BMI >27 kg/m2)
-
Penn State [41]
Harris–Benedict: kcal/d = 0.85 (HBE) + 175 (Tmax) + 33 (Ve) − 6344
Mifflin: kcal/d = 0.96 (MSJ) + 167 (Tmax) + 31 (Ve) − 6212
-
Cunningham [42]:
kcal/d = 370 + 21.6 (kg of fat-free mass)
-
Diabetes modified [42]
kcal/d = 71.761 − 2.34 (a) + 10 (w) + 146 (if diabetic) + 257.3 (if men)
-
American College of Chest Physicians (ACCP) [43]
25–30 kcal/kg of actual weight or 21 kcal/kg IBW
where a, age; h, height (cm); Tmax, maximum temperature in a 24 h period; Ve, minute ventilation; w, actual weight (kg).
Indirect calorimetry remains the ‘gold standard’ method for measuring REE [44,45]. However, its use is limited by cost, availability of proper equipment and trained personnel, patient ventilatory status (FiO2 >60% generally excluded), and ability to use handheld device in non-ventilated patients [46].
When predictive equations must be used, determining how to use an obese person’s weight to avoid introducing bias into the calculations is problematic. Using actual body weight is likely to overestimate caloric needs, given that adipose tissue is felt to be less metabolically active than FFM [47,48]. On the other hand, using IBW will likely underestimate caloric needs because it does not appropriately reflect the increased amount of lean body mass present in the obese patients. Adjustment of weight (see above) to account for these differences estimates that 25–50% of excess mass (actual weight − IBW) is likely to be metabolically active tissue in addition to IBW [49]. This strategy is also flawed and has not yet been adopted for routine use over BMI and IBW [50•].
Several recent studies have examined the usefulness of prediction equations for REE specifically in obese patients. Anderegg et al. [51] compared five different prediction methods (Mifflin–St. Jeor, Harris–Benedict, Ireton–Jones, and 21 or 25 kcal/kg/d) with indirect calorimetry measures in 36 obese patients receiving nutrition support. They found the HBE using ABW (25%) and a stress factor (1.2 for ward patients, 1.5 for ICU patients) most closely predicted REE within ±10% of measured value, but only 50% of the time. Stucky et al. [42] compared HBE, Cunningham, and diabetes-adjusted equations in 28 mechanically ventilated obese trauma and burn patients. All three prediction equations underestimated REE before incorporation of a 20% injury factor (1.2), with the HBE (using actual body weight) most closely approximating caloric needs. Frankenfield et al. [52] measured REE in 202 patients, about half of whom were critically ill, when validating eight different prediction equations. They found the Penn State equation (using HBE from adjusted weight) estimated REE with the least amount of bias across all age and BMI subgroups and with accuracy rates of 70 and 59%, respectively, in young (18–59 years) and elderly (≥60 years) obese patients.
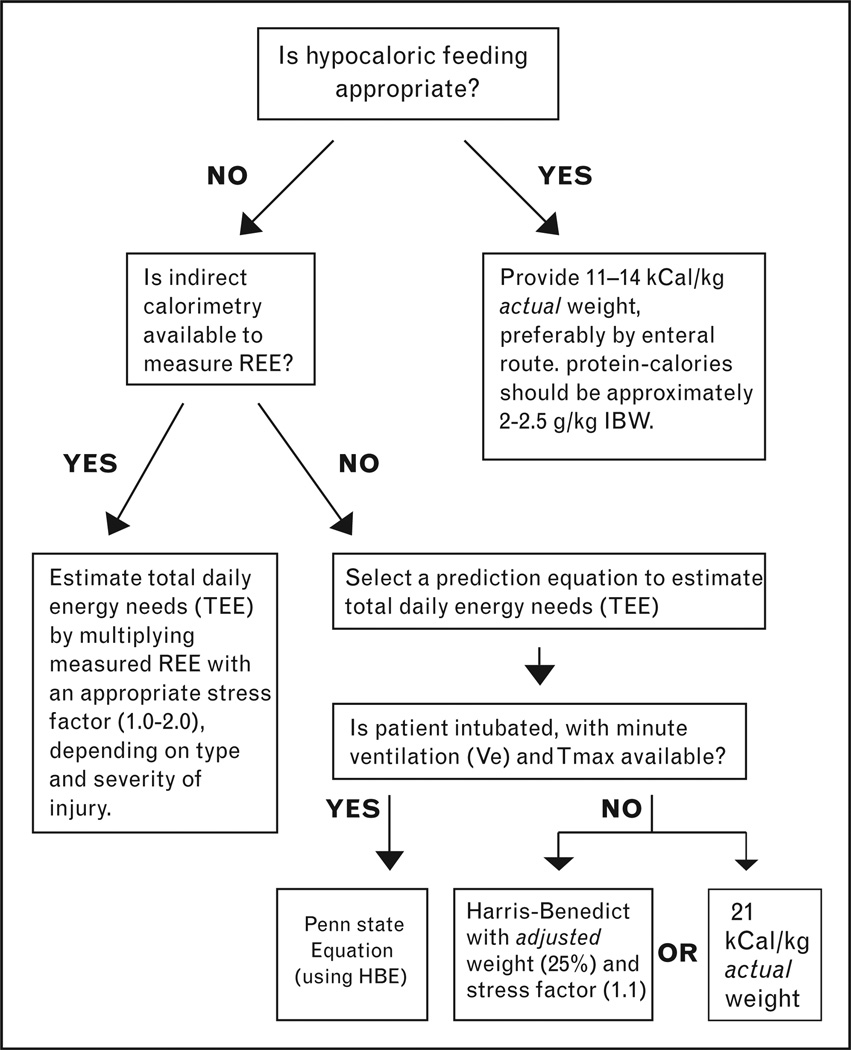
No available equation reliably estimates REE, though the Penn State and HBEs have the strongest evidence supporting their use in the obese ICU patient population [42,48,51–55]. We recommend using the Penn State method in ventilator-dependent obese patients, or HBE with actual body weight and a stress factor (1.1) if the patient is spontaneously breathing. The American Diabetes Association (ADA) recommends against using HBE in ICU patients, but it is better to err on the side of underfeeding the obese and, therefore, not unreasonable to select an equation biased toward underestimation [56]. An alternative method is to provide approximately 21 kcal/kg of actual body weight, a value corroborated by measured REE values in several studies [42,48,51]. For example, the Anderegg study reported REE as 20.4 ± 5 kcal/kg actual body weight per day in ventilated, obese patients. Additionally, Zauner et al. [48] found that once adjusted for weight, REE actually decreased with increasing BMI in critically ill patients; measured REE was 24.8 ± 5.5 kcal/kg/d in normal weight individuals, 20.4 ± 2.6 kcal/kg/d in obese, and only 16.3 ± 2.3 kcal/kg/d in morbidly obese patients. If the average obese ICU patient has an REE of 14–25 kcal/kg/d, then using the 1997 ACCP guideline 25–30 kcal/kg/d of actual body weight would routinely overpredict caloric needs and should be abandoned in this population. Ultimately, all methods for estimation are subject to bias and unacceptably high amounts of error and should only be used when indirect calorimetry is unavailable (Fig. 1).
Figure 1.
Algorithm for determining feeding needs in critically ill obese patients
HBE, Harris–Benedict equation; REE, resting energy expenditure; Tmax, maximum temperature in a 24 h period.
Hypocaloric feeding
Initiation of nutrition support in ICU patients is frequently delayed due to prioritization of other aspects of care such as hemodynamic management of shock, stabilization of mechanical ventilation, urgent surgery, or contraindication to placement of access for enteral or parenteral feeding [57]. Additionally, enteral feeding often fails to provide an adequate amount of calories and nutrients in the critically ill population due to patient intolerance of appropriate tube feeding volumes; this is especially true among obese patients, who are at increased risk for having conditions (see above) predisposing to enteral feeding failure. These are examples of unintentional failure to provide adequate nutrition, whereas ‘hypocaloric feeding’, refers specifically to permissive underfeeding of a patient and is derived from the classical protein-sparing modified fast [58]. There is no standard method for hypocaloric feeding, but generally involves providing 30–70% estimated daily caloric needs in conjunction with a higher proportion of protein calories (often 50–60% of total calories) in order to minimize glucose loads while sparing lean body mass from catabolism. Calorie-restricted nutrition (even briefly) can markedly improve insulin sensitivity and glycemic control, in addition to preventing metabolic consequences of overfeeding, such as hypercapnea, fluid retention, and hypertriglyceridemia. Weight loss and reduction in fat mass is another beneficial effect of this intervention, but should never be the primary objective for nutrition support during critical illness.
Multiple studies have demonstrated positive outcomes in the ICU related to reduced caloric intake [34,59–60]. Dickerson et al. [34] showed that hypocaloric enteral feeding in obese surgical patients was associated with improved nitrogen balance, shorter length of stay in the ICU, and decreased use of antibiotics. Although their study was not specific to obese patients, Krishnan et al. [59] found improved ICU outcomes, including mortality, return of spontaneous ventilation, and nosocomial sepsis rates among patients receiving approximately 9–18 kcal/kg/d (33–65% of the ACCP target). The strongest evidence against hypocaloric feeding was provided by Villet et al. [61], who found a higher rate of infections and poor outcomes associated with increasing negative energy balance in a prospective study of 48 ICU patients. However, only 20 patients (41%) had BMI more than 27 kg/m2 and therefore findings may not fully represent the obese subgroup. Additionally, negative outcomes may reflect initial starvation, as patients went an average of 3 ICU days before receiving any nutrition support.
The 2009 Consensus statement issued jointly by the Society of Critical Care Medicine (SCCM) and the American Society for Parenteral and Enteral Nutrition (ASPEN) endorses hypocaloric feeding of critically ill obese patients with enteral feeds, with the goal to provide no more than 60–70% of target energy requirements or 11–14 kcal/kg actual body weight per day [50•]. Based on nitrogen balance data from studies on hypocaloric feeding, the ASPEN/SCCM guidelines also recommend administration of protein in the range of at least 2.0 g/kg IBW per day for class I and II obese patients and at least 2.5 g/kg IBW per day for class III obesity. There are relatively few contraindications to hypocaloric feeding, other than conditions precluding the use of high-protein nutrition, such as progressive renal failure or hepatic encephalopathy, or conditions in which full caloric (dextrose) loads are preferred, including history of hypoglycemia, diabetic ketoacidosis, or severe immunocompromised state. Otherwise, hypocaloric nutrition should always be considered for the obese ICU patient.
Obstacles to nutrition support in obese patients
Care becomes challenging just by virtue of the physical presence of obesity, particularly in individuals with BMI of more than 40 kg/m2. Many hospitals have specialized equipment designed for severely obese patients, including wider beds with greater load-bearing capacity and adaptive slings/devices that assist in lifting and mobilizing patients. Some tasks may require multiple team members and/or additional resources/time to accomplish with a severely obese patient, including routine aspects of care, such as changing bed linens, bathing, toileting, or turning a patient [62–64]. Decreased individual mobility and impaired ability of staff to assist mobility place morbidly obese patients at increased risk for developing skin-fold infections, pressure ulcers, and even aspiration [65].
Obtaining enteral access for feeding is performed using the same approach as in nonobese patients, but often becomes technically challenging in the obese. Feeding tube placement requiring imaging for guidance is difficult in patients weighing over 300–350 lbs (136–160 kg) due to limits of fluoroscopy or roentogram table. Percutaneous placement of feeding access either surgically or endoscopically is associated with much higher rates of complications in the obese, including hernia, wound infection, and ileus [65,66]. Likewise, severe obesity often limits the ability to easily obtain central venous access for parenteral feeding due to loss of superficial landmarks that are obscured by subcutaneous adipose tissue; this is especially true for the subclavian site. The internal jugular vein is the preferable site for central line placement in the extremely obese, and use of ultrasound guidance can further assist in accurate location when cannulating the vein. Sedation and Trendelenburg positioning typically used during placement of a central line can significantly impair gas exchange due to reduced functional reserve capacity (especially with worsening degree of central adiposity) and possible airway compromise. Careful monitoring by the ICU team and consideration for consultation with an anesthesiologist prior to line placement is recommended.
Conclusion
Obese patients present ICU clinicians with a unique set of challenges not encountered in less obese patients. Careful consideration must be given to energy and nutrient requirement calculations in this population, as prediction equations for REE are highly unreliable. Indirect calorimetry should always be the preferred method for measuring REE, but when it is unavailable or impractical, the Penn State equation and adjusted HBE have the strongest evidence to support their use. Alternatively, 21 kcal/kg of actual bodyweight is a reasonable strategy, though this has yet to be validated in practice. Hypocaloric feeding containing at least 2.0 g/kg IBW per day protein (1.3–1.5 g/kg actual weight) is an approach to nutrition support that prevents complications of overfeeding, such as hyperglycemia and fluid retention, while preserving FFM and promoting steady weight loss. Further investigation, including randomized prospective controlled trials, is warranted to validate this method as standard practice for critically ill obese patients, particularly in mild-moderately obese patients in whom maintenance of excess body mass may confer a protective effect.
Acknowledgements
The authors would like to thank Ashley Bourland, BA, Megan Ruth, PhD, and Lorrie Young, RD, MS, CNSD, LDN for their editorial assistance.
Dr C.A. is a Consultant for Novo Nordisk, Arena Pharmaceuticals, Merck Pharmaceuticals, Amylin Pharmaceuticals, GI Dynamics, Johnson & Johnson Inc., Sanofi Aventis Groupe. Orexigen Pharmaceuticals, Pfizer. She receives research funding from Amylin Pharmaceuticals, Sanofi Aventis Groupe, Pfizer, Orexigen Therapeutics, MetaProteomics LLC, the Dr Robert C. and Veronica Atkins Foundation, and Arena Pharmaceuticals.
Footnotes
The authors have no conflicts to disclose.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 220).
- 1.Yach D, Suckler D, Brownel K. Epidemiologic and economic consequences of the global epidemics of obesity and diabetes. Nat Med. 2006;12:62–66. doi: 10.1038/nm0106-62. [DOI] [PubMed] [Google Scholar]
- 2.Runge CF. Economic consequences of the obese. Diabetes. 2007;56:2668–2672. doi: 10.2337/db07-0633. [DOI] [PubMed] [Google Scholar]
- 3.Must A, Spadano J, Coakley EH, et al. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 4.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 5.Friedenberg FK, Xanthopoulos M, Foster GD, et al. The association between gastroesophageal reflux disease and obesity. Am J Gastroenterol. 2008;103:2111. doi: 10.1111/j.1572-0241.2008.01946.x. [DOI] [PubMed] [Google Scholar]
- 6.Field AE, Coakley EH, Must A, et al. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med. 2001;161:1581–1586. doi: 10.1001/archinte.161.13.1581. [DOI] [PubMed] [Google Scholar]
- 7.Fogarty AW, Glancy C, Jones S, et al. A prospective study of weight change and systemic inflammation over 9 y. Am J Clin Nutr. 2008;87:30–35. doi: 10.1093/ajcn/87.1.30. [DOI] [PubMed] [Google Scholar]
- 8.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 9.United States Census Bureau. [Accessed 3 September 2009];U.S. and world population clocks. Updated in real time. http://www.census.gov.
- 10.Ogden CL, Carroll MD, McDowell MA, Flegal KM. Obesity among adults in the United States: no statistically significant changes since 2003–2004. NCHS Health Brief. 2007 November;(No. 1) [PubMed] [Google Scholar]
- 11.Wang Y, Beydoun MA. The obesity epidemic in the United States – gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 12.National Heart, Lung, and Blood Institute Obesity Education Initiative Expert Panel. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. NIH publications. 2000 http://www.nhlbi.nih.gov/guidelines/obesity/prctgd_c.pdf.
- 13.Yaegashi M, Zuriqat M, Noack S, et al. Outcome of morbid obesity in the intensive care unit. J Intensive Care Med. 2005;20:147–154. doi: 10.1177/0885066605275314. [DOI] [PubMed] [Google Scholar]
- 14.Bercault N, Boulain T, Kuteifan K, et al. Obesity-related excess mortality rate in an adult intensive care unit: a risk-adjusted matched cohort study. Crit Care Med. 2004;32:998–1003. doi: 10.1097/01.ccm.0000119422.93413.08. [DOI] [PubMed] [Google Scholar]
- 15.Neville AL, Brown CV, Weng J, et al. Obesity is an independent risk factor of mortality in severely injured blunt trauma patients. Arch Surg. 2004;139:983–987. doi: 10.1001/archsurg.139.9.983. [DOI] [PubMed] [Google Scholar]
- 16. Dossett LA, Dageforde LA, Swenson BR, et al. Obesity and site-specific nosocomial infection risk in the intensive care unit. Surg Infect. 2009;10:137–143. doi: 10.1089/sur.2008.028. Prospective trial demonstrating increased bloodstream and catheter-related infections in obese patients.
- 17.Smith RL, Chong TW, Hedrick TL, et al. Does body mass index affect infection-related outcomes in the intensive care unit? Surg Infect. 2007;8:581–588. doi: 10.1089/sur.2006.079. [DOI] [PubMed] [Google Scholar]
- 18.Oliveros H, Villamor E. Obesity and mortality in critically ill adults: a systematic review and meta-analysis. Obesity. 2008;16:515–521. doi: 10.1038/oby.2007.102. [DOI] [PubMed] [Google Scholar]
- 19. Akkinusi ME, Pineda LA, El Sohl AA. Effect of obesity on intensive care morbidity and mortality: a meta-analysis. Crit Care Med. 2008;36:151–158. doi: 10.1097/01.CCM.0000297885.60037.6E. Meta-analysis looking at critical care outcomes across different BMI classes, which found some increased morbidity in obese patients but no association with mortality, and even noted a decreased mortality rate in mild-moderately overweight patients.
- 20. Frat JP, Gissot V, Ragot S, et al. Impact of obesity in mechanically ventilated patients: a prospective study. Intensive Care Med. 2008;34:1991–1998. doi: 10.1007/s00134-008-1245-y. One of the few existing prospective studies looking at morbidity and mortality outcomes in obese ICU patients; unlike the majority of retrospective analyses, they reported no association between obesity and several major outcomes with the exception of intubation-related morbidity.
- 21.Dossett LA, Heffernan D, Lightfoot M, et al. Obesity and pulmonary complications in critically injured adults. Chest. 2008;134:974–980. doi: 10.1378/chest.08-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mullen JT, Moorman DW, Davenport DL. The obesity paradox: body mass index and outcomes in patients undergoing nonbariatric general surgery. Ann Surg. 2009;250:166–172. doi: 10.1097/SLA.0b013e3181ad8935. [DOI] [PubMed] [Google Scholar]
- 23.Mizock BA. Alterations in fuel metabolism in critical illness: hyperglycaemia. Best Pract Res Clin Endocrinol Metab. 2001;15:533–551. doi: 10.1053/beem.2001.0168. [DOI] [PubMed] [Google Scholar]
- 24.Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycemia. Lancet. 2009;373:1798–1807. doi: 10.1016/S0140-6736(09)60553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Honiden S, McArdle JR. Obesity in the intensive care unit. Clin Chest Med. 2009;30:581–599. doi: 10.1016/j.ccm.2009.05.007. A systematic, detailed review of the physiologic changes relevant to the critically ill obese patient.
- 26.Jeevanandam M, Young DH, Schiller WR. Obesity and the metabolic response to severe multiple trauma in man. J Clin Invest. 1991;87:262–269. doi: 10.1172/JCI114980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leonidou L, Michalaki M, Gogos CA, et al. Stress induced hyperglycemia in patients with severe sepsis: a compromising factor for survival. Am J Med Sci. 2008;336:467–471. doi: 10.1097/MAJ.0b013e318176abb4. [DOI] [PubMed] [Google Scholar]
- 28.Van den Berghe G, Wilmer A, Hermans G, et al. Intensive medical therapy in the medical ICU. NEJM. 2006;354:449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 29. Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists (AACE) and American Diabetes Association (ADA) consensus statement on inpatient glycemic control. Endocrine Practice. 2009;15:353–369. doi: 10.4158/EP09102.RA. Recommendations on management of inpatient blood glucose control based on most currently available data, with a discussion of targets specifically for critically ill patients.
- 30.Abdul-Ghani MA, Muller FL, DeFronzo RA, et al. Deleterious action of FA metabolites on ATP synthesis: possible link between lipotoxicity, mitochondrial dysfunction, and insulin resistance. Am J Physiol Endocrinol Metab. 2008;295:E678–E685. doi: 10.1152/ajpendo.90287.2008. [DOI] [PubMed] [Google Scholar]
- 31.Schiffelers SL, Saris WH, van Baak MA. The effect of an increased free fatty acid concentration on thermogenesis and substrate oxidation in obese and lean men. Int J Obes Relat Metab Disord. 2001;25:33–38. doi: 10.1038/sj.ijo.0801528. [DOI] [PubMed] [Google Scholar]
- 32.Stevens RD, Dowdy DW, Michaels RK, et al. Neuromuscular dysfunction acquired in critical illness: a systematic review. Intensive Care Med. 2007;33:1876–1891. doi: 10.1007/s00134-007-0772-2. [DOI] [PubMed] [Google Scholar]
- 33.Reid CL, Campbell IT, Little RA. Muscle wasting and energy balance in critical illness. Clin Nutr. 2004;23:273–280. doi: 10.1016/S0261-5614(03)00129-8. [DOI] [PubMed] [Google Scholar]
- 34.Dickerson RN, Boschert KJ, Kudsk KA. Hypocaloric enteral tube feeding in critically ill obese patients. Nutrition. 2002;18:241–246. doi: 10.1016/s0899-9007(01)00793-6. [DOI] [PubMed] [Google Scholar]
- 35.Dickerson RN. Hypocaloric feeding of obese patients in the intensive care unit. Curr Opin Nutr Metab Care. 2005;8:189–196. doi: 10.1097/00075197-200503000-00014. [DOI] [PubMed] [Google Scholar]
- 36.Biolo G, Agostini F, Simunic B, et al. Positive energy balance is associated with accelerated muscle atrophy and increased erythrocyte glutathione turnover during 5 wk of bed rest. Am J Clin Nutr. 2008;88:950–958. doi: 10.1093/ajcn/88.4.950. [DOI] [PubMed] [Google Scholar]
- 37.Harris JA, Benedict FG. A biometric study of basal metabolism in man. Publication 279. Washington, DC: Carnegie Institute of Washington; 1919. [Google Scholar]
- 38.Mifflin MD, St. Jeor ST, Hill LA, et al. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51:241–247. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- 39.Ireton-Jones C, Jones JD. Improved equations for predicting energy expenditure in patients: the Ireton-Jones equations. Nutr Clin Pract. 2002;17:29–31. doi: 10.1177/011542650201700129. [DOI] [PubMed] [Google Scholar]
- 40.Ireton-Jones CS, Turner WW, Leipa GU, et al. Equations for estimation of energy expenditures in patients with burns with special reference to ventilatory status. J Burn Care Rehab. 1992;13:330–333. doi: 10.1097/00004630-199205000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Frankenfield DC, Coleman A, Alam S, Cooney RN. Analysis of estimation methods for resting metabolic rate in critically ill adults. J Parenter Enteral Nutr. 2009;33:27–36. doi: 10.1177/0148607108322399. [DOI] [PubMed] [Google Scholar]
- 42.Stucky CCH, Moncure M, Hise M, et al. How accurate are resting energy expenditure prediction equations in obese trauma and burn patients? JPEN. 2008;32:420. doi: 10.1177/0148607108319799. [DOI] [PubMed] [Google Scholar]
- 43.Cerra FB, Benitez MR, Blackburn G, et al. Applied nutrition in ICU patients: a consensus statement of the American College of Chest Physicians. Chest. 1997;111:769–778. doi: 10.1378/chest.111.3.769. [DOI] [PubMed] [Google Scholar]
- 44.Makita K, Nunn JF, Royston B. Evaluation of metabolic measuring instruments for use in critically ill patients. Crit Care Med. 1990;18:638–644. doi: 10.1097/00003246-199006000-00013. [DOI] [PubMed] [Google Scholar]
- 45.Epstein CD, Peerless JR, Martin JE, Malangoni MA. Comparison of methods of measurements of oxygen consumption in mechanically ventilated patients with multiple trauma: the Fick method versus indirect calorimetry. Crit Care Med. 2000;28:1363–1369. doi: 10.1097/00003246-200005000-00017. [DOI] [PubMed] [Google Scholar]
- 46.Wells JC, Fuller NJ. Precision and accuracy in a metabolic monitor for indirect calorimetry. Eur J Clin Nutr. 1998;52:536–540. doi: 10.1038/sj.ejcn.1600604. [DOI] [PubMed] [Google Scholar]
- 47.Ravussin E, Burnand B, Schutz Y, Jequier E. Twenty-four hour energy expenditure and resting metabolic rate in obese, moderately obese and control subjects. Am J Clin Nutr. 1982;35:566–573. doi: 10.1093/ajcn/35.3.566. [DOI] [PubMed] [Google Scholar]
- 48.Zauner A, Schneeweiss B, Kneidinger N, et al. Weight adjusted resting energy is not constant in critically ill patients. Int Care Med. 2006;32:428–434. doi: 10.1007/s00134-005-0056-7. [DOI] [PubMed] [Google Scholar]
- 49.Krenitsky J. Adjusted body weight, pro: evidence to support the use of adjusted body weight in calculating calorie requirements. Nutr Clin Pract. 2005;20:468–473. doi: 10.1177/0115426505020004468. [DOI] [PubMed] [Google Scholar]
- 50. McClave SA, Martindale RG, Vanek VW, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) JPEN. 2009:277–316. doi: 10.1177/0148607109335234. This consensus statement from expert American groups discusses recommended ‘standard of care’ practices for alimenting the ICU patient, with discussion of management aspects specific to obese patients, including hypocaloric feeding.
- 51.Anderegg BA, Worrall C, Barbour E, et al. Comparison of resting energy expenditure prediction methods with measured resting energy expenditure in obese, hospitalized adults. J Parenter Enteral Nutr. 2009;33:168–175. doi: 10.1177/0148607108327192. [DOI] [PubMed] [Google Scholar]
- 52.Frankenfield DC, Rowe WA, Smith JS, et al. Validation of several established equations for resting metabolic rate in obese and nonobese people. J Am Diet Assoc. 2003;103:1152–1159. doi: 10.1016/s0002-8223(03)00982-9. [DOI] [PubMed] [Google Scholar]
- 53.Alves V, da Rocha EE, Gonzalez MC, et al. Assessment of resting energy expenditure of obese patients: comparison of indirect calorimetry with formulae. Clin Nutr. 2009;28:299–304. doi: 10.1016/j.clnu.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 54.Boullata J, Williams J, Cottrell F, et al. Accurate determination of energy needs in hospitalized patients. J Am Diet Assoc. 2007;107:393–401. doi: 10.1016/j.jada.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 55.Hoher JA, Teixeira PJ, Hertx F, Moreira JS. A comparison between ventilation modes: how does activity level affect energy expenditure estimates? J Parenter Enteral Nutr. 2008;32:176–183. doi: 10.1177/0148607108314761. [DOI] [PubMed] [Google Scholar]
- 56.ADA Executive Summary Guidelines. ‘Critical illness evidence based nutrition practice guidelines’. 2006 September; www.adaevidencelibrary.com. [Google Scholar]
- 57.Artinian V, Krayem H, DiGiovine B. Effects of early enteral feeding on the outcome of critically ill mechanically ventilated medical patients. Chest. 2006;129:960–967. doi: 10.1378/chest.129.4.960. [DOI] [PubMed] [Google Scholar]
- 58.Palghi A, Reed JL, Greenburg I, et al. Multidisciplinary treatment of obesity with a protein-sparing modified fast: results in 668 outpatients. Am J Public Health. 1985;75:1190–1194. doi: 10.2105/ajph.75.10.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krishnan JA, PArce PB, Martinez A. Caloric intake in medical ICU patients: consistency of care with guidelines and relationship to clinical outcomes. Nutr Clin Pract. 2004;19:645–646. doi: 10.1177/0115426504019006645. [DOI] [PubMed] [Google Scholar]
- 60.Choban PS, Burge JC, Scales D, Flancbaum L. Hypoenergetic nutrition support in hospitalized obese patients: a simplified method for clinical application. Am J Clin Nutr. 1997;66:546–550. doi: 10.1093/ajcn/66.3.546. [DOI] [PubMed] [Google Scholar]
- 61.Villet S, Chiolero RL, Bollmann MD, et al. Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin Nutr. 2005;24:502–509. doi: 10.1016/j.clnu.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 62.Hahler B. Morbid obesity: a nursing care challenge. Medsurg Nurs. 2002;11:85–90. [PubMed] [Google Scholar]
- 63.Davidson JE, Kruse MW, Cox DH, Duncan R. Critical care of the morbidly obese. Crit Care Nurs. 2003;26:105–116. doi: 10.1097/00002727-200304000-00005. [DOI] [PubMed] [Google Scholar]
- 64.Winkelman C, Maloney B. Obese ICU patients: resource utilization and outcomes. Clin Nurs Res. 2005;14:303. doi: 10.1177/1054773805275288. [DOI] [PubMed] [Google Scholar]
- 65.Alexander JW. Wounds in the morbidly obese. Obes Surg. 2005;15:1276–1277. doi: 10.1381/096089205774512393. [DOI] [PubMed] [Google Scholar]
- 66.Wheaton G, Marden P, Colleypreist B, et al. Understanding why patients die after gastrostomy tube insertion: a retrospective analysis of mortality. JPEN. 2009;33:375–379. doi: 10.1177/0148607108327156. [DOI] [PubMed] [Google Scholar]