Abstract
Objective
The cell morphology, gene expression, and matrix synthesis of articular chondrocytes are known to vary with depth from the tissue surface. The objective of this study was to investigate if chondrocytes from different zones respond to in vitro oscillatory tensile loading in distinct ways and whether tensile strain, which is most prevalent near the articular surface, would preferentially stimulate superficial zone chondrocytes.
Design
Chondrocytes were separately isolated from the superficial, middle, and deep zones of articular cartilage and seeded into three-dimensional fibrin hydrogel constructs. An intermittent protocol of oscillatory tensile loading was applied for 3 days, and the effects on extracellular matrix (ECM) synthesis were assessed by measuring the incorporation of radiolabed precursors, size-exclusion gel chromatography, and western blotting.
Results
Tensile loading was found to be a potent stimulus for proteoglycan synthesis only in superficial zone chondrocytes. Although overall biosynthesis rates by deep zone chondrocytes were unaffected by tensile loading, the molecular characteristics of proteins and proteoglycans released to the culture medium were significantly altered so as to resemble those of superficial zone chondrocytes.
Conclusions
Oscillatory tensile loading differentially affected subpopulations of articular chondrocytes in three-dimensional fibrin hydrogel constructs. Cells isolated from deeper regions of the tissue developed some characteristics of superficial zone chondrocytes after exposure to tensile loading, which may indicate an adaptive response to the new mechanical environment. Understanding how exogenous mechanical stimuli can differentially influence chondrocytes from distinct tissue zones will yield important insights into mechanobiological processes involved in cartilage tissue development, maintenance, disease, and repair.
Introduction
Articular cartilage is a stratified tissue with well characterized depth-dependent patterns of cellular morphology1–3, extracellular matrix ultrastructure4,5, and material properties6–8. For example, the superficial region has a dense network of collagen fibers primarily aligned parallel to the joint surface, whereas collagen fiber orientation is more random in the middle region and perpendicular to the underlying subchondrdal bone in the deepest areas of the tissue9. The proteoglycan content, is lowest in the superficial zone and increases through the middle and deep regions4,10. These compositional differences are due, at least in part, to metabolic specialization of the chondrocytes resident in each tissue zone11. Consistent with proteoglycan synthesis rates in situ3 and the overall proteoglycan distribution found in articular cartilage, chondrocytes from the deep zone synthesize more extracellular matrix proteoglycans than cells from the superficial zone during both two and three dimensional in vitro culture11–14. Gene expression profiles for freshly isolated chondrocytes from these distinct zones exhibit consistent differences15,16.
The depth-dependent differences in extracellular matrix composition and organization of articular cartilage may also be functional adaptations to the local mechanical environments of each tissue zone. In the middle and deep zones, normal loading elevates local fluid pressure, and the high concentration of proteoglycans, in concert with associated fluid, serves to effectively carry this stress while limiting strain in the solid matrix17. Nearer the articular surface, shear and tension are more significant components of the local mechanical environment due to a combination of compression and a sliding motion during articulation18–20. The structure, density, and orientation of the collagen fibers in the superficial zone create a network particularly well suited for resisting these forces. Thus, chondrocytes resident in the superficial zone of articular cartilage may experience compression, shear, and tension as part of their normal mechanical environment, whereas cells from deeper within the tissue are subjected to primarily compressive stress.
As a reflection of the complex mechanical environment found in articular cartilage, many forms of mechanical stimulation, such as static and dynamic compression21–24, hydrostatic pressure25–27, tissue shear28–31, and oscillatory tension32–34 have been used to better understand cartilage mechanobiology in a variety of model systems. Briefly, static compression inhibits metabolic activity in cartilage tissue explants21, whereas dynamic compression22–24 and hydrostatic pressure25–27 can stimulate chondrocyte extracellular matrix metabolism and gene expression. In addition, tensile34 and shear strains28,29,31 can preferentially stimulate collagen synthesis at both the protein and gene level. To date much of the work in cartilage mechanobiology has utilized tissue explants or cells isolated from multiple cartilage zones. However, in vitro dynamic compression elicited distinct responses from subpopulations of chondrocytes isolated from different tissue zones35,36. In addition, in vitro loading designed to mimic joint articulation preferentially stimulated gene expression and protein synthesis of proteoglycan 4 (PRG4), which is thought to provide lubrication to the joint surface and is found primarily in the superficial zone30,37–40. Finally, cyclic tensile strain, but not cyclic hydrostatic pressure, of alginate constructs seeded with middle and deep zone chondrocytes upregulated gene expression of types I and II collagen as well as superficial zone protein34. These studies provide evidence that chondrocytes from different tissue zones can exhibit distinct responses to mechanical loading, and that mechanical loading regimes typifying the articular surface can promote characteristics of superficial zone chondrocytes.
The current studies examined the effects of oscillatory tensile loading on chondrocytes derived from specific zones of articular cartilage using a fibrin hydrogel construct system. Fibrin was selected as the scaffold material because it has been extensively used for articular cartilage repair strategies41–43 as well as for three-dimensional in vitro culture of chondrocytes44–46. Our objective was to analyze the extracellular matrix biosynthesis of chondrocytes originating from different tissue zones by assessing both total production of cartilage matrix molecules as well as molecular characteristics of these newly synthesized molecules. We hypothesized that cells from distinct zones would respond differently to oscillatory tensile loading, and that extracellular matrix production by superficial zone chondrocytes would be preferentially stimulated compared to that of cells from deeper within the tissue.
Materials and Methods
TISSUE HARVEST AND CELL ISOLATION
Articular chondrocytes from distinct tissue zones were isolated from 2–4 week old bovine stifle joints as described previously32, with modifications based on information available in the literature47,48 and pilot studies conducted in our laboratory. The intact femoral-patellar groove and femoral condyles were removed using a small hand saw. Rectangular osteochondral blocks were then cut from the tissue, rinsed in PBS supplemented with antibiotic-antimycotic (Invitrogen) and divided into discrete zones using a sliding microtome. The top 250 μm was taken as the superficial zone, tissue ranging from 500–1000 μm from the surface was taken as the middle zone, and tissue 1250–2000 μm from the surface was taken as the deep zone (Figure 1A). Slices from the deep zone excluded tissue with significant vascularization or regions of calcified cartilage. Chondrocytes from each tissue zone were then enzymatically isolated using 0.2% collagenase in high glucose DMEM supplemented with 50 μg/mL gentamicin sulfate, 100 μg/mL kanamycin sulfate, 50 μg/mL penicillin, 50 μg/mL streptomycin, 100 μg/mL neomycin, and 0.25 μg/mL Fungizone (Invitrogen) for approximately 20 hours at 37°C with gentle agitation. The cell suspensions were passed through a sterile 74 μm mesh, cell counts and viability were determined using a Beckman Coulter Vi-Cell XR, and cell size was determined with a Beckman Coulter Multisizer III.
Figure 1.
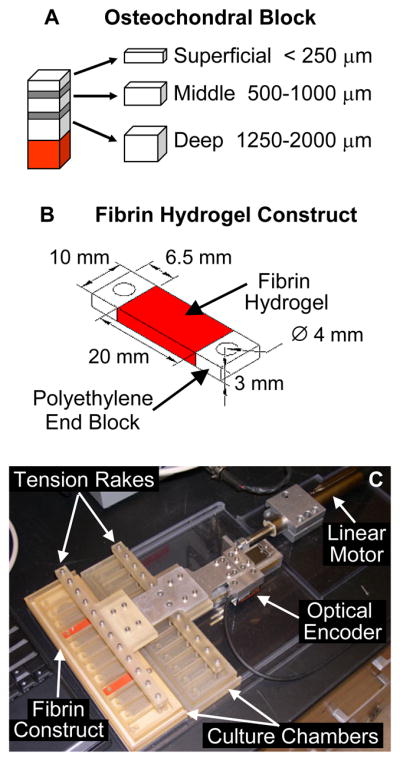
(A) Schematic of zone-specific articular chondrocyte harvest procedure. Osteochondral blocks were sectioned into three zones as shown. Areas shown in gray represent transitional sections between zones that were discarded. The area shown in red represents vascularized regions and calcified cartilage layers, which were also discarded. (B) Sketch of a fibrin hydrogel construct for oscillatory tensile loading studies. (C) Photograph of the Oscillatory Tensile Loading Device.
FIBRIN HYDROGEL CONSTRUCT SEEDING AND CULTURE
Immediately following isolation, chondrocytes were seeded into either cylindrical fibrin hydrogels or rectangular fibrin hydrogel constructs. Bovine fibrinogen (Sigma-Aldrich) was solubilized in high glucose DMEM with 10% FBS (Hyclone, Logan, UT) and 2 mg/mL ε-aminocaproic acid (Sigma-Aldrich) and added to suspensions of chondrocytes from individual cartilage zones. Bovine thrombin (MP Biomedicals) in 40 mM calcium chloride was aliquoted into custom rectangular polycarbonate molds, which were fitted with porous polyethylene end blocks (15–45 μm pore size, Porex Technologies) creating a 20 × 10 × 3 mm region where the cell-fibrinogen solution was added (Figure 1B). The cell-fibrinogen solution infiltrated the porous space of the end blocks before the enzymatic action of the thrombin induced gelation, thereby forming a well-integrated interface between the hydrogel and the end blocks. The molds were incubated for 90 minutes at 37°C to allow for complete hydrogel formation. Final concentrations in the hydrogel constructs were 50 mg/mL fibrin, 50 U/mL thrombin, and 5×106 chondrocytes/mL. The process for creating the cylindrical hydrogels was identical except that cylindrical molds (11mm diameter × 3mm deep) without end blocks were used.
Cylindrical fibrin hydrogels were cultured for up to 15 days with 2 mL of medium that was changed every two days. High glucose DMEM supplemented with 10% FBS, 2 mg/mL ε-aminocaproic acid, 50 μg/mL ascorbic acid, 0.4 mM L-proline, (Sigma-Aldrich), 10 mM non-essential amino acids, 10 mM HEPES buffer, and antibiotic-antimycotic (Invitrogen) was used for all studies. During the final 24 hours of culture for each time point, medium was additionally supplemented with 10 μCi/mL L-5-3H-proline (GE Healthcare) and 5 μCi/mL 35S-sodium sulfate (MP Biomedicals). Upon removal from culture, hydrogels were washed in PBS with 1.0 mM L-proline (Sigma-Aldrich) and 0.8 mM sodium sulfate (Fisher Scientific) to remove unincorporated radiolabeled precursors. The wet mass of each hydrogel was measured, and the hydrogels were frozen.
OSCILLATORY TENSILE LOADING
Rectangular fibrin hydrogel constructs were cultured for up to 6 days in free swelling conditions at 37°C and 5.0% CO2 in rectangular culture plates (Nalgene Nunc) with 3 mL of medium (supplemented as described above) that was changed every two days. A subset of constructs from each group was randomly selected after two days of culture and the medium for these constructs was supplemented with 10 μCi/mL L-5-3H-proline and 5 μCi/mL 35S-sodium sulfate for an additional 24 hours. Upon removal from culture, constructs were washed as described above, the end blocks were removed, construct wet mass was recorded, and samples were frozen. This set of constructs, designated “3 Day”, served as an early time point control. On day 6 of culture the remaining constructs were randomly assigned to a well in either the “unloaded” or “tension” culture chambers. Constructs were transferred into the culture chambers by positioning one of the end blocks over the stationary peg in each well of the chambers. Fresh culture medium was added, and constructs were allowed to equilibrate for one additional day, resulting in a total preculture of 7 days. A computer-controlled, custom bioreactor updated from an earlier design32 was then used to apply an intermittent oscillatory tensile loading protocol that had been developed from several preliminary studies (Figure 1C). To interface the loading device with the constructs, the stainless steel pins of the tension rake were inserted into the second end block of each construct. The tension rake was then attached to the linear motor, which generated a 1.0 Hz sinusoid with 5% ± 5% amplitude. This motion profile was applied for 12 hours, followed by a 12 hour recovery period when constructs were held at 0% displacement. This loading protocol was repeated 3 times, yielding a total culture duration of 10 days (Table 1). Culture medium was changed every day during the final hour of the recovery period and a portion of the conditioned medium was collected. Control constructs in unloaded culture chambers were held at 0% displacement. During the final 24 hours of the loading protocol, culture medium supplemented with radiolabeled precursors as described above was used.
Table 1.
Summary of culture conditions for tensile loading study
| Group Name | Preculture Time | Loading Time | Total Culture Time |
|---|---|---|---|
| 3 Day | 3 days | --- | 3 days |
| Unloaded | 7 days | 3 days | 10 days |
| Tension | 7 days | 3 days | 10 days |
BIOCHEMICAL COMPOSITION ANALYSES
Frozen hydrogels were lyophilized, measured for solid mass, and digested overnight at 60°C using 0.5 mg/mL proteinase K (EMD Chemicals) in 100 mM ammonium acetate (Sigma-Aldrich). 3H-proline and 35S-sulfate incorporation rates (indicators of total protein and sulfated glycosaminoglycan (sGAG) synthesis, respectively) were assessed using a liquid scintillation counter. Total DNA and sGAG contents were measured using Hoechst 33258 dye49 and 1,9-dimethylmethylene blue dye50, respectively (Sigma-Aldrich). sGAG released into the culture medium was also assessed for all collected media samples, and cumulative release was determined by summing the release for each sample. Further characterization of the macromolecules released into the medium was performed using size exclusion liquid chromatography. Portions of the conditioned media from the final day of culture were first processed using a Hi-Trap Sephadex G-25 Superfine column (GE Healthcare) to remove unincorporated radiolabeled precursors. 7 M Urea (JT Baker) plus 50 mM Tris acetate (Fisher Scientific) was used to equilibrate the columns and 8 M Urea plus 50 mM Tris acetate was used as the eluant. Fractions in the void volume were pooled and concentrated using Amicon Ultra-4 10 kDa centrifugal filters (Millipore). Samples were then run on a 30 × 1 cm Econo-Column (BioRad) packed with Sepharose CL-4B (Sigma-Aldrich) and equilibrated with 4 M Guanidine-HCl (Shelton Scientific) plus 50 mM sodium acetate (Sigma-Aldrich). 500 μL fractions were collected at a flow rate of 0.35 mL/minute and analyzed for 35S and 3H content with a liquid scintillation counter.
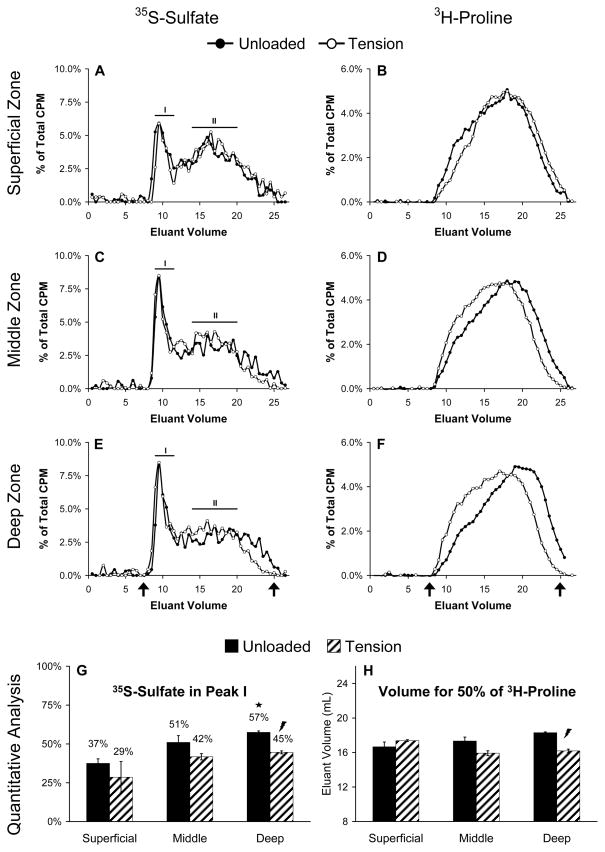
A quantitative assessment of scintillation counter data from the size exclusion chromatography was undertaken to determine the fraction of large proteoglycans (peak I) versus smaller proteoglycans or proteoglycan fragments (peak II, see Figure 5) released to the culture media. The background level of each sample was determined by averaging the values of the final 8 fractions in each run. After subtracting out the background, the CPM corresponding to 35S was summed in each peak, and the percentage of the 35S in each peak was determined. To quantify the molecular weight distribution of newly synthesized and released proteins, the total macromolecular 3H was first determined by summing the CPM values corresponding to 3H for each background-subtracted sample. The eluant volume at which 50% of the 3H had passed through the column was then determined via linear interpolation.
Figure 5.
Analysis and quantification of proteoglycans and proteins released to the medium using size exclusion liquid chromatography. Data from (A, B) Superficial zone constructs, (C, D) Middle zone constructs, and (E, F) Deep zone constructs are shown in rows and are the mean percentage of total CPM in each elution fraction for three replicates per group. Lines labeled I and II (A, C, E) designate peaks corresponding to large and small proteoglycans, respectively. Arrows (E, F) indicate the void and total volumes for the column. Quantitative data are shown as the percentage of 35S-Sulfate found in peak I (G) or as the volume at which 50 percent of the total 3H-Proline eluted from the column (H) and are mean ± SEM, n = 3. ★ indicates significant difference from Superficial;
 indicates Tension significantly different from Unloaded; p < 0.05.
indicates Tension significantly different from Unloaded; p < 0.05.
Finally, to access the specific proteoglycan content of the culture medium processed via gel chromatography, fractions from peak II containing smaller molecular weight proteoglycans were pooled and concentrated using 10 kDa centrifugal filters. Proteoglycans from pooled samples were precipitated overnight at 4°C by adding 3 volumes of ice cold 100% ethanol plus 5 mM sodium acetate. Precipitates were then deglycosylated as previously described51 by sequential digestion with chondroitinase ABC (Calbiochem) and keratinases I and II (Sigma and Seikagaku, respectively) and 10 μL volumes were loaded into 4–12% gradient Tris-glycine gels (Invitrogen). Samples were electrophoretically separated, transferred to nitrocellulose, and blotted with primary antibodies against the G1 or G3 domains of aggrecan (provided by J. Sandy, Tampa, FL). Blots were incubated with an alkaline phosphatase secondary antibody (Sigma-Aldrich), exposed to ECF substrate (GE Healthcare), and imaged on a Fuji FLA3000 imaging system.
STATISTICAL ANALYSES
Chondrocyte size data and column chromatography data were analyzed using a two-sided paired Student’s t test, and all other data were analyzed using a general linear model and Tukey’s test for post hoc analysis with significance set at p<0.05. Data representing the contents of the column chromatography fractions were transformed using an arcsine function to ensure normality52. In all cases, interaction terms were included in the models where appropriate and the criterion for significance was satisfied. Unless otherwise noted, the sample size was 6 for all groups and data are presented as mean ± standard error of the mean.
Results
CHONDROCYTE CHARACTERIZATION AND FREE SWELLING CULTURE
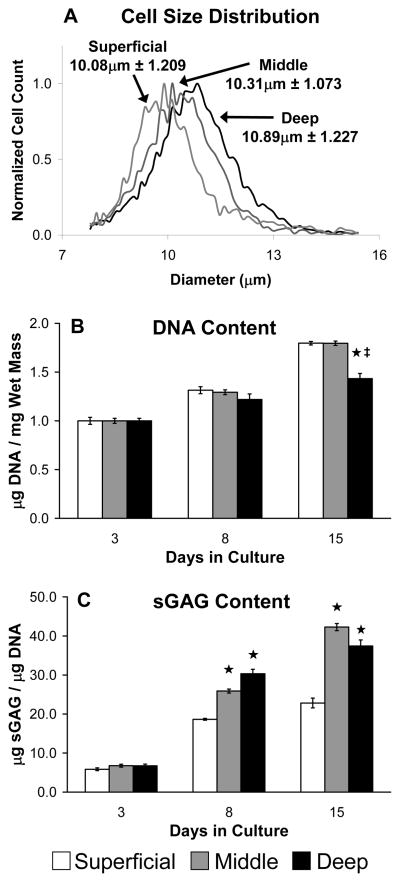
Consistent with published data3,12,13,35,53, chondrocyte size was significantly larger with increasing distance from the articular surface as indicated by cell size distributions (Figure 2A, p<0.001). Data presented for the free swelling culture study are from one of two independent experiments yielding similar results. Fibrin hydrogel DNA content increased with time in culture for chondrocytes from all zones (Figure 2B, p<0.001), while hydrogel contraction, as assessed by changes in water content, was less than 0.5% over the 15 day culture period (data not shown). Additionally, after 15 days hydrogels containing chondrocytes from the superficial and middle zones had significantly higher cell densities than those with deep zone chondrocytes (Figure 2B, p<0.002). DNA data are normalized to the average DNA contents for each group after 3 days in culture to account for small differences (< 10%) in initial cell seeding density. Total sGAG content also increased significantly with time in culture for all hydrogels (Figure 2C, p<0.001). After 8 days, middle and deep zone hydrogels contained higher levels of sGAG than superficial zone hydrogels (p<0.001), and this trend persisted through 15 days of culture. Data from the analysis of radiolabeled precursor incorporation (not shown) were consistent with gross biochemical content, as middle and deep zone hydrogels generally had elevated 3H-proline and 35S-sulfate incorporation rates compared to superficial zone hydrogels.
Figure 2.
Characterization of zone-specific chondrocytes. (A) Initial cell diameter distribution prior to hydrogel seeding; data are mean ± SD, n ≥ 3800 cells. (B) Hydrogel DNA content normalized to Day 3 values and (C) hydrogel sGAG content in free swelling culture; data are mean ± SEM. ★ indicates significant difference from Superficial; ‡ indicates significant difference from Middle; p < 0.05.
OSCILLATORY TENSILE LOADING
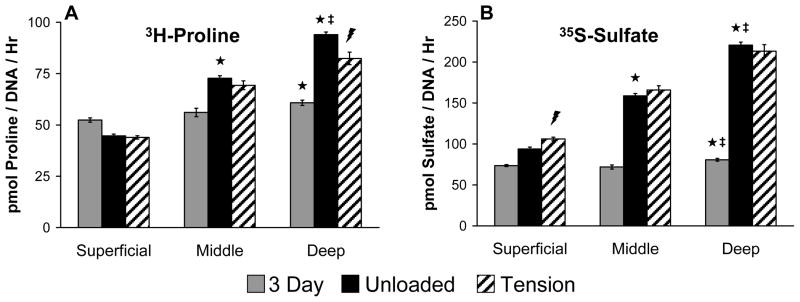
The effects of tensile loading on zone-specific chondrocytes were investigated by preculturing constructs for 7 days in free swelling conditions and then subjecting them to 3 days of intermittent oscillatory tensile loading. Consistent with previous data, chondrocytes from all zones proliferated from days 3 to 10 in culture (p<0.001, not shown), and superficial zone constructs had a higher cell density compared to constructs containing cells from the deep zone (p<0.05, not shown). Tensile loading did not affect cell density for any of the groups. However, extracellular matrix synthesis rates, as indicated by 3H-proline and 35S-sulfate incorporation, varied over time in culture, with tissue zone, and with the application of oscillatory tensile loading (Figures 3A,B). As seen previously, 3H and 35S incorporation rates were higher in constructs containing chondrocytes originating from deeper regions of the tissue (p<0.001). Oscillatory tensile loading decreased 3H-proline incorporation by 12.3% in deep zone constructs (p<0.001). In contrast, 35S-sulfate incorporation was increased 12.9% by loading in superficial zone constructs (p<0.005).
Figure 3.
Biosynthesis rates of zone-specific chondrocytes in fibrin hydrogel constructs subjected to intermittent oscillatory tensile loading; data are mean ± SEM. ★ indicates significant difference from Superficial; ‡ indicates significant difference from Middle;
 indicates Tension significantly different from Unloaded; p < 0.05.
indicates Tension significantly different from Unloaded; p < 0.05.
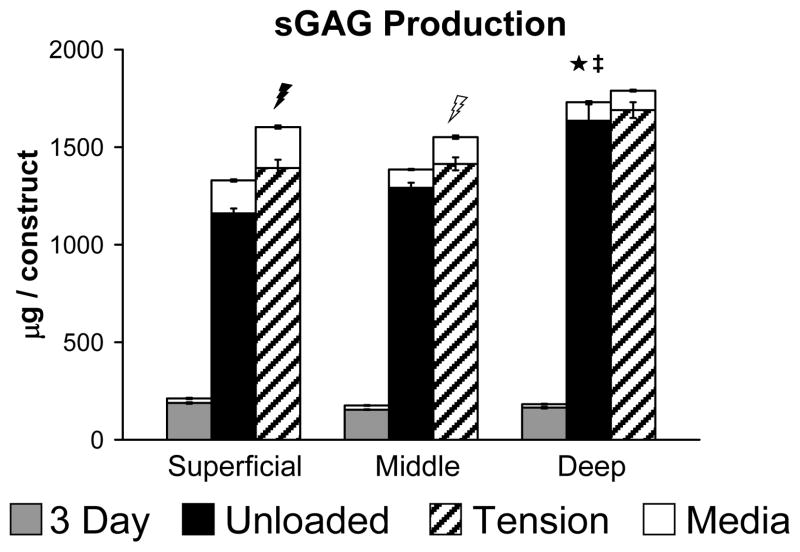
After 10 days in culture, deep zone constructs had retained significantly higher amounts of sGAG compared to superficial or middle zone constructs (Figure 4, black bars, p<0.001), however, superficial zone constructs released more sGAG into the culture medium than either middle or deep zone constructs (Figure 4, open bars, p<0.001). Total sGAG synthesis (retained in constructs plus released to media) was also significantly higher in deep zone constructs compared with middle or superficial zone constructs (Figure 4, black plus open bars, p<0.001). Oscillatory tensile loading significantly increased the amount of sGAG retained in the construct by 20.1% (filled bars) as well as released to the media by 23.8% (open bars) in superficial zone constructs (p<0.02). Release of sGAG to the media was also significantly increased in the middle zone constructs by 46.0% (p<0.001), but no significant effect of tension was detected for deep zone constructs. Tensile loading also increased total sGAG production (retained in construct plus released to media) in superficial zone constructs by 20.6% (p<0.003).
Figure 4.
Total sGAG produced by zone-specific chondrocytes in fibrin hydrogel constructs subjected to intermittent oscillatory tensile loading; data are mean ± SEM. Filled bars represent sGAG accumulated in the constructs, while open bars represent sGAG released to the culture medium. ★ indicates significant difference from Superficial; ‡ indicates significant difference from Middle;
 indicates Tension significantly different from Unloaded in all cases (construct, media, and total);
indicates Tension significantly different from Unloaded in all cases (construct, media, and total);
 indicates Tension significantly different from Unloaded only for release to culture medium; p < 0.05.
indicates Tension significantly different from Unloaded only for release to culture medium; p < 0.05.
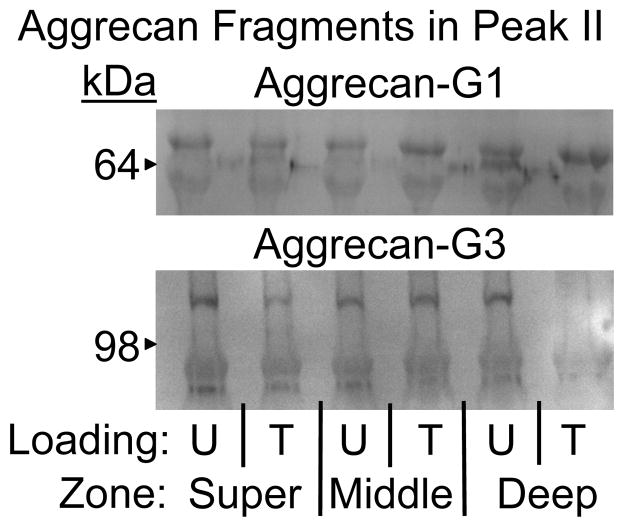
To further characterize proteoglycans released from the hydrogel constructs, size exclusion gel chromatography and western blot analyses were performed on conditioned culture media. Proteoglycans separated into two populations based on hydrodynamic size, as evidenced by two major peaks in the liquid chromatography data (Figure 5A,C,E). The first peak, designated I, eluted near the void volume of the column and contained large molecules up to several thousand kDa, presumably intact aggrecan. In contrast, the second peak, designated II, eluted much later and therefore consisted of smaller macromolecules, perhaps small proteoglycans or aggrecan fragments produced by enzymatic processing. Chondrocytes from the deep zone incorporated a significantly larger fraction of 35S into large proteoglycans released to the culture medium compared with chondrocytes from the superficial zone (Figure 5G, p<0.008), but oscillatory tensile loading caused more 35S to be incorporated into smaller proteoglycans by deep zone chondrocytes (p<0.019). Western blot analysis confirmed that Peak II fractions from all three chondrocyte subpopulations contained multiple aggrecan fragments known to be generated by enzymatic activity51 (Figure 6). Additionally, compared to unloaded controls, lanes corresponding to deep zone chondrocytes subjected to tension contained less intense staining for C-terminal (G3) aggrecan fragments.
Figure 6.
Western blot analysis of Peak II fractions containing proteoglycans released to the culture medium on the final day of culture. Upper, multiple aggrecan fragments bearing the G1 domain (~65 and ~90 kDa); Lower, multiple aggrecan fragments bearing the G3 domain (~70 and ~110 kDa); U = Unloaded and T = Tension. Equal volumes of media were loaded in each lane.
The chromatography elution profiles for proteins containing 3H-proline also revealed intriguing results (Figure 5B,D,F). Regardless of loading, cells from the superficial zone released proteins that were similar in size and elution profile (Figure 5B,H). In contrast, proteins released by cells from the middle and deep zones were affected by oscillatory tensile loading, as evidenced by shifts in both the quantity and size of proteins released to the culture medium (Figure 5D,F). In fact, the eluant volume where 50% of the 3H labeled proteins in the culture media from loaded deep zone constructs had passed through the column was significantly lower compared to unloaded constructs (Figure 5H, p<0.011). This effect of loading was not seen as simply a change in the mean hydrodynamic size of proteins, but a shift in the shape of the elution profile.
Discussion
Although it is well established that chondrocyte morphology and phenotype vary throughout the depth of articular cartilage, it is not well understood how these subpopulations respond to exogenous mechanical stimuli. Our studies investigated phenotypic differences among cells isolated from specific zones of articular cartilage and demonstrated their distinct biosynthetic responses to intermittent oscillatory tensile loading. Chondrocytes from all three tissue zones proliferated and synthesized extracellular matrix molecules during three-dimensional fibrin hydrogel culture, but did so to different degrees. Extracellular matrix synthesis was greater in constructs containing chondrocytes from deeper within the tissue, consistent with numerous studies using many different species in a variety of culture conditions3,5,11–14,35,47,54. Furthermore, three days of intermittent oscillatory tensile loading did not affect 3H-proline incorporation rates for superficial or middle zone constructs, but did reduce 3H incorporation in deep zone constructs. Conversely, tensile loading increased 35S-sulfate incorporation and sGAG accumulation only in superficial zone constructs. Applying dynamic compression with a loading platen smaller than a cartilage sample, thereby inducing tensile strains at the tissue surface adjacent to the platen, has also been shown to preferentially enhance proteoglycan synthesis only in the superficial zone55. Interestingly, these findings were in contrast to a report using three-dimensional agarose culture where both static and dynamic compression inhibited glycosaminoglycan synthesis by superficial zone chondrocytes35. Although differences in the cellular environment between agarose and fibrin may play a role, as various extracellular microenvironments have been shown to differentially affect zone-specific chondrocytes53, this difference in response to mechanical loading is noteworthy because it indicates that superficial zone chondrocyte extracellular matrix synthesis may be dependent on the local mechanical environment. Hence, superficial zone chondrocytes may respond to tensile loading in a fundamentally different manner compared with compressive loading, indicating that distinct cellular signaling pathways may be involved.
Analysis of conditioned culture media by gel chromatography and western blots yielded intriguing results and provided further insight into zone-specific chondrocyte mechanobiology not otherwise apparent when measuring total protein or proteoglycan synthesis. Medium from loaded deep zone constructs contained higher levels of newly synthesized proteins compared to unloaded samples (p<0.05). Interestingly, this increase in 3H-proline found in the medium was comparable to the decrease of 3H-proline retained in the constructs, and thus tensile loading did not affect overall protein synthesis by deep zone chondrocytes. However, the gel chromatography elution profiles of proteins released by deep zone chondrocytes were dramatically altered by tensile loading as evidenced by a shift toward larger proteins. Middle zone chondrocytes exhibited a similar shift, but no effect was found in superficial zone chondrocytes. Mechanical loading induces fluid flow in these constructs, and thus increases transport of freely diffusible proteins to the medium. However, if increased transport was the only mechanism governing changes in protein release to the media, a shift in the magnitude, but not necessarily the shape, of the elution profile would be expected. Therefore, oscillatory tensile loading promoted the synthesis and release of larger proteins exclusively by chondrocytes from deeper regions of the tissue. Furthermore, chondrocytes typically incorporate the majority of 3H-proline into collagen molecules56, and therefore, this shift likely represents an increase in the size of collagen molecules being assembled and released to the culture medium. The superficial zone in native articular cartilage has enhanced tensile mechanical properties due to the collagen network architecture4,7,57, which is thought to be a functional adaptation to the shear and tensile forces generated at the tissue surface during joint motion1. Thus, in vitro tensile loading may induce chondrocytes from deeper regions of articular cartilage to assemble larger collagen molecules in response to the new mechanical environment.
Oscillatory tensile loading also influenced proteoglycan synthesis and release in a zone-dependent manner. 35S-sulfate incorporation and total sGAG production by superficial zone chondrocytes, but not middle or deep zone chondrocytes, were increased with loading. Therefore, tension was a potent stimulus for proteoglycan synthesis only in superficial zone cells. Additionally, consistent with previous studies11, medium from superficial zone constructs contained a higher proportion of small proteoglycans relative to large proteoglycans, and this was unchanged with tensile loading. Thus, tensile loading stimulated proteoglycan synthesis in superficial zone chondrocytes, but did not alter the molecular weight characteristics of these molecules. In contrast, following oscillatory tensile loading, chondrocytes from the deep zone incorporated a more substantial percentage of 35S-sulfate into lower molecular weight proteoglycans in a manner remarkably similar to the superficial zone cells. Thus, tension differentially affected proteoglycan metabolism in a more intricate manner than modulating total production. These zone-dependent responses to tensile loading may be related to differences in the tissue composition and mechanical environment found through the depth of native tissue. Deeper regions in articular cartilage predominately contain the large proteoglycan aggrecan, whereas areas nearer to the tissue surface contain a mixture of large and small proteoglycans and a lower overall fixed charge density. This contributes to differences in the compressive mechanical properties of tissue from various depths and may represent adaptation to the mechanical environments generated during joint articulation. Thus, alterations in the ratio of large to small proteoglycans may also indicate an adaptation to the local mechanical environment, potentially leading to differences in the extracellular matrix composition and organization in engineered tissue constructs. For superficial zone chondrocytes, which may experience some tensile strain in vivo, tension increased overall proteoglycan metabolism but did not affect the characteristics of the proteoglycans produced and released to the culture medium. However, chondrocytes from deeper within the tissue typically experience little mechanical tension in situ; thus, the change in the characteristics of the proteoglycans synthesized by deep zone chondrocytes may indicate an adaptation to the altered mechanical environment. This finding could have resulted from increased synthesis of smaller dermatin sulfate proteoglycans, the generation of aggrecan fragments through increased enzyme activity, or synthesis of other proteoglycans not typically found in deeper regions of cartilage tissue, such as PRG4. Western blots of the culture medium did not indicate enhanced degradation of aggrecan molecules with the application of intermittent tensile loading. Furthermore, cyclic tensile loading20, a combination of dynamic compression and articular motion37–39, dynamic tissue shear30, and continuous passive motion40 have all been shown to stimulate PRG4 gene expression or protein synthesis. Interestingly, several of these studies used cells from either full thickness cartilage37–39 or solely from the middle and deep zones20, indicating that chondrocytes originating from deeper within cartilage tissue may take on attributes of superficial zone chondrocytes when exposed to a mechanical environment more characteristic of the articular surface. Further work is necessary to ascertain the exact nature of the changes seen in the proteoglycans released to the media by deep zone chondrocytes. However, taken together these data indicate that oscillatory tensile loading promoted a “superficial zone chondrocyte” phenotype that was maintained in cells originating from near the articular surface and induced in chondrocytes isolated from deeper within the tissue.
These studies demonstrate differential responses of chondrocytes from distinct articular cartilage zones to tensile loading and have important implications for cartilage mechanobiology and tissue engineering. The finding that chondrocytes exhibit depth-dependent proteoglycan and protein synthesis characteristics in response to tensile loading suggests that chondrocytes are highly tuned to their mechanical environment. These results lend further support to the concept that chondrocytes from distinct regions are highly specialized, but also indicate that the local mechanical environment may be responsible for these distinctions. Hence, chondrocyte origin is an important consideration when developing strategies for cartilage repair, as cellular subpopulations may respond in different ways to the same exogenous stimuli. Once implanted engineered cartilage tissues will experience a complex mechanical environment and thus understanding the unique behaviors of chondrocytes from distinct tissue zones will help direct strategies targeting the successful restoration of damaged or diseased articular cartilage.
Acknowledgments
The authors thank Dr. John Sandy for generously providing the primary antibodies for aggrecan detection. This work was funded by the National Institutes of Health (NIAMS AR048253), an ARCS Foundation fellowship (EJV), an NIH Cellular and Tissue Engineering Program grant (CGW), and Medtronic Foundation and George Foundation fellowships (CGW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buckwalter JA, Mankin HJ. Articular cartilage: tissue design and chondrocyte-matrix interactions. Instr Course Lect. 1998;47:477–86. [PubMed] [Google Scholar]
- 2.Youn I, Choi JB, Cao L, Setton LA, Guilak F. Zonal variations in the three-dimensional morphology of the chondron measured in situ using confocal microscopy. Osteoarthritis Cartilage. 2006;14:889–97. doi: 10.1016/j.joca.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 3.Wong M, Wuethrich P, Eggli P, Hunziker E. Zone-specific cell biosynthetic activity in mature bovine articular cartilage: a new method using confocal microscopic stereology and quantitative autoradiography. J Orthop Res. 1996;14:424–32. doi: 10.1002/jor.1100140313. [DOI] [PubMed] [Google Scholar]
- 4.Mow VC, Ratcliffe A, Mow VC, Hayes WC. Basic Orthopaedic Biomechanics. Philadelphia: Lippincott-Raven Publications; 1997. Structure and Function of Articular Cartilage and Meniscus; pp. 113–77. [Google Scholar]
- 5.Bayliss MT, Venn M, Maroudas A, Ali SY. Structure of proteoglycans from different layers of human articular cartilage. Biochem J. 1983;209:387–400. doi: 10.1042/bj2090387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schinagl RM, Gurskis D, Chen AC, Sah RL. Depth-dependent confined compression modulus of full-thickness bovine articular cartilage. J Orthop Res. 1997;15:499–506. doi: 10.1002/jor.1100150404. [DOI] [PubMed] [Google Scholar]
- 7.Roth V, Mow VC. The intrinsic tensile behavior of the matrix of bovine articular cartilage and its variation with age. J Bone Joint Surg Am. 1980;62:1102–17. [PubMed] [Google Scholar]
- 8.Mow VC, Guo XE. Mechano-Electrochemical Properties of Articular Cartilage: Their Inhomogeneities and Anisotropies. Annu Rev Biomed Eng. 2002;4:175–209. doi: 10.1146/annurev.bioeng.4.110701.120309. [DOI] [PubMed] [Google Scholar]
- 9.Speer DP, Dahners L. Collagenous Architecture of Articular-Cartilage - Correlation of Scanning Electron-Microscopy and Polarized-Light Microscopy Observations. Clin Orthop Relat Res. 1979:267–75. [PubMed] [Google Scholar]
- 10.Poole AR, Kojima T, Yasuda T, Mwale F, Kobayashi M, Laverty S. Composition and structure of articular cartilage: a template for tissue repair. Clin Orthop Relat Res. 2001:S26–S33. doi: 10.1097/00003086-200110001-00004. [DOI] [PubMed] [Google Scholar]
- 11.Aydelotte MB, Greenhill RR, Kuettner KE. Differences between sub-populations of cultured bovine articular chondrocytes. II. Proteoglycan metabolism. Connect Tissue Res. 1988;18:223–34. doi: 10.3109/03008208809016809. [DOI] [PubMed] [Google Scholar]
- 12.Siczkowski M, Watt FM. Subpopulations of chondrocytes from different zones of pig articular cartilage. Isolation, growth and proteoglycan synthesis in culture. J Cell Sci. 1990;97 (Pt 2):349–60. doi: 10.1242/jcs.97.2.349. [DOI] [PubMed] [Google Scholar]
- 13.Archer CW, McDowell J, Bayliss MT, Stephens MD, Bentley G. Phenotypic modulation in sub-populations of human articular chondrocytes in vitro. J Cell Sci. 1990;97 (Pt 2):361–71. doi: 10.1242/jcs.97.2.361. [DOI] [PubMed] [Google Scholar]
- 14.Kim TK, Sharma B, Williams CG, Ruffner MA, Malik A, McFarland EG, et al. Experimental model for cartilage tissue engineering to regenerate the zonal organization of articular cartilage. Osteoarthritis Cartilage. 2003;11:653–64. doi: 10.1016/s1063-4584(03)00120-1. [DOI] [PubMed] [Google Scholar]
- 15.Darling EM, Hu JC, Athanasiou KA. Zonal and topographical differences in articular cartilage gene expression. J Orthop Res. 2004;22:1182–7. doi: 10.1016/j.orthres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Darling EM, Athanasiou KA. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J Orthop Res. 2005;23:425–32. doi: 10.1016/j.orthres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Soltz MA, Ateshian GA. Interstitial fluid pressurization during confined compression cyclical loading of articular cartilage. Ann Biomed Eng. 2000;28:150–9. doi: 10.1114/1.239. [DOI] [PubMed] [Google Scholar]
- 18.Askew MJ, Mow VC. The biomechanical function of the collagen fibril ultrastructure of articular cartilage. Trans ASME J Biomech Eng. 1978;100:105–15. [Google Scholar]
- 19.Kelly PA, O’Connor JJ. Transmission of rapidly applied loads through articular cartilage. Part 1: Uncracked cartilage. Proc Inst Mech Eng [H] 1996;210:27–37. doi: 10.1243/PIME_PROC_1996_210_388_02. [DOI] [PubMed] [Google Scholar]
- 20.Wong M, Carter DR. Articular cartilage functional histomorphology and mechanobiology: a research perspective. Bone. 2003;33:1–13. doi: 10.1016/s8756-3282(03)00083-8. [DOI] [PubMed] [Google Scholar]
- 21.Gray ML, Pizzanelli AM, Grodzinsky AJ, Lee RC. Mechanical and physicochemical determinants of the chondrocyte biosynthetic response. J Orthop Res. 1988;6:777–92. doi: 10.1002/jor.1100060602. [DOI] [PubMed] [Google Scholar]
- 22.Sah RL, Kim YJ, Doong JY, Grodzinsky AJ, Plaas AH, Sandy JD. Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res. 1989;7:619–36. doi: 10.1002/jor.1100070502. [DOI] [PubMed] [Google Scholar]
- 23.Buschmann MD, Gluzband YA, Grodzinsky AJ, Hunziker EB. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci. 1995;108 (Pt 4):1497–508. doi: 10.1242/jcs.108.4.1497. [DOI] [PubMed] [Google Scholar]
- 24.Lee DA, Bader DL. Compressive strains at physiological frequencies influence the metabolism of chondrocytes seeded in agarose. J Orthop Res. 1997;15:181–8. doi: 10.1002/jor.1100150205. [DOI] [PubMed] [Google Scholar]
- 25.Hall AC, Urban JPG, Gehl KA. The effects of hydrostatic-pressure on matrix synthesis in articular-cartilage. J Orthop Res. 1991;9:1–10. doi: 10.1002/jor.1100090102. [DOI] [PubMed] [Google Scholar]
- 26.Parkkinen JJ, Ikonen J, Lammi MJ, Laakkonen J, Tammi M, Helminen HJ. Effects of Cyclic Hydrostatic Pressure on Proteoglycan Synthesis in Cultured Chondrocytes and Articular Cartilage Explants. Arch Biochem Biophys. 1993;300:458–65. doi: 10.1006/abbi.1993.1062. [DOI] [PubMed] [Google Scholar]
- 27.Smith RL, Rusk SF, Ellison BE, Wessells P, Tsuchiya K, Carter DR, et al. In vitro stimulation of articular chondrocyte mRNA and extracellular matrix synthesis by hydrostatic pressure. J Orthop Res. 1996;14:53–60. doi: 10.1002/jor.1100140110. [DOI] [PubMed] [Google Scholar]
- 28.Jin M, Frank EH, Quinn TM, Hunziker EB, Grodzinsky AJ. Tissue shear deformation stimulates proteoglycan and protein biosynthesis in bovine cartilage explants. Arch Biochem Biophys. 2001;395:41–8. doi: 10.1006/abbi.2001.2543. [DOI] [PubMed] [Google Scholar]
- 29.Jin M, Emkey GR, Siparsky P, Trippel SB, Grodzinsky AJ. Combined effects of dynamic tissue shear deformation and insulin-like growth factor I on chondrocyte biosynthesis in cartilage explants. Arch Biochem Biophys. 2003;414:223–31. doi: 10.1016/s0003-9861(03)00195-4. [DOI] [PubMed] [Google Scholar]
- 30.Nugent GE, Aneloski NM, Schmidt TA, Schumacher BL, Voegtline MS, Sah RL. Dynamic shear stimulation of bovine cartilage biosynthesis of proteoglycan 4. Arthritis Rheum. 2006;54:1888–96. doi: 10.1002/art.21831. [DOI] [PubMed] [Google Scholar]
- 31.Waldman SD, Spiteri CG, Grynpas MD, Pilliar RM, Kandel RA. Long-term intermittent shear deformation improves the quality of cartilaginous tissue formed in vitro. J Orthop Res. 2003;21:590–6. doi: 10.1016/S0736-0266(03)00009-3. [DOI] [PubMed] [Google Scholar]
- 32.Vanderploeg EJ, Imler SM, Brodkin KR, Garcia AJ, Levenston ME. Oscillatory tension differentially modulates matrix metabolism and cytoskeletal organization in chondrocytes and fibrochondrocytes. J Biomech. 2004;37:1941–52. doi: 10.1016/j.jbiomech.2004.02.048. [DOI] [PubMed] [Google Scholar]
- 33.Connelly JT, Vanderploeg EJ, Levenston ME. The influence of cyclic tension amplitude on chondrocyte matrix synthesis: experimental and finite element analyses. Biorheology. 2004;41:377–87. [PubMed] [Google Scholar]
- 34.Wong M, Siegrist M, Goodwin K. Cyclic tensile strain and cyclic hydrostatic pressure differentially regulate expression of hypertrophic markers in primary chondrocytes. Bone. 2003;33:685–93. doi: 10.1016/s8756-3282(03)00242-4. [DOI] [PubMed] [Google Scholar]
- 35.Lee DA, Noguchi T, Knight MM, O’Donnell L, Bentley G, Bader DL. Response of chondrocyte subpopulations cultured within unloaded and loaded agarose. J Orthop Res. 1998;16:726–33. doi: 10.1002/jor.1100160615. [DOI] [PubMed] [Google Scholar]
- 36.Lee DA, Noguchi T, Frean SP, Lees P, Bader DL. The influence of mechanical loading on isolated chondrocytes seeded in agarose constructs. Biorheology. 2000;37:149–61. [PubMed] [Google Scholar]
- 37.Grad S, Lee CR, Gorna K, Gogolewski S, Wimmer MA, Alini M. Surface motion upregulates superficial zone protein and hyaluronan production in chondrocyte-seeded three-dimensional scaffolds. Tissue Eng. 2005;11:249–56. doi: 10.1089/ten.2005.11.249. [DOI] [PubMed] [Google Scholar]
- 38.Grad S, Lee CR, Wimmer MA, Alini M. Chondrocyte gene expression under applied surface motion. Biorheology. 2006;43:259–69. [PubMed] [Google Scholar]
- 39.Grad S, Gogolewski S, Alini M, Wimmer MA. Effects of simple and complex motion patterns on gene expression of chondrocytes seeded in 3D scaffolds. Tissue Eng. 2006;12:3171–9. doi: 10.1089/ten.2006.12.3171. [DOI] [PubMed] [Google Scholar]
- 40.Nugent-Derfus GE, Takara T, O’Neill JK, Cahill SB, Gortz S, Pong T, et al. Continuous passive motion applied to whole joints stimulates chondrocyte biosynthesis of PRG4. Osteoarthritis Cartilage. 2007;15:566–74. doi: 10.1016/j.joca.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hendrickson DA, Nixon AJ, Grande DA, Todhunter RJ, Minor RM, Erb H, et al. Chondrocyte-Fibrin Matrix Transplants for Resurfacing Extensive Articular-Cartilage Defects. J Orthop Res. 1994;12:485–97. doi: 10.1002/jor.1100120405. [DOI] [PubMed] [Google Scholar]
- 42.van Susante JLC, Buma P, Homminga GN, van den Berg WB, Veth RPH. Chondrocyte-seeded hydroxyapatite for repair of large articular cartilage defects. A pilot study in the goat. Biomaterials. 1998;19:2367–74. doi: 10.1016/s0142-9612(98)00158-6. [DOI] [PubMed] [Google Scholar]
- 43.Fussenegger M, Meinhart J, Hobling W, Kullich W, Funk S, Bernatzky G. Stabilized autologous fibrin-chondrocyte constructs for cartilage repair in vivo. Ann Plas Surg. 2003;51:493–8. doi: 10.1097/01.sap.0000067726.32731.E1. [DOI] [PubMed] [Google Scholar]
- 44.Ting V, Sims CD, Brecht LE, McCarthy JG, Kasabian AK, Connelly PR, et al. In vitro prefabrication of human cartilage shapes using fibrin glue and human chondrocytes. Ann Plast Surg. 1998;40:413–20. doi: 10.1097/00000637-199804000-00016. [DOI] [PubMed] [Google Scholar]
- 45.Fortier LA, Lust G, Mohammed HO, Nixon AJ. Coordinate upregulation of cartilage matrix synthesis in fibrin cultures supplemented with exogenous insulin-like growth factor-I. J Orthop Res. 1999;17:467–74. doi: 10.1002/jor.1100170403. [DOI] [PubMed] [Google Scholar]
- 46.Hunter CJ, Mouw JK, Levenston ME. Dynamic compression of chondrocyte-seeded fibrin gels: effects on matrix accumulation and mechanical stiffness. Osteoarthritis Cartilage. 2004;12:117–30. doi: 10.1016/j.joca.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 47.Klein TJ, Schumacher BL, Schmidt TA, Li KW, Voegtline MS, Masuda K, et al. Tissue engineering of stratified articular cartilage from chondrocyte subpopulations. Osteoarthritis Cartilage. 2003;11:595–602. doi: 10.1016/s1063-4584(03)00090-6. [DOI] [PubMed] [Google Scholar]
- 48.Schmidt TA, Schumacher BL, Klein TJ, Voegtline MS, Sah RL. Synthesis of proteoglycan 4 by chondrocyte subpopulations in cartilage explants, monolayer cultures, and resurfaced cartilage cultures. Arthritis Rheum. 2004;50:2849–57. doi: 10.1002/art.20480. [DOI] [PubMed] [Google Scholar]
- 49.Kim YJ, Sah RL, Doong JY, Grodzinsky AJ. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem. 1988;174:168–76. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- 50.Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247–8. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 51.Sandy JD, Verscharen C. Analysis of aggrecan in human knee cartilage and synovial fluid indicates that aggrecanase (ADAMTS) activity is responsible for the catabolic turnover and loss of whole aggrecan whereas other protease activity is required for C-terminal processing in vivo. Biochem J. 2001;358:615–26. doi: 10.1042/0264-6021:3580615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sokal RR, Rohlf FJ. Biometry. New York, NY: W.H. Freeman and Company; 1981. [Google Scholar]
- 53.Hwang NS, Varghese S, Lee HJ, Theprungsirikul P, Canver A, Sharma B, et al. Response of zonal chondrocytes to extracellular matrix-hydrogels. FEBS Lett. 2007;581:4172–8. doi: 10.1016/j.febslet.2007.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hidaka C, Cheng C, Alexandre D, Bhargava M, Torzilli PA. Maturational differences in superficial and deep zone articular chondrocytes. Cell Tissue Res. 2006;323:127–35. doi: 10.1007/s00441-005-0050-y. [DOI] [PubMed] [Google Scholar]
- 55.Parkkinen JJ, Lammi MJ, Helminen HJ, Tammi M. Local Stimulation of Proteoglycan Synthesis in Articular-Cartilage Explants by Dynamic Compression In vitro. J Orthop Res. 1992;10:610–20. doi: 10.1002/jor.1100100503. [DOI] [PubMed] [Google Scholar]
- 56.Sah RLY, Doong J-YH, Grodzinsky AJ, Plaas AHK, Sandy JD. Effects of compression on the loss of newly synthesized proteoglycans and proteins from cartilage explants. Arch Biochem Biophys. 1991;286:20–9. doi: 10.1016/0003-9861(91)90004-3. [DOI] [PubMed] [Google Scholar]
- 57.Fithian DC, Kelly MA, Mow VC. Material properties and structure-function relationships in the menisci. Clin Orthop. 1990:19–31. [PubMed] [Google Scholar]