Abstract
There are limited data on successful weight management approaches among adolescents from underserved communities. The primary aim of this study was to obtain preliminary data on the efficacy, safety, and acceptability of a lifestyle intervention with milk-based supplements among adolescents from underserved communities. The secondary aims of this study were to assess change in adiposity indices and metabolic indices and to measure compliance. The authors conducted a 12-week open-labeled lifestyle intervention. Adolescents were taught a structured meal plan, including the use of 2 milk-based supplements daily, and participated in weekly lifestyle counseling. Overweight was defined as a body mass index >85th percentile. Percent total body fat was estimated using bioelectric impedance. Fasting blood samples were used to measure insulin indices and other biochemical safety tests. The sample consisted of 40 adolescents (70% girls, 83% minority). Although there was no significant change in body mass index (median [Q1, Q3]; −0.10 [−0.91, 0.61] kg/m2, P = .26), participants showed a decrease in body mass index z score (−0.03 [−0.08, 0.01] SD, P = .01]), weight z score (−0.04 [−0.11, 0.02] SD, P = .001), and percent total body fat (−1.20 [−2.55, −0.12]%, P = .0001). No new onset of type 2 diabetes mellitus was reported, and plasma vitamin D increased (P < .01). Consumption of milk-based drinks increased from a median of 4.5 to 13.5 servings per week, whereas sugary beverages decreased from 8.0 to 3.8 servings per week. A lifestyle intervention that includes milk-based supplements may safely improve some adiposity indices and decrease intake of sugary beverages among overweight adolescents from underserved areas.
Keywords: adolescents, overweight, obesity, milk-based supplements, meal replacements, behavior modification, lifestyle change
The obesity “epidemic” has not spared the pediatric population as revealed by the most recent National Health and Nutrition Examination Survey (NHANES) that shows that nearly one third of adolescents had a body mass index (BMI) at or above the 85th percentile for age and gender (overweight), with more than half of those considered obese (at or above the 95th percentile).1,2 Despite these trends, there is still a lack of evidence to support current pediatric weight treatment approaches. Although randomized controlled studies of lifestyle changes have shown long-term BMI loss, these studies were drawn from homogeneous and motivated groups and rarely included adolescents and minorities.3 Pediatric studies using a comprehensive behavioral approach4–6 typically report mean weight losses ranging from 1 to 4 kg, especially when participants are younger and motivated. As opposed to weight management in the well-controlled research setting, weight management in the clinical setting is typically associated with BMI z score loss rather than BMI or weight loss.7–9
Milk-based nutritional supplements or meal replacements have been shown to be a valid alternative dietary strategy in the treatment of adult obesity and have also been shown to aid in maintaining weight loss in this population.10–14 These supplements may be a more palatable alternative to skim milk and may limit meal skipping. However, there has been a paucity of studies investigating the use of milk-based nutritional supplements in the pediatric population.
The rise in overweight and obesity has been most dramatic among the African American and Hispanic populations, reaching rates of more than 40%.10 Similar trends have been observed among adolescents at Boston Medical Center (BMC), where approximately half of the adolescents from minority communities served by this urban ambulatory care center are overweight or obese.15 Pediatric patients attending the ambulatory care clinics at BMC are mostly non-Hispanic black (55%) or Hispanic (16%), and nearly half are girls. Those referred for pediatric weight management by either the ambulatory clinics at BMC or community-based health centers rely mostly on public assistance (70%) for reimbursement of their medical needs.15,16
The current epidemic of obesity is best explained by changes in the environment rather than in the gene pool. There is increasing evidence that sugary beverages contribute to the pediatric epidemic of obesity and elevated risk for type 2 diabetes mellitus (T2DM). The consumption of soft drinks in the United States increased by 61% in adults and more than doubled in children and adolescents from 1977 to 1997.11–13 During that period, milk consumption decreased.14,17–19 In particular, 2 studies showed that weight gain in school-age children is directly related to consumption of sugar-sweetened soft drink consumption.18,19 A third study showed that the odds of becoming overweight increased by 60% for each additional can of sugar-sweetened drink a child consumed per day.20 As opposed to sugar-sweetened soft drink consumption, Pereira et al21 observed inverse relationships between frequency of dairy intake and development of obesity in overweight young adults. Thus, interventions that include increasing intake of milk-based beverages should also examine changes in intake of sugary beverages.
We hypothesized that in our ethnically diverse population, providing milk-based nutritional supplements in addition to a lifestyle change intervention would benefit adolescents seeking weight management, especially if they use the milk-based supplements to displace sugary beverages. The primary aim of this open-labeled study was to determine BMI change in overweight and obese adolescents from underserved communities using a lifestyle intervention with milk-based supplements and to assess the safety and acceptability of these supplements. The secondary aims of this study were to assess changes in adiposity indices and metabolic indices and to measure compliance. We also stratified the sample of adolescents by gender and BMI status.
Methods
Study Design/Participants/Intervention
This was a 12-week open-labeled lifestyle intervention using milk-based supplements targeting overweight and obese adolescents. Participants were recruited into this study through their primary care physician, from flyers posted around the BMC campus, or from advertisements in a local newspaper. Participants were originally considered eligible based on their age (12–17.9 years) and BMI (≥85th and <97th percentiles).22 However given the prevalence of obesity in our clinic population, we expanded our criteria to include BMIs >97th percentiles. In this study, we used the Institute of Medicine23,24 criteria to classify adolescents as overweight or obese based on their BMI.24
Exclusion criteria included lactose intolerance, T2DM (fasting glucose >126 mg/dL),25 and reported medications and medical conditions known to affect growth and development. These medical conditions included reported significant cardiac disease, disordered eating, untreated hypothyroidism, malignancy, genetic obesity-associated syndromes (eg, Prader-Willi), psychiatric conditions, participation in a weight management program in the past 6 months, use of weight loss medications and supplements, current smoking, and alcohol or drug addiction/abuse within the previous 2 years. After meeting eligibility criteria, 1 parent or guardian signed the informed consent, and the participant signed an assent. This study was approved by the Boston University School of Medicine Institutional Review Board.
A run-in period of 3 days was used to evaluate compliance. At that time, a registered dietitian met with each adolescent to assess his or her caloric needs and to provide him or her with a meal plan. Counseling included portion size, meal scheduling, meal planning, and appropriate use and timing of the supplements. Participants were instructed to use the milk-based supplement as a snack or as a drink during a meal. Alternatively, the supplements could be used as a meal replacement when meals were skipped (such as breakfast or lunch). Participants were required to keep food records and return the labels of the supplement to assess for compliance. Only those adolescents who returned completed food records and consumed 2 supplements per day for the 3 days were enrolled.
Study participants were instructed on a structured meal plan of 3 meals and 2 snacks per day, including 2 milk-based supplements (Conopco, Inc, dba Slim-Fast Foods Company: 325 mL [11 oz], 220 kcal, 10 g protein, 40 g carbohydrate, 2.5–3.0 g fat, 5 g dietary fiber, multivitamins, and minerals). The supplement has a glycemic index (GI) of 43 and a glycemic load (GL) of 17.2 for each 11 ounces.26
Specially designed labels were created to enhance the appearance of the product to be more appealing to an adolescent. A strong focus on structured meals, including fruits, vegetables, whole grains, and lean protein, was reemphasized at all visits with the goal of reducing caloric intake by 250 to 500 calories per day. Supplements were provided weekly, and their labels as well as 3-day food records were collected at the following visit to measure compliance. The participants received lifestyle counseling at weekly group sessions. Most of these group sessions included culturally appropriate food demonstrations. Content for the sessions was developed and adapted based on the LEARN Program for Weight Control approach to meet the specific needs of adolescents.27 The intensive lifestyle intervention sessions were based on a goal-centered approach to weight loss.28 The participants were expected to attend 9 of 12 “intensive” sessions. Parental involvement was encouraged but not required.
Data collected at baseline and at the 12-week visit included a physical examination with blood pressure, anthropometric measures (weight, height, waist circumference, percent body fat), and 12-hour fasting laboratory tests (hematology, serum chemistry, and levels of lipids, thyroid-stimulating hormone [TSH], insulin, glucose, hemoglobin A1C [HbA1C], C-reactive protein [CRP], homocysteine, and 25-OH vitamin D). All laboratory tests were performed at the BMC laboratory. Height was measured to the nearest 0.5 cm by using a wall-mounted stadiometer. After voiding, body weight was measured in light clothing without shoes to the nearest 0.1 kg on a digital scale (Seca model 708; Seca Mess und Wiegetechnik, Vogel & Halke GmbH & Co, Hamburg, Germany). Waist circumference was measured at the minimum circumference between the iliac crest and the rib cage.29 In addition, bioelectrical impedance analysis (BIA; RJL Systems, Clinton Township, Michigan) was used to estimate percent body fat.
Dietary intake and acceptability of the supplement were assessed using the Block Kids’ Food Frequency Questionnaire (FFQ) at baseline and at the end of the intervention. An exit interview was performed regarding program satisfaction, type of supplement preference, flavor preference, and peer acceptance and acceptability.
Efficacy measures included change in BMI (kg/m2). Safety measures included change in height z score to assess growth during the 12-week period as well as hemoglobin and hematocrit as a measure of nutritional adequacy. Other measures included changes in adiposity measures such as BMI z scores (SD), weight (kg and z scores), and percent total body fat from BIA, as well as metabolic biomarkers such as levels of insulin, glucose, HbA1C, lipids, CRP, homocysteine, and vitamin D. Finally, measures of compliance included the food and walking logs as well as the number of supplement labels returned.
Statistical Analysis
Adolescents were divided according to the sample median BMI at baseline: those above a BMI of 37.3 versus those at or below 37.3. They were also divided by loss in BMI z score: those with a z score loss and those with no change or gain in z score. Finally, the adolescents were divided according to the ratio of returned labels to dispensed labels: those above 0.76 (compliant) versus those at or below 0.76 (noncompliant).
Carbohydrate metabolism indices included fasting insulin and glucose, hemoglobin HbA1C, and the homeostasis model assessment of insulin resistance (HOMA-IR). Insulin resistance was estimated using the HOMA-IR, which is calculated based on fasting serum levels of insulin and glucose30:
This measure has been validated in children and adolescents.31 Diabetes was defined as a glucose >126 mg/dL. Changes in outcome variables were computed as the differences between measurements at baseline and the end of the intervention.
Participants were considered noncompliant if they missed more than 3 sessions. Compliance with physical activity (walking) and meal planning was measured by food and physical activity records returned, whereas compliance with milk-based supplements was measured using the supplement labels returned. The total beverage intake per week was computed as the sum of servings per week. Variables were divided into categories prior to stratification by gender, BMI, and compliance measures for secondary analysis.
For matched baseline to follow-up comparisons of continuous measures, the signed rank test was employed, whereas Wilcoxon’s rank-sum test was used for group comparisons; nonparametric procedures were used because of the non-normality of several of the variables examined. The chi-square test of homogeneity and Fisher’s exact test were used for group comparisons of categorical data, whereas McNemar’s exact test was used for paired categorical comparisons. All analyses were performed using SAS for Windows Release 8.02.
Calculation of GI and GL of the supplement and comparisons with skim milk and sugary beverages were obtained. The GI compares the ability of foods with the same amount of carbohydrate to raise blood glucose. The GL (GL = GI/100 × carbohydrate grams) also takes into account the amount of carbohydrate ingested.
Results
A total of 104 adolescents had a BMI ≥85th percentile and consented to participate in the study. After the run-in period, a total of 69 overweight or obese participants were enrolled in the treatment portion of this study, and 40 completed the 12-week lifestyle change intervention in addition to receiving 2 milk-based supplements per day. The 29 remaining participants were excluded from the analysis because they did not come for follow-up. Study completers (n = 40) were 70% female (28 vs 12 male), with 55% (n = 22) African American, 20% (n = 8) Hispanic, 18% (n = 7) white, and 8% (n = 3) other. Baseline characteristics did not vary significantly between completers (n = 40) and noncompleters (n = 29; data not shown). In this sample, 46% of the food records and 15% of the walking logs were completed and returned. As 69% of labels from the milk-based supplements were returned, we assumed a 69% compliance rate with use of milk-based supplements.
Baseline and 12-week laboratory and anthropometric characteristics are summarized in Table 1. The main measure of efficacy of the intervention, BMI, did not change during the study period (P = .26). Height measurements, a measure of safety, increased over that 12-week period (P < .05), and the participants remained on their z score trajectory for height (P = .85). The other measures of safety of the intervention were hemoglobin and hematocrit, which also did not change significantly.
Table 1.
Change in Measurements Among Completers
| Median (Q1–Q3) | |||
|---|---|---|---|
| Baseline | 12 Weeks | P Valuea | |
| Weight, kg | 101.6 (85.9–126.7) | 100.1 (87.6–127.7) | .55 |
| Height, cm | 165.2 (161.2–170.0) | 166.4 (161.4–171.0) | .003 |
| BMI, kg/m2 | 38.1 | 37.9 | .26 |
| Body fat, % | 48.7 (35.9–52.0) | 46.2 (35.0–51.5) | .0001 |
| Height z score, SD | 0.6 (–0.1–1.3) | 0.6 (–0.1–1.2) | .85 |
| Weight z score, SD | 2.6 (2.1–3.2) | 2.5 (2.0–3.2) | .001 |
| BMI z score, SD | 2.4 (2.1–2.7) | 2.3 (2.1–2.7) | .01 |
| Glucose, mg/dL | 80.0 (75.0–88.0) | 80.5 (75.0–88.0) | .50 |
| Hematocrit, % | 39.5 (36.6–40.8) | 37.6 (35.7–40.6) | .11 |
| Hemoglobin, g/dL | 13.0 (12.0–13.8) | 12.6 (12.1–13.6) | .24 |
| Insulin, µU/mL | 11.0 (8.0–17.0) | 22.0 (8.0–32.0) | .004 |
| HOMA-IR | 2.0 (1.5–3.4) | 4.6 (1.4–6.1) | .01 |
| HbA1C, % | 5.3 (5.2–5.6) | 5.7 (5.5–6.0) | .0001 |
| Vitamin D, ng/mL | 14.0 (10.8–17.6) | 18.0 (12.9–23.0) | .0001 |
| C-reactive protein, mg/dL | 0.4 (0.2–0.6) | 0.4 (0.2–0.5) | .45 |
| Homocysteine, µmol/L | 7.3 (6.1–9.1) | 7.8 (6.3–8.8) | .31 |
| Total cholesterol, mg/dL | 159 (142–185) | 168 (138–181) | .80 |
| Triglycerides, mg/dL | 84 (61–109) | 90 (63–114) | .07 |
| HDL, mg/dL | 45 (38–50) | 43 (38–53) | .75 |
| LDL, mg/dL | 98 (73–118) | 94 (77–113) | .44 |
HOMA-IR = (Insulin µU/mL × Glucose mmol/L)/22.5. Glucose mmol/L = Glucose mg/dL × 0.055. BMI, body mass index; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; LDL, low-density lipoprotein.
Testing the null that the median difference is 0.
Acceptability of the supplement was measured by food frequency data (Block FFQ), which indicate that compared with before the intervention, participants consumed less sugary beverages per week at the end of the 12-week period. Median sugary beverage consumption decreased from 8.0 to 3.8 servings per week (Table 2). As opposed to other milk-based products found in the FFQs, milk-based supplements were estimated separately using returned labels. Although milk-based supplement intake increased throughout the duration of the intervention (P < .05), that of milk products recorded in the FFQs did not differ significantly (P < .05; Table 2). Once we added the servings of milk-based supplements as 1 serving per can to those of other milk-based products obtained from the FFQs, we observed an increase in servings of milk per week (from 4.5 to 13.5 servings).
Table 2.
Change in Food Frequency Among Completers
| Median (Q1–Q3) | |||
|---|---|---|---|
| Baseline | 12 Weeks | P Valuea | |
| Sweetened beverages per week | 8.0 (1.5–12.3) | 3.8 (1.0–8.5) | .049 |
| Milk per weekb | 4.5 (0.0–16.5) | 2.8 (0.0–7.0) | .13 |
| Milkb and meal replacements per week | 4.5 (0.0–16.5) | 13.5 (9.0–20.5) | .0003 |
| Milk and meal replacements × 1.375 per weekc | 4.5 (0.0–16.5) | 18.2 (11.1–25.1) | <.0001 |
Testing the null that the median difference is 0.
Excludes milk from meal replacements.
One meal replacements contains 1.375 servings of milk.
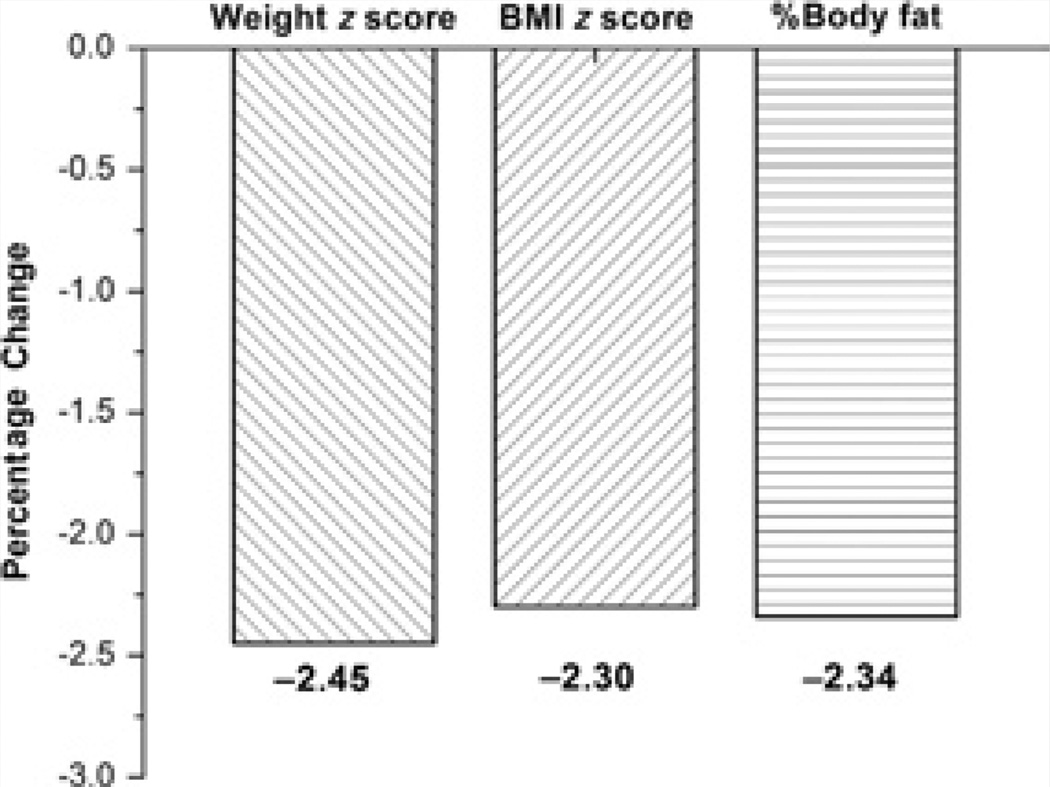
During this 12-week period, HbA1C, insulin, and HOMA-IR increased (P < .05), but no new onset of type 2 diabetes was observed. We observed an increase in serum 25-OH vitamin D levels (P < .01). This translated to a decrease in the prevalence of 25-OH vitamin D deficiency (<20 ng/mL) from 86.8% to 55.3%. Weight z score, BMI z score, and percent total body fat decreased during the study period (P < .05). On average, participants lost 2.3% of baseline body fat, 2.5% of baseline z score for weight, and 2.3% of baseline z score for BMI (P < .05; Figure 1). After stratification by gender, changes in weight z score, BMI z score, and total body fat were found to be significantly lower in girls but not in boys.
Figure 1.
Average percentage change in percent body fat and z score for weight and body mass index (BMI).
Of the 40 study participants, 12 did not complete an exit interview. Although the percentage of non-Hispanic black participants who completed the exit interview was higher than the other racial and ethnic groups, other baseline characteristics were similar for those with and without an exit interview. To interpret the answers from the exit interview, we divided participants based on their BMI z score change into successful (BMI z score difference < median) and unsuccessful (BMI z score ≥ median; Table 3). Successful participants reported being able to adhere to eating the foods outlined by their meal plan more often than unsuccessful participants (P = .02). Unsuccessful participants reported that it was difficult for them to bring the shakes to school more often than successful participants (P = .05). Although skipping breakfast is common in overweight adolescents, this is not evaluated as part of the Block questionnaire. However, we were delighted to observe that all participants interviewed (n = 28) chose to take 1 of their 2 milk-based supplements as their breakfast. This pattern was confirmed using data from participants who completed food records.
Table 3.
Responses Given on Exit Interview by Body Mass Index (BMI) Change
| Median (Q1–Q2) | |||
|---|---|---|---|
| Question | Loss in BMI z Score (n = 14) |
No Loss in BMI z Score (n = 14) |
P Value |
| 1. I would recommend this program to my friends. | 8.0 (5.0–9.0) | 7.5 (5.0–9.0) | .89 |
| 2. I was able to adhere to eating the foods outlined by my meal plan. | 8.5 (6.0–9.0) | 6.0 (5.0–8.0) | .02 |
| 3. I enjoyed the groups. | 8.0 (8.0–9.0) | 8.0 (5.0–10.0) | .86 |
| 4. It was difficult for me to bring the shakes to school. | 1.0 (1.0–6.0) | 4.5 (2.0–9.0) | .05 |
| 5. I felt embarrassed drinking the shakes around my friends and fellow classmates. | 1.0 (1.0–2.0) | 5.5 (1.0–9.0) | .06 |
| 6. I felt that the shakes and groups helped me. | 9.0 (7.0–9.0) | 8.0 (4.0–10.0) | .45 |
| 7. If I had the chance to participate in this program again I would. | 9.0 (5.0–9.0) | 7.5 (4.0–10.0) | .80 |
| n (%) | n (%) | P Value | |
| 8. What flavor of drink did you enjoy the most? Chocolate Strawberry Vanilla |
9 (64) 3 (21) 2 (14) |
7 (50) 2 (14) 5 (36) |
.58 |
| 9. Choose the meals of the day that you most often replaced with our shakes. Circle 2 responses. Breakfast Lunch Snack Supper/dinner |
14 (100) 8 (57) 2 (14) 2 (14) |
14 (100) 4 (29) 3 (21) 3 (21) |
— .13 .99 .99 |
Possible response for questions 1 through 7 ranged from 1 = strongly disagree to 10 = strongly agree.
Discussion
A lifestyle intervention with the use of milk-based nutritional supplements among overweight and obese adolescents in an urban low-income setting can lead to significant favorable changes in z score for BMI and weight as well as percentage of body fat without compromising growth in height. Although there was no change in weight or BMI, it is note-worthy that obese adolescents typically gain more than 2 to 3 units of BMI per year.32 Therefore, the change in BMI over this 6-month study period suggests that the addition of the meal supplement was sufficient to limit gain in BMI although not sufficient to decrease BMI. This is, however, a positive result of this study and can be attributed to both the supplements and the behavioral program that accompanied the supplements.7–9 We also have shown that supplement usage was accompanied by a displacement or decrease in sugary beverage consumption and a total increase in milk product consumption. Our findings are in agreement with adult studies that demonstrate milk-based nutritional supplement therapy as a convenient, safe, and efficacious method for weight management.33,34
The intervention elicited a decrease in anthropometric parameters in girls but not in boys, which has been noted in other studies of adolescent populations.35 Although the explanation could rely on different causal mechanisms between boys and girls, it is plausible that girls are more susceptible to negative psychological effects of overweight and obesity and are thus more responsive to the intervention. However, the median weight measures were similar in magnitude and may reflect a small sample size (girls, n = 28; boys, n = 12). It was noted that percent body fat decreased significantly in girls, but we cannot exclude the potential role of enhanced physical activity. We could not differentiate the added effect of activity because of the lack of compliance with completed physical activity logs.
Although weight z score, BMI z score, and percent body fat decreased significantly after the 12-week intervention, we observed increases in carbohydrate metabolism indices. Typically, one would expect a decrease in the biomarkers of carbohydrate metabolism while obesity indices are improving. Possible explanations for this rise would include a significant elevation in glycemic load from the supplement and other food items. However, this hypothesis is not corroborated by the results, suggesting that the use of the supplement displaced intake of sugary beverages, which have a similar GI and GL as the supplement,36 and the lack of BMI gain. For the same volume, another source of protein and vitamin D, skim milk, has a GI of 32 and a GL of 5.3. Skim milk contains 122 kcal per 11 ounces, which corresponds to about half the calories provided by the same volume of supplement. Sugary beverages, such as sodas—a poor source of protein, calcium, and vitamin D—have on average a GI of 58, a GL of 16, and a total caloric intake of 150 kcal per 12-ounce can.
We cannot exclude the possibility that the addition of skim milk rather than the milk-based supplement would have affected the GI and GL of the daily food intake of the adolescents as we did not have enough data from the food logs to calculate the overall daily GI and GL of the study sample. The increase in insulin indices, including fasting insulin, HOMA-IR, and HbA1C, may be explained in part by the growth in height of the adolescents.37 The participants’ average height increased significantly during the 12 weeks of the study (Table 1). Several studies have documented that the doubling of growth velocity at puberty is accompanied by a 2- to 3-fold rise in insulin levels.38,39 However, we would not be able to evaluate whether the increase in biomarkers of carbohydrate metabolism was due to the adolescent growth and development rather than their supplement use in this study, as this was an observational open-label study rather than a placebo-controlled randomized trial.
We were interested in the differences between the supplement and the same volume of skim milk in calories and GL because they are both good sources of vitamin D and protein and, in that respect, can be considered equally nutritious. However, we do note that there is quite a large difference between the supplement and skim milk in GL and calories, such that the supplement, if it replaces milk consumption in the study, could potentially add calories and disrupt glycemic control. However, there is some evidence that increased protein intake and milk intake may be associated with improved satiety, decreased hunger, and decreased weight gain.21
As reported in other studies, we observed that the majority (94.7%) of the overweight participants in this study had low vitamin D (<29 ng/mL) at baseline, and more than 75% had vitamin D deficiency (<20 ng/mL).40 After the 12-week intervention, the levels of 25-OH vitamin D increased significantly (Table 1), and the prevalence of vitamin D deficiency dropped by 31.5%.This may be explained in part by the consumption of milk-based supplements and by change in season. Another finding was a significant decrease in hemoglobin and hematocrit in girls. Although this decrease was not relevant clinically, this has been reported mostly among girls with increased menses associated with obesity but also possible gastrointestinal losses associated with increased milk consumption. However, we did not identify any participants with anemia prior to or after the intervention.
Future studies should be performed using milk-based nutritional supplements using a randomized controlled design to show that this therapy in adolescents can quantitatively improve adiposity measures and vitamin D status in this population. If adolescents find milk-based nutritional supplements to be palatable, they may replace other less healthy options. Our exit interview responses suggested that a good meal target for milk-based nutritional supplements would be breakfast. Several studies have suggested that many overweight and obese adolescents in fact skip breakfast, with potential ramifications for weight maintenance as well as intellectual function in school.41,42
Limitations of this study are based on its design, which is an open-label pilot study with a relatively small sample size but providing important trial information. Despite these limitations and low compliance scores with the lifestyle intervention, we were able to demonstrate changes in the participants’ anthropometric parameters among girls. This has also been described in other settings.35 However, we were not able to distinguish the effects of the behavioral treatment from the effects of the addition of nutritional supplement in this study as we did not have a control group for comparison. Although body fat change was estimated using bioelectrical impedance analysis and not the gold standard underwater weighing or dual-energy X-ray absorptiometry (DXA), findings were in agreement with changes in other anthropometric parameters. In addition, the exit interview findings cannot be generalized to the entire group as most of the respondents were non-Hispanic black. Last, we relied on self-report measures of dietary assessment (food records and food frequency questionnaires), which are not as accurate as direct measurements.
The strengths of this study include the recruitment of a challenged group of minorities and a lifestyle intervention. There is a lack of published studies in obese minority adolescents that focus on lifestyle intervention with supplements. This study is unique in that it shows that milk-based nutritional supplements may be used as a safe tool in adolescent weight management and may contribute to changes in anthropometric parameters. We do also acknowledge that our dropout rate is consistent with the 20% to 30% dropout rates that are typically seen in lifestyle studies.7–9
In summary, findings from this pilot study suggest that milk-based nutritional supplements can be safely added to a lifestyle intervention among minority adolescents, at least over a 12-week period. This study is especially important as outcomes from lifestyle interventions in the clinical setting are disappointing in this age group. In addition, the use of milk-based nutritional supplements in this population may result in a decrease in the consumption of sugary beverages, which have been associated with weight gain in adolescents. Longer term safety of nutritional supplements in this population still needs to be determined. There is a need to conduct trials of milk-based versus placebo supplements in conjunction with a lifestyle intervention in overweight and obese adolescents, especially those in minority populations who are suffering the greatest increase in overweight prevalence over the past 40 years.
Acknowledgments
This study was funded in part by Conopco, Inc. Study sponsors had no role in the study design, collection, analysis, and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Footnotes
Part of this work was presented at NAASO, The Obesity Society Annual Conference, Las Vegas, Nevada, November 2004.
References
- 1.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA. 2002;288:1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 3.Summerbell CD, Ashton V, Campbell KJ, Edmunds L, Kelly S, Waters E. Interventions for treating obesity in children. Cochrane Database Syst Rev. 2003;3 doi: 10.1002/14651858.CD001872. CD001872. [DOI] [PubMed] [Google Scholar]
- 4.Goldfield GS, Raynor HA, Epstein LH. Treatment of pediatric obesity. In: Wadden TA, Stunkard AJ, editors. Handbook of Obesity Treatment. New York: Guilford; 2002. pp. 532–556. [Google Scholar]
- 5.Strauss RS, Pollack HA. Epidemic increase in childhood overweight, 1986–1998. JAMA. 2001;286:2845–2848. doi: 10.1001/jama.286.22.2845. [DOI] [PubMed] [Google Scholar]
- 6.Haddock CK, Shadish WR, Klesges RC, Stein RJ. Treatments for childhood and adolescent obesity. Ann Behav Med. 1994;16:235–244. [Google Scholar]
- 7.Savoye M, Shaw M, Dziura J, et al. Effects of a weight management program on body composition and metabolic parameters in overweight children: a randomized controlled trial. JAMA. 2007;297:2697–2704. doi: 10.1001/jama.297.24.2697. [DOI] [PubMed] [Google Scholar]
- 8.Reinehr T, de Sousa G, Toschke AM, Andler W. Long-term follow up of cardiovascular disease risk factors in children after an obesity intervention. Am J Clin Nutr. 2006;84:490–496. doi: 10.1093/ajcn/84.3.490. [DOI] [PubMed] [Google Scholar]
- 9.Rodearmel SJ, Wyatt HR, Stroebele N, Smith SM, Ogden LG, Hill JO. Small changes in dietary sugar and physical activity as an approach to preventing excessive weight gain: the America on the move family study. Pediatrics. 2007;120:e869–e879. doi: 10.1542/peds.2006-2927. [DOI] [PubMed] [Google Scholar]
- 10.Robinson TN. Behavioral treatment of childhood and adolescent obesity. Int J Obes. 1999;23(suppl 12):S52–S57. doi: 10.1038/sj.ijo.0800860. [DOI] [PubMed] [Google Scholar]
- 11.Schulze MB, Manson JE, Ludwig DS, et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292:927–934. doi: 10.1001/jama.292.8.927. [DOI] [PubMed] [Google Scholar]
- 12.Apovian CM. Sugar-sweetened soft drinks, obesity, and type 2 diabetes. JAMA. 2004;292:978–979. doi: 10.1001/jama.292.8.978. [DOI] [PubMed] [Google Scholar]
- 13.Block G. Foods contributing to energy intake in the US: data from NHANES III and NHANES 1999–2000. J Food Comp Anal. 2004;17:439–447. [Google Scholar]
- 14.Cavadini C, Siega-Riz AM, Popkin BM. US adolescent food intake trends from 1965 to 1996. Arch Dis Child. 2000;83:18–24. doi: 10.1136/adc.83.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Apovian CM, Lenders C, editors. Overweight and Obesity in Adults and Children: A Clinical Guidebook. New Providence, PA: CRC Press; 2006. [Google Scholar]
- 16.Lenders C, Wright JA, Apovian CM, et al. Weight loss surgery eligibility according to various BMI criteria among adolescents. Obesity. doi: 10.1038/oby.2008.477. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielsen SJ, Popkin BM. Changes in beverage intake between 1977 and 2001. Am J Prev Med. 2004;27:205–210. doi: 10.1016/j.amepre.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Berkey CS, Rockett HR, Field AE, et al. Sugar-added beverages and adolescent weight change. Obes Res. 2004;12:778–788. doi: 10.1038/oby.2004.94. [DOI] [PubMed] [Google Scholar]
- 19.James J, Thomas P, Cavan D, et al. Preventing childhood obesity by reducing consumption of carbonated drinks: cluster randomized controlled trial. BMJ. 2004;328:1237. doi: 10.1136/bmj.38077.458438.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ludwig DS, Peterson KE, Gortmaker SL. Relationship between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet. 2001;357:505–508. doi: 10.1016/S0140-6736(00)04041-1. [DOI] [PubMed] [Google Scholar]
- 21.Pereira MA, Jacobs DR, Jr, Van Horn L, Slattery ML, Kartashov AI, Ludwig DS. Dairy consumption, obesity, and the insulin resistance syndrome in young adults: the CARDIA Study. JAMA. 2002;287:2081–2089. doi: 10.1001/jama.287.16.2081. [DOI] [PubMed] [Google Scholar]
- 22.2000 CDC Growth Charts: United States. Vital and Health Statistics of the Centers for Disease Control and Prevention/National Center for Health Statistics, 2000. Atlanta, GA: Centers for Disease Control and Prevention; 2000. [Google Scholar]
- 23.US Institute of Medicine, Committee on Prevention of Obesity in Children and Youth. Preventing Childhood Obesity: Health in the Balance. Washington, DC: National Academies Press; 2005. [PubMed] [Google Scholar]
- 24.Barlow SE the Expert Committee. Expert Committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120:S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 25.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2002;25(suppl 1):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 26.Frier HI, Kaye WA, Florez A, Leeds AR. Oral glucose tolerance and the glycemic index of liquid meal replacement: preliminary results. J Invest Med. 2002;50:172A. [Google Scholar]
- 27.Brownell KD, Wadden TA. The LEARN Program for Weight Control. Dallas, TX: American Health Publishing Company; 1999. [Google Scholar]
- 28.Wadden TA, Butryn ML. Behavioral treatment of obesity. Endocrinol Metab Clin North Am. 2003;32:981–1003. doi: 10.1016/s0889-8529(03)00072-0. [DOI] [PubMed] [Google Scholar]
- 29.Taylor RW, Jones IE, Williams SM, Goulding A. Evaluation of waist circumference, waist-to-hip ratio, and the measured conicity index as screening tools for high trunk fat mass, as measured by dual-energy X-ray absorptiometry, in children aged 3–19 y. Am J Clin Nutr. 2000;72:490–495. doi: 10.1093/ajcn/72.2.490. [DOI] [PubMed] [Google Scholar]
- 30.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 31.Conwell LS, Trost SG, Brown WJ, Batch JA. Indexes of insulin resistance and secretion in obese children and adolescents: a validation study. Diabetes Care. 2004;27:314–319. doi: 10.2337/diacare.27.2.314. [DOI] [PubMed] [Google Scholar]
- 32.Barlow SE, Dietz WH. Obesity evaluation and treatment: expert committee recommendations. The Maternal and Child Health Bureau, Health Resources and Services Administration and the Department of Health and Human Services. Pediatrics. 1998;102:E29. doi: 10.1542/peds.102.3.e29. [DOI] [PubMed] [Google Scholar]
- 33.Foster GD, Makris AP, Bailer BA. Behavioral treatment of obesity. Am J Clin Nutr. 2005;82(suppl):230S–235S. doi: 10.1093/ajcn/82.1.230S. [DOI] [PubMed] [Google Scholar]
- 34.Noakes M, Foster PR, Keogh JB, Clifton PM. Meal replacements are as effective as structured weight-loss diets for treating obesity in adults with features of metabolic syndrome. J Nutr. 2004;134:1894–1899. doi: 10.1093/jn/134.8.1894. [DOI] [PubMed] [Google Scholar]
- 35.Gortmaker SL, Peterson K, Wiecha J, et al. Reducing obesity via a school-based interdisciplinary intervention among youth: Planet Health. Arch Pediatri Adolesc Med. 1999;153:409–418. doi: 10.1001/archpedi.153.4.409. [DOI] [PubMed] [Google Scholar]
- 36.Foster-Powell K, Holt SHA, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr. 2002;76:5–56. doi: 10.1093/ajcn/76.1.5. [DOI] [PubMed] [Google Scholar]
- 37.Hindmarsh PC, Matthews DR, DiSilvo L, Kurtz AB, Brook CGD. Relation between height velocity and fasting insulin concentration. Am J Dis Child. 1988;63:665–666. doi: 10.1136/adc.63.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guzzaloni G, Grugni G, Minocci A, Moro D, Morabito F. Comparison between beta-cell function and insulin resistance indexes in prepubertal and pubertal obese children. Metabolism. 2002;51:1011–1016. doi: 10.1053/meta.2002.34029. [DOI] [PubMed] [Google Scholar]
- 39.Jiang X, Srinivasan SR, Berenson GS. Relation of obesity to insulin secretion and clearance in adolescents: the Bogalusa Heart Study. Int J Obes Relat Metab Disord. 1996;20:951–956. [PubMed] [Google Scholar]
- 40.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 41.Berkey CS, Rockett HR, Gillman MW, Field AE, Colditz GA. Longitudinal study of skipping breakfast and weight change in adolescents. Int J Obes Relat Metab Disord. 2003;27:1258–1266. doi: 10.1038/sj.ijo.0802402. [DOI] [PubMed] [Google Scholar]
- 42.Rampersaud GC, Pereira MA, Girard BL, Adams J, Metzl JD. Breakfast habits, nutritional status, body weight, and academic performance in children and adolescents. J Am Diet Assoc. 2005;105:743–760. doi: 10.1016/j.jada.2005.02.007. [DOI] [PubMed] [Google Scholar]