Abstract
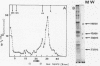
Purified 15 S globin mRNA-protein (mRNP) complexes obtained by EDTA dissociation of duck reticulocytes polyribosomes were digested with the calcium dependant Staphylococcus aureus nuclease (EC 3. 1. 4. 7.). 25% of the globin mRNA sequences were resistant to extensive nuclease digestion as determined by TCA precipitation of the digested 15 S particles labelled in vivo with tritiated uridine. Polyacrylamide gel electrophoresis of the RNA from nuclease digested 15 S particles showed that the protected oligoribonucleotides were distributed into two distinct size classes of 25,000 and 12,000 MW. Comparison between in vitro iodine-labelled 9 S globin mRNA extracted from Staphylococcal nuclease digested 15 S mRNP particles was carried out by fingerprinting. Mapping of T1 ribonuclease digests by high-voltage electrophoresis and homochromatography showed that specific oligoribonucleotides were protected against nuclease attack by proteins of the 15 S mRNP.
Full text
PDF


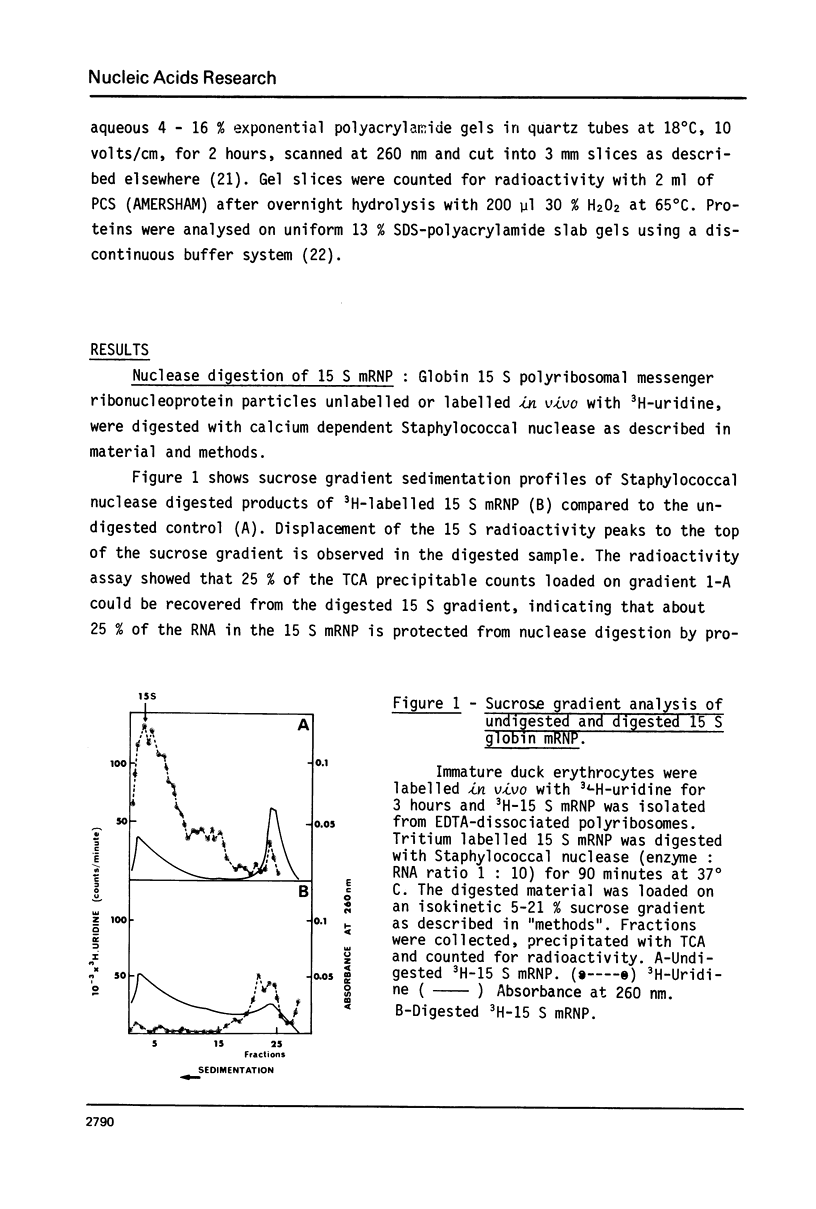
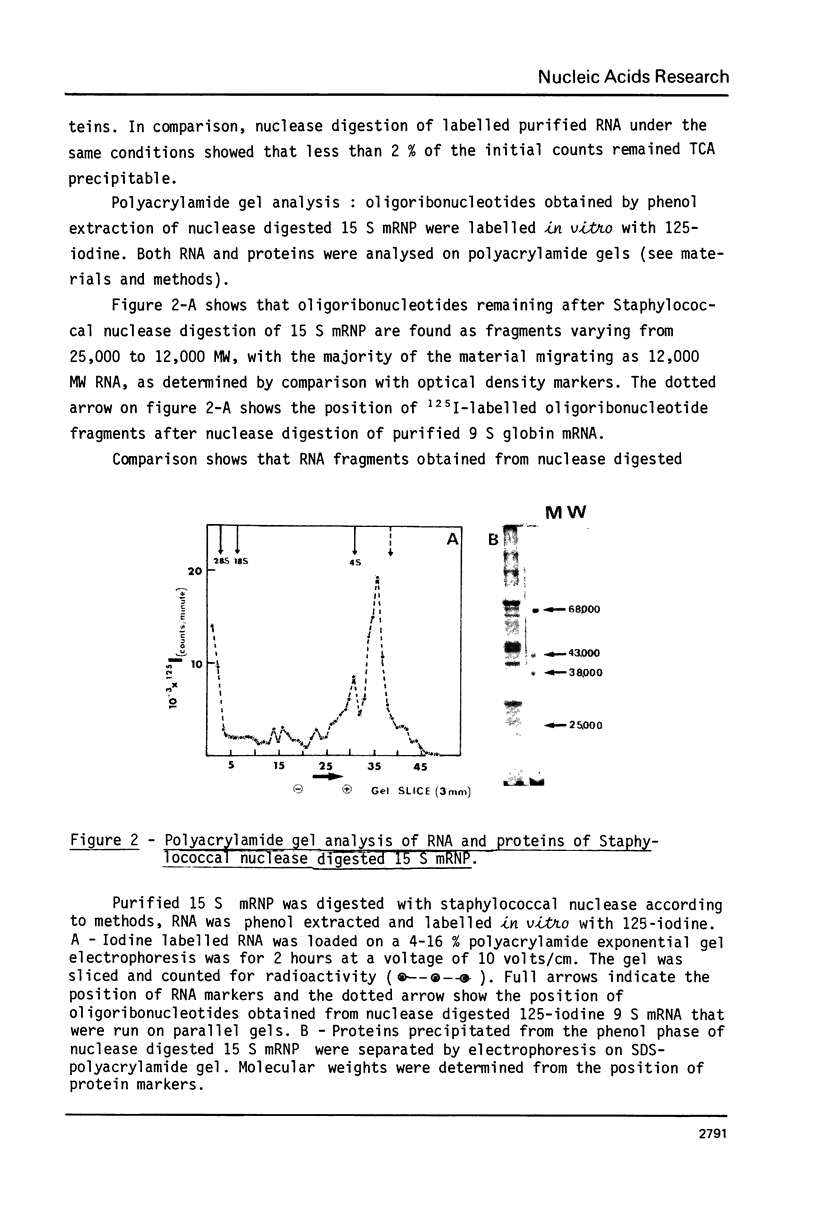






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augenlicht L. H., McCormick M., Lipkin M. Digestion of RNA of chromatin and nuclear ribonucleoprotein by staphylococcal nuclease. Biochemistry. 1976 Aug 24;15(17):3818–3823. doi: 10.1021/bi00662a026. [DOI] [PubMed] [Google Scholar]
- Blobel G. A protein of molecular weight 78,000 bound to the polyadenylate region of eukaryotic messenger RNAs. Proc Natl Acad Sci U S A. 1973 Mar;70(3):924–928. doi: 10.1073/pnas.70.3.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civelli O., Vincent A., Buri J. F., Scherrer K. Evidence for a translational inhibitor linked to globin mRNA in untranslated free cytoplasmic messenger ribonucleoprotein complexes. FEBS Lett. 1976 Dec 15;72(1):71–76. doi: 10.1016/0014-5793(76)80815-0. [DOI] [PubMed] [Google Scholar]
- Clark R. J., Felsenfeld G. Structure of chromatin. Nat New Biol. 1971 Jan 27;229(4):101–106. doi: 10.1038/newbio229101a0. [DOI] [PubMed] [Google Scholar]
- Commerford S. L. Iodination of nucleic acids in vitro. Biochemistry. 1971 May 25;10(11):1993–2000. doi: 10.1021/bi00787a005. [DOI] [PubMed] [Google Scholar]
- Dubochet J., Morel C., Lebleu B., Herzberg M. Structure of globin mRNA and mRNA-protein particles. Use of dark-field electron microscopy. Eur J Biochem. 1973 Jul 16;36(2):465–472. doi: 10.1111/j.1432-1033.1973.tb02931.x. [DOI] [PubMed] [Google Scholar]
- Favre A., Morel C., Scherrer K. The secondary structure and poly(A) content of globin messenger RNA as a pure RNA and in polyribosome-derived ribonucleoprotein complexes. Eur J Biochem. 1975 Sep 1;57(1):147–157. doi: 10.1111/j.1432-1033.1975.tb02285.x. [DOI] [PubMed] [Google Scholar]
- Franklin R. M. Purification and properties of the replicative intermediate of the RNA bacteriophage R17. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1504–1511. doi: 10.1073/pnas.55.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gander E. S., Mueller R. U., Goldenberg S., Morel C. EDTA-and puromycin-derived duck- and rabbit globin-messenger ribonucleoprotein complexes isolated by oligo (dT)-cellulose chromatography. Mol Biol Rep. 1975 Dec;2(4):343–349. doi: 10.1007/BF00357022. [DOI] [PubMed] [Google Scholar]
- Holder J. W., Lingrel J. B. Determination of secondary structure in rabbit globin messenger RNA by thermal denaturation. Biochemistry. 1975 Sep 23;14(19):4209–4215. doi: 10.1021/bi00690a009. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Lane C. D., Grecory C. M., Morel C. Duck-haemoglobin synthesis in frog cells. The translation and assay of reticulocyte 9-S RNA in oocytes of Xenopus laevis. Eur J Biochem. 1973 Apr;34(2):219–227. doi: 10.1111/j.1432-1033.1973.tb02749.x. [DOI] [PubMed] [Google Scholar]
- Mirault M. E., Scherrer K. Isolation of preribosomes from HeLa cells and their characterization by electrophoresis on uniform and exponential-gradient-polyacrylamide gels. Eur J Biochem. 1971 Nov 11;23(2):372–386. doi: 10.1111/j.1432-1033.1971.tb01631.x. [DOI] [PubMed] [Google Scholar]
- Morel C., Gander E. S., Herzberg M., Dubochet J., Scherrer K. The duck-globin messenger-ribonucleoprotein complex. Resistance to high ionic strength, particle gel electrophoresis, composition and visualisation by dark-field electron microscopy. Eur J Biochem. 1973 Jul 16;36(2):455–464. doi: 10.1111/j.1432-1033.1973.tb02930.x. [DOI] [PubMed] [Google Scholar]
- Morel Carlos, Kayibanda Boniface, Scherrer Klaus. Proteins associated with globin messenger RNA in avian erythroblasts: Isolation and comparison with the proteins bound to nuclear messenger-likie RNA. FEBS Lett. 1971 Oct 15;18(1):84–88. doi: 10.1016/0014-5793(71)80413-1. [DOI] [PubMed] [Google Scholar]
- Revel M., Groner Y. Post-transcriptional and translational controls of gene expression in eukaryotes. Annu Rev Biochem. 1978;47:1079–1126. doi: 10.1146/annurev.bi.47.070178.005243. [DOI] [PubMed] [Google Scholar]
- Robertson H. D., Dickson E., Model P., Prensky W. Application of fingerprinting techniques to iodinated nucleic acids. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3260–3264. doi: 10.1073/pnas.70.11.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz H., Darnell J. E. The association of protein with the polyadenylic acid of HeLa cell messenger RNA: evidence for a "transport" role of a 75,000 molecular weight polypeptide. J Mol Biol. 1976 Jul 15;104(4):833–851. doi: 10.1016/0022-2836(76)90185-6. [DOI] [PubMed] [Google Scholar]
- Sonenberg N., Morgan M. A., Merrick W. C., Shatkin A. J. A polypeptide in eukaryotic initiation factors that crosslinks specifically to the 5'-terminal cap in mRNA. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4843–4847. doi: 10.1073/pnas.75.10.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spohr G., Kayibanda B., Scherrer K. Polyribosome-bound and free-cytoplasmic-hemoglobin-messenger RNA in differentiating avian erythroblasts. Eur J Biochem. 1972 Nov 21;31(1):194–208. doi: 10.1111/j.1432-1033.1972.tb02519.x. [DOI] [PubMed] [Google Scholar]
- Sundquist B., Persson T., Lindberg U. Characterization of mRNA-protein complexes from mammalian cells. Nucleic Acids Res. 1977 Apr;4(4):899–915. doi: 10.1093/nar/4.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]