Abstract
Introduction
Antibiotic associated diarrhea (AAD) is a frequently encountered adverse event following antibiotic administration. Evidence suggests that probiotics may be beneficial in preventing and decreasing the severity of AAD.
Material and methods
Adult patients who were prescribed antibiotics for 3-14 days were enrolled from eight Canadian centers. Study treatment was randomized at a 1 : 1 ratio of BIO-K+CL1285® or placebo and was administered within 24 h of initiation to 5 days after termination of antibiotherapy. Patients were followed for 21 days after last dose of study treatment. The primary outcome was severity and incidence of AAD. Severity was measured by the total number of days with diarrhea and incidence was defined as the number of patients with at least one day with diarrhea over the total number of patients enrolled in the study.
Results
216 patients were randomized to BIO-K+ and 221 to placebo. The mean (SD) number of days with diarrhea was 1.19. (3.20) days for the placebo and 0.67 (2.05) days for BIO-K+CL1285® (p = 0.040). Adjusted multivariate linear regression results showed that the duration of diarrhea for BIO-K+CL1285 ® vs. placebo was reduced by 51.5% (b[SE] = 0.515 [0.256], p = 0.045). The incidence of diarrhea was 21.8% for the BIO-K+ and 29.4% for the placebo group (OR = 0.667, p = 0.067). Multivariate logistic regression, showed that the adjusted odds ratio of AAD in patients receiving BIO-K+ vs. placebo was 0.627 (p = 0.037). Study treatment was well tolerated.
Conclusions
BIO-K+ is effective for preventing and reducing the severity of AAD in patients receiving antibiotic therapy in a hospital setting.
Keywords: lactobacillus, antibiotic associated diarrhea, probiotics, acidophilus
Introduction
Diarrhea is one of the most frequently reported adverse events associated with antibiotic treatment. Alterations in the balance and diversity of the composition of the normal intestinal flora have been identified as the two major factors involved in the pathogenesis of Antibiotic Associated Diarrhea [1, 2]. The incidence of AAD varies widely with rates between 10 to 30% and has been identified as the leading cause of diarrhea in hospitalized patients [3]. The onset of AAD can occur as early as a few hours after the first dose or as late as 2 months after discontinuation of antibiotic therapy [1]. Osmotic diarrhea is the most frequent manifestation of AAD and is produced by alterations in both carbohydrate fermentation and bile acid metabolism caused by a decrease in anaerobic flora [4, 5]. The magnitude of these changes is influenced by the type of antibiotic used along with the capability of the intestinal flora to resist to pathogen colonization. In some occasions, the changes in the makeup of the intestinal flora will lead to the proliferation of pathogenic organisms such as Staphylococcus aureus, Candida spp., Klebsiella oxytoca, and Clostridium difficile [5, 6]. Given that the mechanism of action for AAD is based on an acute disruption of the intestinal normal flora, interventions that could counteract this effect that are administered prior to or concurrently with the antibiotic treatment would be efficacious in preventing or reducing the severity of AAD.
The Food and Agricultural Organization of the United Nations and World Health Organization have defined probiotics as “live organisms which when administered in adequate amounts confer a health benefit on the host” [7].There is accumulating evidence that probiotics are beneficial in preventing and shortening the duration of AAD from an increasing number of clinical studies [8–11]. Mechanisms by which probiotics exert their therapeutic effects include:
modulation of the epithelial cell barrier function,
antagonistic activity against pathogenic bacteria either by inhibition of adherence and translocation or by production of antibacterial substances,
modulation of intestinal cytokine production,
anti-inflammatory properties, and
However, in a recent meta-analysis [15] only 13 (52%) out of 25 trials assessed reported a beneficial effect of probiotics in preventing AAD. The disagreement in the results reported in these studies may be due to differences in the patient population, the type and dose of probiotics, the class of antibiotics used, and the duration of the treatment [15].
The role of the Lactobacillus genus on the reduction of AAD has been vastly studied. Hickson and co-workers reported the efficacy of a probiotic drink containing Lactobacillus casei, Lactobacillus bulgaricus, and Streptococcus thermophilus in the reduction of the incidence of AAD and C. difficile-associated diarrhea (CDAD) in a population of 135 elderly hospital patients [9]. In a recent study, a probiotic milk drink containing Lactobacillus rhamnosus GG, Lactobacillus acidophilus La-5 and Bifidobacterium Bb-12 reduced the risk of AAD by 79% in hospitalized adult patients [10]. The strain L. rhamnosus GG was also proven effective in the prevention of AAD in a sample of 188 children treated with oral antibiotics for common childhood infections [16].
In assessing the potential benefits of probiotics in AAD it should be noted that the effect is highly strain specific and generalization of the results to related strains is not valid [10, 17]. It is therefore essential that individual strains are assessed independently and that the results must be interpreted for the specific strain.
BIO K+ CL1285® is a commercially available probiotic with a patented formula containing the L. acidophilus CL1285® strain of human origin, registered at the Pasteur Institute, and a L. casei strain. In a randomized clinical trial the fermented milk formula, formulation of BIO K+ CL1285®, was shown to be efficacious in preventing AAD in hospitalized patients [11]. However, this was a single center study with a relatively small sample size. There is consensus among experts that well designed clinical trials with larger sample size are required to adequately assess and demonstrate the efficacy of probiotics in the management and prevention of AAD [10, 15 17, 18].
Based on the above discussion it is anticipated that administration of BIO K+ CL1285® concurrently with an antibiotic would minimize the destabilization of the intestinal normal flora and be efficacious in reducing AAD. The objective of the present study was to assess the efficacy and safety of BIO K+ CL1285® compared to placebo in reducing the incidence and severity of AAD in patients treated in a hospital setting.
Material and methods
Study design and treatment
This was a multicenter double-blind, randomized, placebo controlled, study that was conducted in eight Canadian centers between March 2006 and October 2007. Patients were randomized at a 1 : 1 ratio to receive BIO K+ or a placebo. The formulation of the BIO K+ CL1285® lactobacilli fermented milk was a combination of 50 × 109 colony forming units of L. acidophilus CL1285® and L. casei (BIO K+ CL1285®, BIO K+ International Inc., Canada). The duration of treatment was between 29 and 40 days. The dosage schedule was 49 g/day for the first 2 days, in order to evaluate the tolerance of the patient to the product, followed by a full dose of 98 g/day for the remaining treatment period. The placebo was prepared by BIO K+ International and was a lactoserum devoid of any microorganisms with similar texture and flavor as to BIO K+. Both the BIO K+ and placebo preparations were administered within 24 h after the first dose of antibiotic and continued once daily within ± 2 h of the administration of the antibiotic treatment and for 5 days following the termination of antibiotic regimen.
Diary cards were provided to patients to record antibiotic and study product use along with the presence of diarrhea during the treatment period. Additional follow up assessments were conducted at 21 days after treatment termination. For the 21 day follow up period patients were instructed to complete the diary cards only when diarrhea occurred.
Testing for CDAD was performed at the discretion of the treating physician and according to the protocol in place at the study centers. CDAD was defined as an episode of diarrhea and positive results for C. difficile Toxin A or B. Data for patients discharged from the hospital before the end of the study were obtained by a self administered diary and standardized telephone interview.
All statistical analyses were conducted on the Intent to Treat (ITT) population that was comprised of any patient that received at least one dose of the study product, a minimum of three days antibiotic treatment and had at least one follow up visit to allow ascertainment of the study outcomes.
Potentially eligible patients signed an informed consent form prior to the performance of any study procedure. The study protocol was approved by the internal review board of each institution and was conducted according to the principles of Declaration of Helsinki.
Study population
In order to be eligible for inclusion in the study patients had to be 18 years or older, treated in an emergency room or hospital ward for a minimum of 12 h, and scheduled to receive antibiotic therapy for a minimum of three days and a maximum of 14 days. All patients were required to have received no more than 24 h of antibiotic therapy before being enrolled in the study. Exclusion criteria included active diarrhea at enrollment, a history of daily consumption of fermented milk and/or yogurt, known lactose intolerance, pregnant/breastfeeding women, an active and uncontrolled intestinal disease, history of an ileostomy, a jejunostomy or a colostomy, an immune-suppressive state or condition, a diagnosis of C. difficile-associated diarrhea within the previous three months, active radiotherapy or chemotherapy, a recent (< 6 months) or planned bone marrow graft or organ transplant, prior antibiotic therapy in the fourteen days prior to study initiation, current or planned administration of metronidazole (alone or in combination) or vancomycin monotherapy for the treatment of an infection, and participation in another clinical trial.
Outcome measures
The primary efficacy outcome measures were the severity and incidence of AAD during the treatment period and the 21 day follow up. In ascertaining the study outcome, a day of diarrhea was defined as one or more episodes of unformed or liquid stool in a 24 h period. Incidence of diarrhea was defined as the proportion of patients with one or more days with diarrhea over the total number of patients in the ITT population. Severity was assessed by duration defined as the total number of days with diarrhea. Secondary efficacy outcome measures included the incidence of CDAD. Safety was assessed by the incidence of treatment-emergent adverse events, which were reported according to the MedDRA (version 10.1) dictionary of terms.
Statistical analysis
Sample size calculations were established in order to detect a 50% reduction in the incidence of diarrhea in favor of the treatment group. Based on available data in the literature and preliminary results on BIO K+ a 20% incidence of diarrhea in the placebo was anticipated. Therefore in order to detect statistical significance with 80% power and 5% significance as well as a relative risk of 0.50, a total of 200 evaluable patients per group were to be enrolled in the study. Allowing for 20% attrition, a total of 500 patients were to be recruited.
Descriptive statistics including the mean and standard deviation for continuous scale variables and frequency distributions for categorical variables were reported for all study variables by treatment group. The between-group difference with respect to the incidence of diarrhea was assessed for statistical significance with the χ2 test. The relative risk as estimated by the logistic regression based odds ratio was used to assess the magnitude and precision of the difference. The statistical significance of the between group difference with respect to the duration of diarrhea was assessed with the Student's t-test for Independent samples. Adjusted analyses were based on Multivariate Binary Logistic regression for incidence of diarrhea and General Linear Models for duration of diarrhea. In the adjusted analyses potential confounding variables that were included as covariates in the multivariate models were age, gender, duration of treatment, duration of antibiotic use, change in antibiotic use, and history of AAD. These covariates were selected a priori because of their potential importance as risk factors and prognostic predictors for diarrhea. The backward selection process based on the Wald statistic with an α tolerance of 0.15 was used to select the final set of covariates included in the multivariate models.
Safety evaluation was conducted on all patients receiving at least one dose of the study treatment and was assessed by the number of events and the number of patients experiencing at least one serious or non-serious treatment related adverse event during the treatment period. Causal relation of the adverse event to the study drug was ascertained by the treating physician.
All analyses were based on the Intent to Treat population. No patients were excluded from the analyses because of protocol violation or non-compliance. There were no replacements of missing values or imputations applied and all analyses were conducted on observed cases. The Statistical Product and Service Solutions (SPSS version 12.0 for Windows) was used for all statistical analyses.
Results
Patient disposition and baseline characteristics
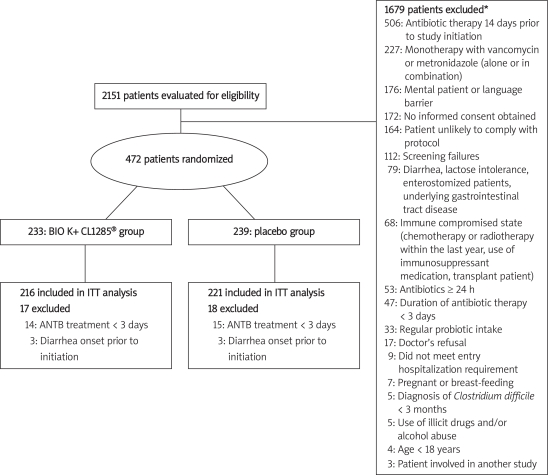
Among the 472 randomized patients, 29 patients were excluded from the ITT analysis due to antibiotic treatment duration of less than 3 days and 6 patients were excluded because diarrhea onset occurred before initiation of study treatment. Therefore a total of 437 (92.6%) were included in the ITT population (Figure 1).
Figure 1.
Patient disposition
*8 patients reported more than one reason for exclusion; ITT – intent to treat, ANTB – antibiotic
The patient demographics and baseline characteristics were similar for the BIO K+ and placebo groups (Table I). More than 50% of the patients were hospitalized with a mean duration of hospital stay of 8.8 and 7.1 for the BIO K+ and placebo groups respectively. The most frequent type of antibiotic received during the study was β-lactams, including cephalosporin and penicillin, by 76.9% of the patients in the BIO K+ group and 78.3% of the patients in the placebo group. The most frequent underlying condition for antibiotic use was respiratory infections for 39.4% of the patients in the BIO K+ group and 38.5% of the patients in the placebo group. The mean (SD) duration of antibiotic treatment was 9.8 (3.9) days for the BIO K+ group and 9.7 (3.8) days for the placebo group. The mean duration of treatment with the study product was approximately 12.0 days for both groups.
Table 1.
Demographics and baseline characteristics
| Treatment group | P-value | ||
|---|---|---|---|
| BIO K+ CL1285® (N = 216) | Placebo (N = 221) | ||
| Age [years], mean (SD) | 59.5 (18.1) | 58.1 (19.1) | 0.409 |
| Age categories [years],N(%) | |||
| Gender, N(%): | |||
| •Male | 117 (54.2) | 107 (48.4) | 0.251 |
| •Female | 99 (45.8) | 114 (51.6) | |
| Distribution of patients per hospital, N(%): | |||
| •Centre Hospitalier Régional de Trois Rivie‘res | 24 (11.1) | 19 (8.6) | |
| •St. Mary's Hospital | 11 (5.1) | 12 (5.4) | |
| •Hôpital Hôtel-Dieu de Saint-Jérôme | 68 (31.5) | 76 (34.4) | |
| •Hamilton General Hospital | 6 (2.8) | 6 (2.7) | |
| •Hôpital Laval | 24 (11.1) | 23 (10.4) | |
| •Kingston General Hospital | 34 (15.7) | 39 (17.6) | |
| •North York General Hospital | 36 (16.7) | 35 (15.8) | |
| •Centre de Santé et de Services Sociaux de Chicoutimi | 13 (6.0) | 11 (5.0) | |
| Type of care, N(%): | |||
| •Hospitalized | 118 (54.6) | 130 (58.8) | 0.386 |
| •Out patients | 98 (45.4) | 91 (41.2) | |
| Hospitalization duration [days], mean (SD) | 8.8 (15.1) | 7.1 (6.4) | 0.302 |
| History of CDAD and/or AAD, N(%) | 3 (1.4) | 5 (2.3) | 0.724 |
| Concomitant medications in the last 15 days, N(%): | |||
| •Laxative | 20 (9.3) | 19 (8.6) | 0.868 |
| •Anti-inflammatory | 54 (25.0) | 50 (22.6) | 0.576 |
| •Antacid | 14 (6.5) | 21 (9.5) | 0.291 |
| •Aspirin | 61 (28.2) | 58 (26.2) | 0.668 |
| •Proton-pump inhibitor | 51 (23.6) | 62 (28.1) | 0.326 |
| •Opiate | 28 (13.0) | 29 (13.1) | 0.999 |
| Duration of antibiotic therapy [days], mean (SD) | 9.76 (3.85) | 9.65 (3.76) | 0.768 |
| Duration of treatment [days], mean (SD) | 12.37 (5.21) | 11.99 (4.93) | 0.429 |
| Antibiotics received during study§, N(%): | |||
| •β-Lactams | 166 (76.9) | 173 (78.3) | 0.732 |
| •Quinolones | 68 (31.5) | 70 (31.7) | 0.999 |
| •Macrolides | 51 (23.6) | 51 (23.1) | 0.910 |
| •Clindamycin | 18 (8.3) | 14 (6.3) | 0.466 |
| •Other: | |||
| -Metronidazole | 1 (0.5) | 0 (0.0) | 0.494 |
| -Septra | 3 (1.4) | 0 (0.0) | 0.120 |
| -Tetracycline | 11 (5.1) | 9 (4.1) | 0.653 |
| -Tobramycine | 1 (0.5) | 1 (0.5) | 0.999 |
| -Vancomycin | 0 (0.0) | 1 (0.5) | 0.999 |
| -Linezolide | 1 (0.5) | 0 (0.0) | 0.494 |
| Underlying infections for antibiotic treatment, N(%): | |||
| •Respiratory | 85 (39.4) | 85 (38.5) | 0.922 |
| •Skin | 49 (22.7) | 50 (22.6) | 0.999 |
| •Urogenital tract | 23 (10.6) | 36 (16.3) | 0.094 |
| •Other | 65 (30.1) | 52 (23.5) | 0.131 |
CDAD – clostridium difficile-associated diarrhea;
§ there were 163 patients who received more than 1 type of antibiotic
Efficacy outcomes
The mean (SD) duration of diarrhea was 0.67 (2.05) [95% CI: 0.39-0.94] days for the BIO K+ group and 1.19 (3.20) [95% CI: 0.77-1.62] days for the placebo group (p = 0.040) (Table II). The backward selection procedure for the multivariate linear regression model assessing between group difference with respect to duration of diarrhea, retained in the model the following variables: treatment group, duration of antibiotic therapy, patient's age and duration of study treatment while gender, change in antibiotic treatment and history of AAD were not retained. The final linear regression model showed a significant treatment effect with a reduction on the adjusted mean number of days with diarrhea in the BIO K+ group vs. the placebo group of 51.5% (b[SE] = 0.515 [0.256]; 95% CI [0.012-1.018]).The adjusted least square mean estimates based on this multivariate linear regression for the number of days with diarrhea were similar to the unadjusted estimates and the between group adjusted difference remained statistically significant (p = 0.045).
Table II. Efficacy outcomes
| Treatment group | Odds ratio (95% CI) | P-value | ||
|---|---|---|---|---|
| BIO K+ CL1285® (N = 216) | Placebo (N = 221) | |||
| Unadjusted analysis | ||||
| Severity: | ||||
| mean (SD) number of days with diarrhea | 0.67 (2.05) | 1.19 (3.20) | NA | 0.040 |
| Incidence: | 21.8 | 29.4 | 0.667 | 0.067 |
| (%) of patients with ≥ 1 day of diarrhea | (0.433-1.030) | |||
| Adjusted analysis* | ||||
| Severity: | 0.67 (0.37) | 1.19 (0.42) | NA | 0.045 |
| mean (SE) number of days with diarrhea§ | ||||
| Incidence: | 21.8 | 31.7 | 0.627 | 0.037 |
| (%) of patients with ≥ 1 day of diarrhea├ | (0.405–0.971) | |||
* Adjusted for age, duration of treatment with study product, duration of treatment with antibiotics
§ Least Square Mean Estimates based on linear regression analysis
├ based on Multi-Variate Logistic Regression Analysis, SD – standard deviation, SE – standard error
The incidence of diarrhea was 21.8% in the BIO K+ group compared to 29.4% in the placebo group. This difference approached statistical significance (odds ratio [95% CI] = 0.667 [0.433-1.030], p = 0.067). However, the study sample was underpowered, with only 40% power, to detect as statistically significant this difference. The final multivariate logistic regression model assessing the between group difference with respect to the incidence of new onset AAD retained the same covariates as the linear regression model, specifically treatment group, duration of study treatment, duration of antibiotic therapy, and patient's age. The results of this analysis showed a statistically significant adjusted odds ratio of diarrhea for the BIO K+ group vs. the placebo group of 0.627 (95% CI [0.405-0.971], p = 0.037).
There were 12.5% of the patients in the BIO K+ group and 19.0% in the placebo group with diarrhea ≥ 2 days (p = 0.062). The proportion of patients with ≥ 3 days diarrhea was 7.9% in the BIO K+ group and 13.6% patients in the placebo group (p = 0.054). These differences approached statistical significance with the study being underpowered, 40 and 42% respectively, to detect these effects as statistically significant The adjusted odds ratios of BIO K+ vs. placebo were 0.554 (95% CI: 0.326-0.940, p = 0.029) for diarrhea duration ≥ 2 days and 0.504 (95% CI: 0.269-0.944, p = 0.032) for diarrhea ≥ 3 days.
There were 16 patients in the BIO K+ group and 30 in the placebo group that underwent CDAD testing. Of these, 1 (6.2%) patient in the BIO K+ group and 4 (13.3%) in the placebo group were positive for the C. difficile toxins (odds ratio = 0.433, p = 0.645). The incidence of bloating was 21.8% in the BIO K+ group vs. 19.9% in the placebo group. For painful cramps the incidence was 14.8% in the BIO K+ and 15.8% in the placebo group while that for bloody stools was 1.9 vs. 4.1% respectively. None of these differences were statistically significant.
Safety
One or more treatment-emergent non serious adverse event (NSAE) was reported by 72 (33.3%) patients in the BIO K+ group and 76 (34.4%) patients in the placebo group. There was no statistically significant difference between the two study groups with respect to the incidence of treatment-emergent NSAEs (Table III). The most frequently reported non serious adverse events were constipation, flatulence and nausea. A total of 38 serious adverse events were reported; 15 in the BIO K+ group and 23 in the placebo group, during the course of the study. None of the serious adverse events were related to the treatment product. There were eight deaths reported during the study, four in the placebo group and three in the BIO K+ group. None of the deaths were causally related to the study product.
Table III.
Treatment-emergent non serious adverse events
| Adverse event, N (%) | Treatment group | |
|---|---|---|
| BIO K+ CL1285 (N=216) | Placebo (N=221) | |
| Gastrointestinal adverse events: | ||
| • Constipation | 12 (5.6) | 8 (3.6) |
| • Flatulence | 7 (3.2) | 13 (5.9) |
| • Nausea | 7 (3.2) | 5 (2.3) |
| • Vomiting | 5 (2.4) | 0 (0.0) |
| • Dyspepsia | 0 (0.0) | 2 (0.9) |
| • Dysphagia | 1 (0.5) | 0 (0.0) |
| • Eructation | 0 (0.0) | 1 (0.5) |
| • Fecal incontinence | 1 (0.5) | 0 (0.0) |
| • Gastro esophageal | 1 (0.5) | 0 (0.0) |
| • reflux disease | ||
| • Glossitis | 0 (0.0) | 1 (0.5) |
| • Reflux gastritis | 1 (0.5) | 0 (0.0) |
| Other adverse events: | ||
| • Vulvovaginal mycotic infection | 0 (0.0) | 2 (0.9) |
| • Muscle spasms | 0 (0.0) | 2 (0.9) |
| • Hyperthermia | 0 (0.0) | 1 (0.5) |
| • Pyrexia | 0 (0.0) | 1 (0.5) |
| • Arthralgia | 1 (0.5) | 0 (0.0) |
| • Headache | 1 (0.5) | 0 (0.0) |
| • Dyspnea | 0 (0.0) | 1 (0.5) |
| • Presence of at least one treatment-emergent non serious adverse event | 72 (33.3) | 76 (34.4) |
Discussion
The results of this randomized, double-blind, placebo-controlled trial have shown that the lactobacilli-fermented milk product BIO K+ CL1285® is effective in reducing the severity and incidence of AAD in patients treated with antibiotics in a hospital setting.
The duration of diarrhea used as a measure of severity of AAD, was 1.19 days in the placebo group and 0.67 days in the BIO K+ group. This is a statistically and clinically significant unadjusted reduction of 43.7% in the mean duration of diarrhea. After adjusting for baseline prognostic variables the results show that, on the average, diarrhea will be reduced by 51.5% in patients treated with BIO K+ compared to those treated with placebo. This reduction in the duration of diarrhea, which is both clinically important and statistically significant, has major implications for patient's quality of life as well as direct and indirect health care costs related to the management of AAD.
The results of this study showed a 33% unadjusted reduction in the risk of diarrhea (incidence) in the patients treated with BIO K+ compared to placebo. Although this treatment effect is clinically important, the study was not sufficiently powered to detect this effect size as statistically significant. The low power was due to higher (29.4%) than the expected 20% incidence of diarrhea in the placebo group. This is a possible weakness of the current study, which is related to the assumptions used for sample size calculations. The multivariate logistic regression analyses controlled for the effect of the covariates, thus reducing within group variance and improving the power of the study. These results showed that after adjusting for the effect of potential confounders, the treatment with BIO K+ resulted in a statistically significant 37.3% reduction in the risk of AAD. While the type of concomitant medication used may be associated with increased risk for diarrhea; the two groups were very well matched with respect to this parameter and therefore there is no concern for confounding on the treatment effect.
The results of the current study are comparable with others that have reported the effectiveness of probiotics in reducing the incidence of AAD [9–11, 16, 17, 19–21]. However, the majority of these studies have been conducted on smaller number of patients. Conversely, the current study was conducted in several centers across Canada and in a larger and more diverse patient sample that is representative of the target population. The pathogenesis of antibiotic related diarrhea is based on the disruption of the intestinal normal flora. Probiotics including lactobacilli are efficacious in restoring normal flora and thus preventing or reducing the duration of diarrhea. A similar mechanism of action for the benefits of probiotics has been noted for Crohn's disease[22], irritable bowel syndrome [23], ulcerative colitis [24], and patients undergoing ileal pouch anal anastomosis [25].
The major strength of the present study is inherited in the double blind, placebo controlled design. Given the merit of double blind, controlled trial, the results of the current study provide evidence that the BIO K+ CL1285® lactobacilli-fermented probiotic is effective in the prevention of antibiotic associated diarrhea. These results have important implications for the general target population treated with antibiotics in a hospital setting and show that treatment with BIO K+ CL1285® will reduce the duration of diarrhea by approximately one half and the risk for diarrhea by more than one third. This will result in significantly improved patient quality of life and health care cost savings by reduced hospitalizations and health care resource utilization.
In recent years there has been an increase in the incidence of C. difficile infection in hospital settings, with the elderly hospitalized patients being at increased risk. In fact between 10-20% of C. difficile cases are observed in the elderly patients [26]. In the current study, despite the low overall incidence of C. difficile, a lower rate was observed in the lactobacilli compared to the placebo group. It should be noted, however, that testing for C. difficile was not conducted on all patients and that testing was dependent on the decision of the treating physician and hospital protocols. Further research on the assessment of the efficacy of BIO K+ in the prevention of CDAD is required as this nosocomial infection remains an important health issue worldwide.
Despite the high proportion of patients reporting at least one adverse event in each group, the majority (80%) of the events were mild in severity and the incidence was similar for the placebo and treatment groups. These results show that BIO K+ was overall safe and well tolerated.
In conclusion, this study demonstrates that probiotic prophylaxis with BIO K+ CL1285® is safe and efficacious in reducing the incidence and duration of antibiotic associated diarrhea in patients receiving antibiotic treatment in a hospital setting.
Acknowledgments
Dr. A. Poirier (Centre hospitalier régional de Trois-Rivie‘res, Trois-Rivie‘res, Québec), Dr. Y. Pesant (Centre de recherche médicale St. Jérôme, St. Jérôme, Québec), Dr. P. Rochette (Hôpital Laval, Québec), Dr. M. Sivilotti (Kingston General Hospital, Kingston, Ontario), Dr. A. Worster (Hamilton Health Sciences, Hamilton, Ontario), Dr. Magdy Elkashab (North York General Hospital, Toronto, Ontario), and Dr. D. Grimard (Centre de santé et services sociaux de Chicoutimi, Chicoutimi, Québec) contributed to the study as Principal Investigators in their respective centers. Dr. L. Othmen (BIO K+ International Inc., Laval, Quebec) for her contribution to the trial, data completion and verification.
Conflict of interest
John S. Sampalis, Eliofotisti Psaradellis and Emmanouil Rampakakis are employees of JSS Medical Research Inc.; JSS Medical Research Inc. was paid by BIO K+ International Inc. to conduct and manage this study. JSS Medical Research Inc. was responsible for analyzing and interpreting the data as well as writing and reviewing the manuscript.
The study was funded by a grant-in-aid of research from BIO K+ International Inc.
References
- 1.Högenauer C, Hammer HF, Krejs GJ, Reisinger EC. Mechanisms and management of antibiotic-associated diarrhea. Clin Infect Dis. 1998;27:702–10. doi: 10.1086/514958. [DOI] [PubMed] [Google Scholar]
- 2.Young VB, Schmidt TM. Antibiotic-associated diarrhea accompanied by large-scale alterations in the composition of the fecal microbiota. J Clin Microbiol. 2004;42:1203–6. doi: 10.1128/JCM.42.3.1203-1206.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiström J, Norrby SR, Myhre EB, et al. Frequency of antibiotic-associated diarrhoea in 2462 antibiotic-treated hospitalized patients: a prospective study. J Antimicrob Chemother. 2001;47:43–50. doi: 10.1093/jac/47.1.43. [DOI] [PubMed] [Google Scholar]
- 4.McFarland LV. Epidemiology, risk factors and treatments for antibiotic-associated diarrhea. Dig Dis. 1998;16:292–307. doi: 10.1159/000016879. [DOI] [PubMed] [Google Scholar]
- 5.Kaltenbach G, Heitz D. Antibiotic-associated diarrhea in the elderly [French] Rev Med Interne. 2004;25:46–53. doi: 10.1016/j.revmed.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Beaugerie L, Petit JC. Microbial-gut interactions in health and disease. Antibiotic-associated diarrhoea. Best Pract Res Clin Gastroenterol. 2004;18:337–52. doi: 10.1016/j.bpg.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Report of a joint FAO/WHO Working Group. Ontario, Canada, London: FAO/WHO; Guidelines for the Evaluation of Probiotics in Food. ftp://ftp.fao.org/es/esn/food/wgreport2.pdf. 2002. Ref Type: Data File. [Google Scholar]
- 8.Pedone CA, Bernabeau AO, Postaire ER. The effect of supplementation with milk fermented by Lactobacillus casei (strain DN-114 001) on acute diarrhea in children attending day care centers. Int J Clin Pract. 1999;53:179–84. [PubMed] [Google Scholar]
- 9.Hickson M, D'Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335:80. doi: 10.1136/bmj.39231.599815.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wenus C, Goll R, Loken EB, Biong AS, Halvorsen DS, Florholmen J. Prevention of antibiotic-associated diarrhoea by a fermented probiotic milk drink. Eur J Clin Nutr. 2008;62:299–301. doi: 10.1038/sj.ejcn.1602718. [DOI] [PubMed] [Google Scholar]
- 11.Beausoleil M, Fortier N, Guenette S, et al. Effect of a fer-mented milk combining lactobacillus acidophilus Cl1285 and lactobacillus casei in the prevention of antibiotic-associated diarrhea: a randomized, double-blind, placebo-controlled trial. Can J Gastroenterol. 2007;21:732–6. doi: 10.1155/2007/720205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones JL, Foxx-Orenstien AE. The role of probiotics in inflammatory bowel disease. Dig Dis Sci. 2007;52:607–11. doi: 10.1007/s10620-006-9225-y. [DOI] [PubMed] [Google Scholar]
- 13.Gionchetti P, Rizzello F, Campieri M. Probiotics in gastroenterology. Curr Opin Gasteroenterology. 2002;18:235–9. doi: 10.1097/00001574-200203000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Boirivant M, Strober W. The mechanism of action of probiotics. Curr Opin Gasteroenterology. 2007;23:679–92. doi: 10.1097/MOG.0b013e3282f0cffc. [DOI] [PubMed] [Google Scholar]
- 15.McFarland LV. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastro-enterol. 2006;101:812–22. doi: 10.1111/j.1572-0241.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- 16.Vanderhoof JA, Whitney DB, Antonson DL, Hanner TL, Lupo JV, Young RJ. Lactobacillus GG in the prevention of antibiotic-associated diarrhea in children. J Pediatr. 1999;135:564–8. doi: 10.1016/s0022-3476(99)70053-3. [DOI] [PubMed] [Google Scholar]
- 17.D'Souza AL, Rajkumar C, Cooke J, Bulpitt CJ. BMJ. Probiotics in prevention of antibiotic associated diarrhoea: meta-analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cremonini F, Di Caro S, Nista EC, et al. Meta-analysis: the effect of probiotic administration on antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2002;16:1461–7. doi: 10.1046/j.1365-2036.2002.01318.x. [DOI] [PubMed] [Google Scholar]
- 19.McFarland LV, Surawicz CM, Greenberg RN, et al. Prevention of beta-lactam-associated diarrhea by Saccharomyces boulardii compared with placebo. Am J Gastroenterol. 1995;90:439–48. [PubMed] [Google Scholar]
- 20.Wunderlich PF, Braun L, Fumagalli I, et al. Double-blind report on the efficacy of lactic acid-producing Enterococcus SF68 in the prevention of antibiotic-associated diarrhoea and in the treatment of acute diarrhoea. J Int Med Res. 1989;17:333–8. doi: 10.1177/030006058901700405. [DOI] [PubMed] [Google Scholar]
- 21.Surawicz CM, Elmer GW, Speelman P, McFarland LV, Chinn J, van Bellle G. Prevention of antibiotic-associated diarrhea by Saccharomyces boulardii: a prospective study. Gastroenterology. 1989;96:981–8. doi: 10.1016/0016-5085(89)91613-2. [DOI] [PubMed] [Google Scholar]
- 22.Rahimi R, Nikfar S, Rahimi F, et al. A meta-analysis of the benefit of probiotics in maintaining remission of human ulcerative colitis: evidence for prevention of disease relapse and maintenance of remission. Dig Dis Sci. 2008;53:2524–31. doi: 10.1007/s10620-007-0171-0. [DOI] [PubMed] [Google Scholar]
- 23.Nikfar S, Rahimi R, Rahimi F, Abdollahi M. Efficacy of probiotics in irritable bowel syndrome: a meta-analysis of randomized, controlled trials. Dis Colon Rectum. 2008;51:1775–80. doi: 10.1007/s10350-008-9335-z. [DOI] [PubMed] [Google Scholar]
- 24.Rahimi R, Nikfar S, Rezaie A, Abdollahi M. A meta-analysis of the benefit of probiotics in maintaining remission of human ulcerative colitis: evidence for prevention of disease relapse and maintenance of remission. Arch Med Sci. 2008;4:185–90. [Google Scholar]
- 25.Elahi B, Nikfar S, Derakshani S, Vafaie M, Abdollahi M. On the benefit of probiotics in the management of pouchitis in patients underwent ileal pouch anal anastomosis: a meta-analysis of controlled clinical trials. Dig Dis Sci. 2007;53:1278–84. doi: 10.1007/s10620-007-0006-z. [DOI] [PubMed] [Google Scholar]
- 26.Kelly CP, Pothoulakis C, Lamont JT. Clostridium difficile colitis. N Engl J Med. 1994;330:257–62. doi: 10.1056/NEJM199401273300406. [DOI] [PubMed] [Google Scholar]