Abstract
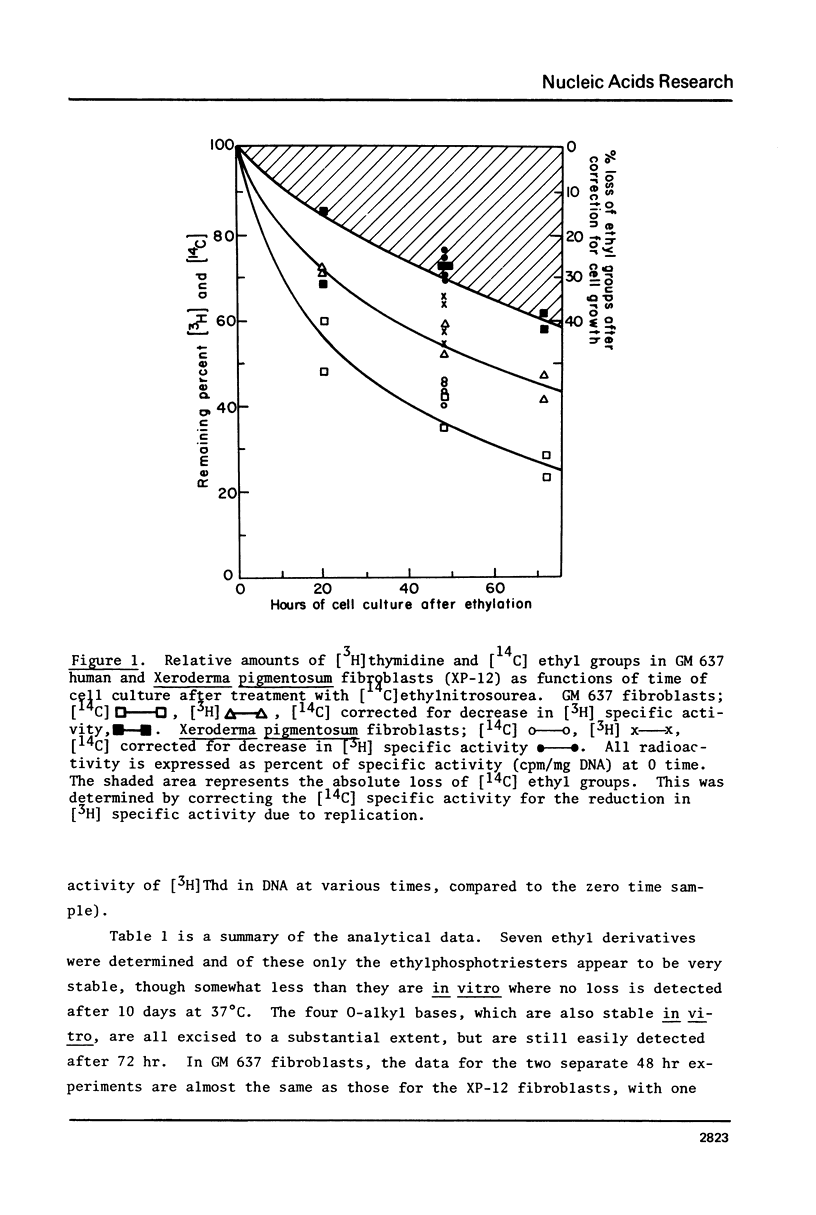
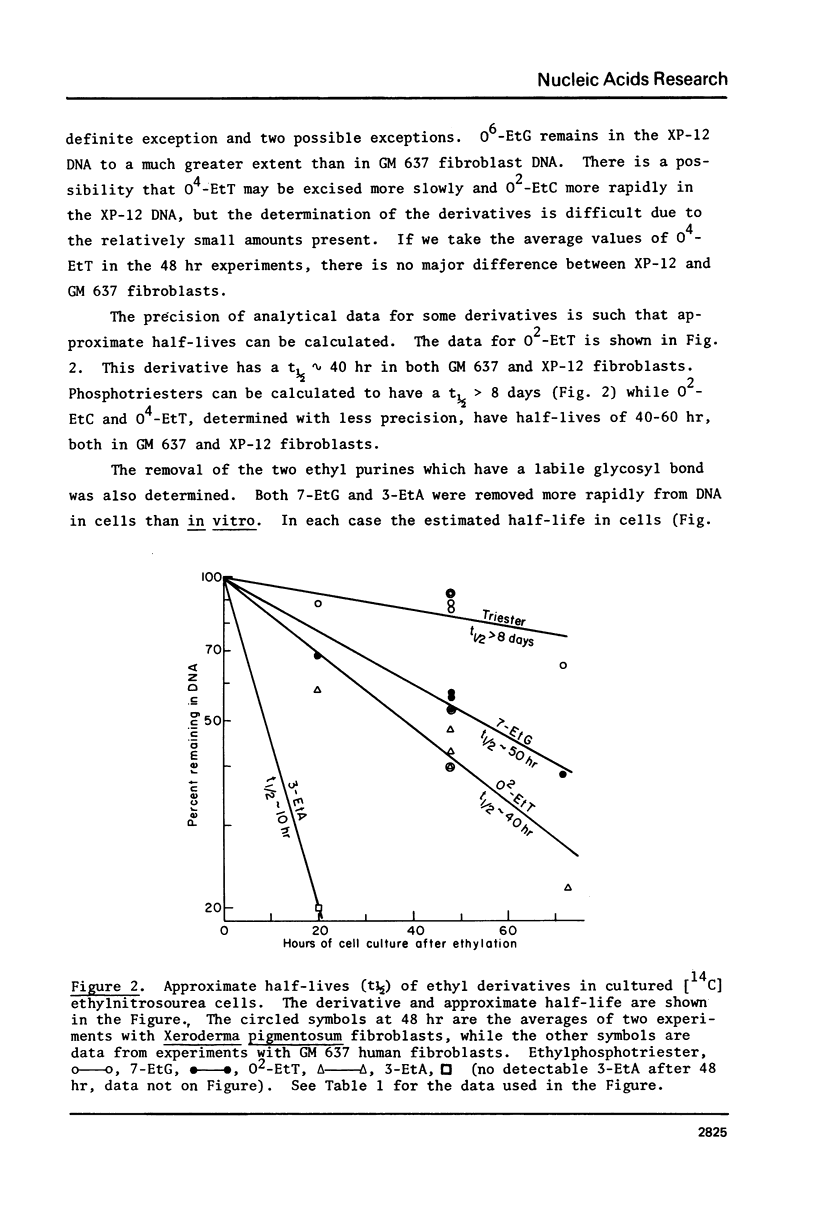
The potent carcinogen, ethylnitrosourea, has been shown to ethylate oxygens, in preference to nitrogens, in the DNA of cultured cells. We have now studied the removal of seven ethyl derivatives in replicating cells. The following findings are reported. 1) The absolute amounts of 02-EtT, 04-EtT and 02-EtC are decreased in cellular DNA after correction for cell growth. However the rate of decrease diminishes after approximately 20 hr and after more than two cell doublings 20--40% of each derivative persists. This decrease is presumed to be due to enzymes since these derivatives are stable in isolated DNA. 2) The amount of ethyl phosphotriesters remains almost unchanged during 72 hr of cell culture. 3) The unstable purine derivatives, 7-EtG and 3-EtA, are both removed from cellular DNA with a rate faster than can be accounted for by the lability of the glycosyl bond. 4) Both GM 637 fibroblasts and Xeroderma pigmentosum fibroblasts (12-RO) (XP-12) have similar ability to remove ethyl products, except for O6-ethyl G which persists to a greater extent in XP12 cells. 5) The implications of the in vivo persistence of ethylated bases is discussed in regard to recent demonstrations that O2-EtT, O4-ET, O2-EtC and O6-EtG are all mutagenic.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodell W. J., Banerjee M. R. The influence of chromatin structure on the distribution of DNA repair synthesis studied by nuclease digestion. Nucleic Acids Res. 1979 Jan;6(1):359–370. doi: 10.1093/nar/6.1.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodell W. J. Nonuniform distribution of DNA repair in chromatin after treatment with methyl methanesulfonate. Nucleic Acids Res. 1977 Aug;4(8):2619–2628. doi: 10.1093/nar/4.8.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootsma D., Mulder M. P., Cohen J. A., Pot F. Different inherited levels of DNA repair replication in xeroderma pigmentosum cell strains after exposure to ultraviolet irradiation. Mutat Res. 1970 May;9(5):507–516. doi: 10.1016/0027-5107(70)90035-7. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. DNA repair with purines and pyrimidines in radiation- and carcinogen-damaged normal and xeroderma pigmentosum human cells. Cancer Res. 1973 Feb;33(2):362–369. [PubMed] [Google Scholar]
- Cleaver J. E. Defective repair replication of DNA in xeroderma pigmentosum. Nature. 1968 May 18;218(5142):652–656. doi: 10.1038/218652a0. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Nucleosome structure controls rates of excision repair in DNA of human cells. Nature. 1977 Dec 1;270(5636):451–453. doi: 10.1038/270451a0. [DOI] [PubMed] [Google Scholar]
- Day R. S., 3rd, Scudiero D., Dimattina M. Excision repair by human fibroblasts of DNA damaged by r-7, t-8-dihyroxy-t-9,10-oxy-7,8,9,10- tetrahydrobenzo(a)pyrene. Mutat Res. 1978 Jun;50(3):383–394. doi: 10.1016/0027-5107(78)90043-x. [DOI] [PubMed] [Google Scholar]
- Feldman G., Remsen J., Shinohara K., Cerutti P. Excisability and persistence of benzo(a)pyrene DNA adducts in epithelioid human lung cells. Nature. 1978 Aug 24;274(5673):796–798. doi: 10.1038/274796a0. [DOI] [PubMed] [Google Scholar]
- Fraval H. N., Rawlings C. J., Roberts J. J. Increased sensitivity of UV-repair-deficient human cells to DNA bound platinum products which unlike thymine dimers are not recognized by an endonuclease extracted from Micrococcus luteus. Mutat Res. 1978 Jul;51(1):121–132. doi: 10.1016/0027-5107(78)90014-3. [DOI] [PubMed] [Google Scholar]
- Gerchman L. L., Ludlum D. B. The properties of O 6 -methylguanine in templates for RNA polymerase. Biochim Biophys Acta. 1973 May 10;308(2):310–316. doi: 10.1016/0005-2787(73)90160-3. [DOI] [PubMed] [Google Scholar]
- Goth-Goldstein R. Repair of DNA damaged by alkylating carcinogens is defective in xeroderma pigmentosum-derived fibroblasts. Nature. 1977 May 5;267(5606):81–82. doi: 10.1038/267081a0. [DOI] [PubMed] [Google Scholar]
- Kleihues P., Margison G. P. Exhaustion and recovery of repair excision of O6-methylguanine from rat liver DNA. Nature. 1976 Jan 15;259(5539):153–155. doi: 10.1038/259153a0. [DOI] [PubMed] [Google Scholar]
- Kriek E. Persistent binding of a new reaction product of the carcinogen N-hydroxy-N-2-acetylaminofluorene with guanine in rat liver DNA in vivo. Cancer Res. 1972 Oct;32(10):2042–2048. [PubMed] [Google Scholar]
- Lawley P. D., Warren W. Specific excision of ethylated purines from DNA of Escherichia coli treated with N-ethyl-N-nitrosourea. Chem Biol Interact. 1975 Jul;11(1):55–57. doi: 10.1016/0009-2797(75)90066-6. [DOI] [PubMed] [Google Scholar]
- Lieberman M. W., Dipple A. Removal of bound carcinogen during DNA repair in nondividing human lymphocytes. Cancer Res. 1972 Sep;32(9):1855–1860. [PubMed] [Google Scholar]
- Loveless A. Possible relevance of O-6 alkylation of deoxyguanosine to the mutagenicity and carcinogenicity of nitrosamines and nitrosamides. Nature. 1969 Jul 12;223(5202):206–207. doi: 10.1038/223206a0. [DOI] [PubMed] [Google Scholar]
- Mehta J. R., Ludlum D. B. Synthesis and properties of O6-methyldeoxyguanylic acid and its copolymers with deoxycytidylic acid. Biochim Biophys Acta. 1978 Dec 21;521(2):770–778. doi: 10.1016/0005-2787(78)90316-7. [DOI] [PubMed] [Google Scholar]
- Miller P. S., Braiterman L. T., Ts'o P. O. Effects of a trinucleotide ethyl phosphotriester, Gmp(Et)Gmp(Et)U, on mammalian cells in culture. Biochemistry. 1977 May 3;16(9):1988–1996. doi: 10.1021/bi00628a036. [DOI] [PubMed] [Google Scholar]
- Miller P. S., Fang K. N., Kondo N. S., Ts'o P. O. Syntheses and properties of adenine and thymine nucleoside alkyl phosphotriesters, the neutral analogs of dinucleoside monophosphates. J Am Chem Soc. 1971 Dec;93(24):6657–6665. doi: 10.1021/ja00753a054. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Dimethylnitrosamine inhibits enzymatic removal of O6-methylguanine from DNA. Nature. 1978 Jul 13;274(5667):182–184. doi: 10.1038/274182a0. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Formation and metabolism of alkylated nucleosides: possible role in carcinogenesis by nitroso compounds and alkylating agents. Adv Cancer Res. 1977;25:195–269. doi: 10.1016/s0065-230x(08)60635-1. [DOI] [PubMed] [Google Scholar]
- Riazuddin S., Lindahl T. Properties of 3-methyladenine-DNA glycosylase from Escherichia coli. Biochemistry. 1978 May 30;17(11):2110–2118. doi: 10.1021/bi00604a014. [DOI] [PubMed] [Google Scholar]
- Schendel P. F., Robins P. E. Repair of O6-methylguanine in adapted Escherichia coli. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6017–6020. doi: 10.1073/pnas.75.12.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow R. B., Regan J. D. Defective repair of N-acetoxy-2-acetylaminofluorene-induced lesions in the DNA of xeroderma pigmentosum cells. Biochem Biophys Res Commun. 1972 Jan 31;46(2):1019–1024. doi: 10.1016/s0006-291x(72)80243-2. [DOI] [PubMed] [Google Scholar]
- Shinohara K., Cerutti P. A. Excision repair of benzo[a]pyrene-deoxyguanosine adducts in baby hamster kidney 21/C13 cells and in secondary mouse embryo fibroblasts C57BL/6J. Proc Natl Acad Sci U S A. 1977 Mar;74(3):979–983. doi: 10.1073/pnas.74.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shooter K. V. DNA phosphotriesters as indicators of cumulative carcinogen-induced damage. Nature. 1978 Aug 10;274(5671):612–614. doi: 10.1038/274612a0. [DOI] [PubMed] [Google Scholar]
- Singer B. All oxygens in nucleic acids react with carcinogenic ethylating agents. Nature. 1976 Nov 25;264(5584):333–339. doi: 10.1038/264333a0. [DOI] [PubMed] [Google Scholar]
- Singer B., Bodell W. J., Cleaver J. E., Thomas G. H., Rajewsky M. F., Thon W. Oxygens in DNA are main targets for ethylnitrosourea in normal and xeroderma pigmentosum fibroblasts and fetal rat brain cells. Nature. 1978 Nov 2;276(5683):85–88. doi: 10.1038/276085a0. [DOI] [PubMed] [Google Scholar]
- Singer B., Fraenkel-Conrat H., Kuśmierek J. T. Preparation and template activities of polynucleotides containing O2- and O4-alkyluridine. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1722–1726. doi: 10.1073/pnas.75.4.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B. Sites in nucleic acids reacting with alkylating agents of differing carcinogenicity of mutagenicity. J Toxicol Environ Health. 1977 Jul;2(6):1279–1295. doi: 10.1080/15287397709529530. [DOI] [PubMed] [Google Scholar]
- Singer B. The chemical effects of nucleic acid alkylation and their relation to mutagenesis and carcinogenesis. Prog Nucleic Acid Res Mol Biol. 1975;15(0):219–284. [PubMed] [Google Scholar]
- Smerdon M. J., Tlsty T. D., Lieberman M. W. Distribution of ultraviolet-induced DNA repair synthesis in nuclease sensitive and resistant regions of human chromatin. Biochemistry. 1978 Jun 13;17(12):2377–2386. doi: 10.1021/bi00605a020. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Hanawalt P. C. Repair replication in cultured normal and transformed human fibroblasts. Biochim Biophys Acta. 1976 Oct 4;447(2):121–132. doi: 10.1016/0005-2787(76)90335-x. [DOI] [PubMed] [Google Scholar]
- Venitt S., Tarmy E. M. The selective excision of arylalkylated products from the DNA of Escherichia coli treated with the carcinogen 7-bromomethylbenz(a)anthracene. Biochim Biophys Acta. 1972 Nov 16;287(1):38–51. doi: 10.1016/0005-2787(72)90328-0. [DOI] [PubMed] [Google Scholar]


