Abstract
This study examined the relationship between obesity and asthma symptom perception in 200 youth with asthma. Repeated subjective and objective peak flow measurements were summarized using the Asthma Risk Grid (Klein et al., 2004), resulting in Accurate, Symptom Magnification and Danger Zone scores. Analyses were stratified by age and included ethnicity.
For younger children, obesity was not significantly related to perception scores. For older children, a significant obesity-by-ethnicity interaction for Accurate Symptom Perception scores indicated that obese white children had lower accuracy than white nonobese children, while there was no difference for obese versus nonobese minority children. Obesity was also related to higher Symptom Magnification scores regardless of ethnicity for older children.
These findings suggest that obesity may complicate asthma management by interfering with the ability to accurately perceive symptoms for some patients. More remains to be learned about the role of sociodemographic factors underlying this relationship.
Keywords: Asthma, Body mass index, Obesity, Symptom perception, Children
1. Introduction
Pediatric asthma and obesity are two of the most prevalent and problematic public health challenges. Research has reported a complex and poorly understood overlap between these conditions. Asthma remains the most common chronic illness in childhood, affecting nearly 6.5 million children in the United States, and resulting in high healthcare costs, functional limitation and a disproportionate impact on lower-income families, urban communities, and ethnic minorities (National Asthma Education and Prevention Program, 2007). The high rates and risks associated with asthma have persisted, despite considerable advances in pharmacologic treatment, requiring further research to understand the complex factors contributing to asthma morbidity (Lara et al., 2002).
Concurrent to the rise in asthma, pediatric obesity rates have increased remarkably over the past few decades (Barlow, 2007; Spear et al., 2007). The prevalence of obesity among children and adolescents ages 6–19 years tripled between 1980 and 2000 (Flegal et al., 2002). Pediatric obesity poses significant health risks, including cardiovascular, endocrine, and mental health co-morbidities, and contributes to subsequent risk of adult obesity, morbidity, and mortality (Krebs, Jacobson, and American Academy of Pediatrics Committee on Nutrition, 2003). Accordingly, prevention and treatment of pediatric obesity and asthma are public health priorities, and further research examining the overlap between these issues is needed (Story, 2007; Weiss and Shore, 2004).
Several lines of research have investigated the common pathways between asthma and obesity, with varying results. First, the risk of developing asthma may be increased by preexisting obesity (Flaherman and Rutherford, 2006). Several potential mechanisms for this relationship have been studied, including mechanical, inflammatory, gene-by-environment, and hormonal influences (see reviews by Weiss and Shore, 2004 and Shore, 2008). Secondly, asthma may set the stage for subsequent obesity (Forrest and Leeds, 2007; Gennuso et al., 1998). For example, children with asthma may fear that engaging in physical activity could initiate asthma symptoms and adopt sedentary lifestyles, contributing to the propensity for excessive weight gain (Glazebrook et al., 2006).
Regardless of causal pathways, it appears that individuals facing the dual burden of asthma and obesity experience more complicated illness course and compromised outcomes (Guerra et al., 2004; Sin et al., 2002; Sturdy et al., 2002; Taylor et al., 2008; von Mutius, 2002). Certain sociodemographic characteristics may influence the asthma–obesity relationship, though empirical data in this area remain sparse. A stronger relationship between the two conditions has been found in females relative to males (Jacobson et al., 2008; Pérez-Perdomo et al., 2003) and in younger versus older children (Abramson et al., 2008). Moreover some pediatric asthma studies have found higher rates of obesity in ethnic minority participants (e.g., Tantisira et al., 2003; Lara et al., 2006), although none have assessed directly whether ethnic disparities exist in the asthma–obesity relationship. Further research is needed to determine the specific role of sociodemographic factors, such as ethnicity and socioeconomic status, in the overlap between asthma and obesity.
1.1. The role of symptom perception in the asthma and obesity overlap
Due to the fluctuating course of asthma, the ability to perceive the onset and severity of symptoms is a key to optimal illness management (Fritz et al., 1996; McQuaid et al., 2007). Failure to sense compromised breathing may result in increased asthma morbidity, severe exacerbations and an increased risk of mortality (Feldman et al., 2007; Magadle et al., 2002; Strunk et al., 1985). Overestimation of symptoms can lead to unnecessary increases in the use of quick relief medications (Apter et al., 1997; Main et al., 2003), heightening the risk of iatrogenic consequences, and overutilization of health care services (Dirks and Schraa, 1983; Davis et al., 2009).
More remains to be learned about the risk factors for inaccurate symptom perception in children. Child age (Guyatt et al., 1997), cognitive-related factors (e.g., attention, Koinis Mitchell et al., 2009), asthma severity (Yoos et al., 2003), and ethnicity (Fritz et al., in press) have been associated with the ability to perceive asthma symptoms. Emerging research suggests that obesity may also contribute to variability in asthma symptom perception, particularly in overestimation of asthma symptoms. While some investigations have found actual differences in asthma severity between obese and normal weight individuals with asthma (e.g., Bibi et al., 2004), numerous studies of adults (Lavoie et al., 2006; Schachter et al., 2001; Sin et al., 2002; Thomson et al., 2003) and children (Belamarich et al., 2000; Bibi et al., 2004; Pianosi and Davis, 2004; Schachter et al., 2003; Wickens et al., 2005) with asthma found that obese individuals reported higher rates of asthma symptoms (e.g., wheezing and dyspnea) and asthma morbidity (e.g., activity limitation, compromised quality of life) than nonobese patients, while generally the obese groups did not have significantly more pulmonary compromise by objective measures such as Peak Expiratory Flow Rate (PEF) or Forced Expiratory Volume in 1 second (FEV1). Further evidence to suggest a relationship between body composition and symptom perception comes from the field of heartbeat perception, where overweight status has been associated with poorer perception (e.g., Rouse et al., 1988). Results from these reports suggest that obesity is a potentially important factor contributing to variability in asthma symptom perception, although more empirical research is needed.
The current study sought to build upon existing research in the association between perception of asthma symptoms and obesity by directly assessing the relationship between pediatric asthma symptom perception and obesity using an established symptom perception methodology. An additional aim was to explore the role of patient characteristics that may play a part in the relationship between obesity and symptom perception in children with asthma.
1.2. Methods
The current report describes a secondary data analysis from a study assessing symptom perception in children with asthma. A detailed description of the study protocol has been published previously (Klein et al., 2004) and is summarized below.
1.3. Participants
Study recruitment was accomplished through hospital clinics, doctor referrals, community advertising, and an asthma summer camp. Eligibility criteria included (1) age between 7 and 17 years, (2) presence of physician-diagnosed asthma for at least 6 months prior to enrollment, and (3) children and caregivers able to complete the study protocol in English. Exclusionary conditions included a pulmonary condition other than asthma (e.g., cystic fibrosis) and significant cognitive delay as indicated by school placement.
Participants included in this analysis were 200 children (mean age = 12 years, SD = 2.2) and a primary caregiver that was most often the biological mother (89%), but was sometimes the father (6%) or other adult (5%). Forty-seven percent of children were female. Average time since asthma diagnosis was 8.2 years (SD = 3.3). Almost one-third (30.5%) of participants were from ethnic/racial minority backgrounds, specifically Black/African American (11%), Hispanic/Latino (11%), or other/biracial (8.5%), while the remainder (69.5%) were white/non-Hispanic.
Data were collected at three U.S. sites: Brown Medical School (RI), University of Texas Medical School (TX), and National Jewish Medical and Research Center (CO). No site differences were found for age, gender or obesity status. There were site differences in asthma severity, with a higher proportion of moderate and severe cases in CO relative to TX and RI (Chi-square = 13.7, p < .05). Ethnic diversity also differed by site, with a smaller proportion of ethnic minorities in the RI sample relative to the other sites (Chisquare = 7.5, p < .05).
The sample for the current study constitutes a subgroup of participants that had valid symptom perception and BMI data. Forty-five subjects from the larger sample were excluded due to insufficient symptom perception data (details below). Nine additional children at the underweight level, defined as below the 5th BMI percentile, were excluded for the purposes of categorical analyses comparing obese to nonobese, normal weight children. The fifty-four excluded participants did not differ from included subjects on demographic variables (gender, ethnicity, SES, and age) or on asthma severity.
1.4. Procedures
Children with asthma and a primary caregiver (henceforth referred to as the parent) completed the informed consent/assent process in accordance with Institutional Review Board guidelines. Participants completed an initial study session that included questionnaires and orientation to the AM2 handheld spirometer, which children used at home for the subsequent 5–6 weeks. At the end of this monitoring period, families returned to the lab for a brief follow up visit and toreturntheAM2.Compensationwas provided at each session. All measures and methods are described below.
1.5. Measures
1.5.1. Sociodemographic information
Parents provided demographic information including the child’s age, gender, race/ethnicity, and parent occupation. A measure of socioeconomic status (SES) was derived from parent information using the National Opinion Research Council (NORC) rating of occupational prestige (Nakao and Treas, 1992). In households with two working caregivers the highest NORC score was used. Scores ranged from 21.9 to 86.1 (mean = 51.4, SD = 14.8), indicating a wide range of occupational prestige in this sample.
1.5.2. Body mass index (BMI)
Children’s height was measured using a standard wall chart. Weight was obtained using either calibrated scale measurement or parent report, when necessary. Body mass index and age- and sex-specific BMI percentiles were generated using the Centers for Disease Control and Prevention’s growth charts and accompanying SAS coding schemes (http://www.cdc.gov/nccdphp/dnpa/growth-charts). Using the most recent Expert Committee Recommendations by the American Academy of Pediatrics (Barlow, 2007), 59.5% of included children were normal weight (BMI ≧ 5% and <85%), 21% were overweight (BMI ≧ 85% and <95%) and 19.5% were obese (BMI ≧ 95%). Participants were classified into obese versus nonobese categories based on the 95th percentile for BMI. This cut off was used in accordance with studies documenting the substantially elevated risk of serious comorbidities associated with the obese classification (Cook et al., 2003, 2008; Calcaterra et al., 2008; Sen et al.,2008).Additional studies documenting significantly higher use of medical services in obese patients with asthma, with equivalent pulmonary function values between weight groups (e.g., Thomson et al., 2003; Belamarich et al., 2000) suggest that this classification is a meaningful way to explore potential links between body weight and asthma symptom perception.
1.5.3. Asthma severity
Two pediatric asthma specialists independently rated each child’s asthma severity in accordance with NIH criteria that were current at the time of the study (National Asthma Education and Prevention Program, 1997; National Institutes of Health, 2002). Rating discrepancies were minimal and reconciled by discussion. By this method 7.5% of children were classified as mild intermittent, 60% were mild persistent, 24% were moderate persistent and 5.5% were severe persistent.
1.5.4. Objective lung function
Lung function measurements were obtained using the AM2 handheld spirometer (Viasys, Yorba Linda, CA, USA). Peak expiratory flow (PEF) values recorded during each AM2 use across the assessment period were converted to “percent personal best”. The proportion of personal best values falling below 80% over total blows recorded was also calculated to provide an index of compromise across the assessment period.
1.5.5. Symptom perception assessment
In addition to recording objective lung function, the AM2 has the capacity to present pre-programmed questions and to store date/time stamped responses and corresponding spirometric values for later downloading. For the present study, a prompt appeared on the screen cueing children to enter a subjective assessment of their lung functioning at that moment in the form of a “guess” of their current PEF. Once the guess was entered children were prompted to complete three successive forced maximal exhalations, or ‘blows’. The best of three blows as determined by the highest PEF value was locked into the device with corresponding subjective data.
During the study visit, research staff oriented children and parents to the proper use of the AM2 using a standard study script and written instructions. Research assistants first demonstrated and then coached children to enter subjective peak flow ratings and to perform forced, sustained expirations with maximal effort into the device. Training continued until children demonstrated proficiency with both tasks. Children were instructed to use the device twice daily before asthma medications and additionally at any time they experienced asthma symptoms. During the at-home monitoring period participants received periodic phone or mail contacts to encourage daily use of the device and review proper blowing technique. Human subjects considerations precluded blinding the children to their peak flow measurements. Though this raises the possibility of a learning effect over the course of the monitoring period, previous studies using this modality have not found evidence of spontaneous learning secondary to study participation (Fritz et al., 1996).
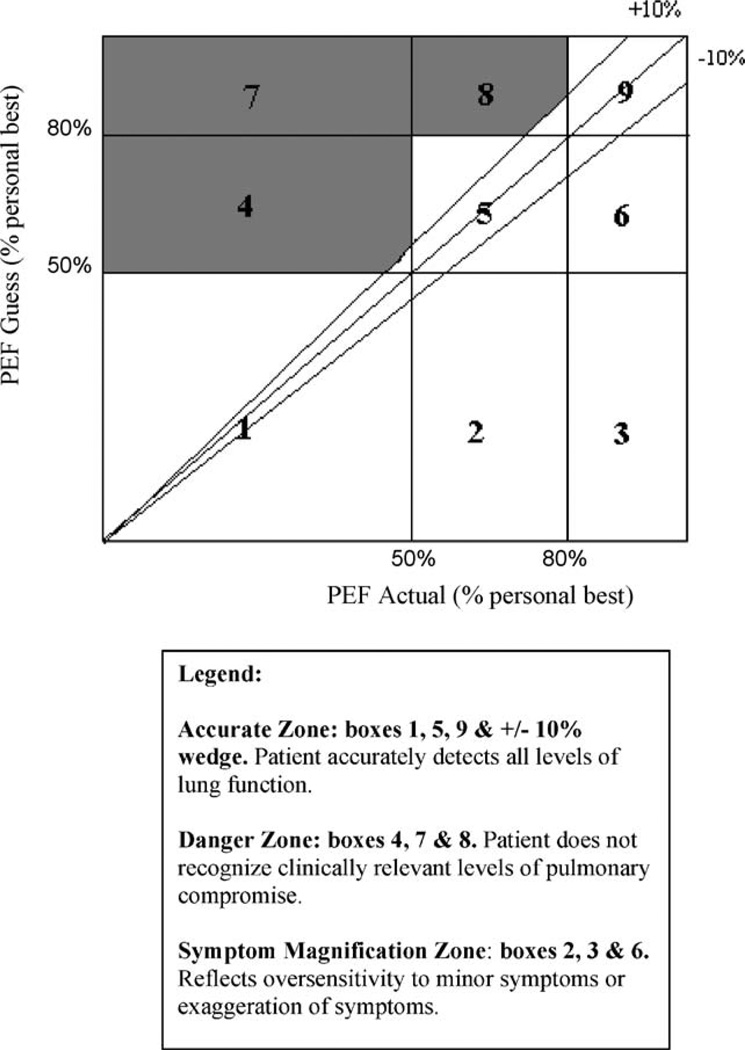
Multiple subjective–objective assessments were collected during the monitoring period and summarized using the Asthma Risk Grid, which has been described previously (Fritz et al., 1996; Klein et al., 2004) and appears in Fig. 1. Briefly, for each participant, subjective and objective values are converted to percentages of personal best and plotted on vertical and horizontal axes, respectively. Each point falls into one of three zones (Fig. 1). Accurate Zone (AZ) describes subjective assessments that closely match objective pulmonary functioning. The Symptom Magnification Zone (SMZ) reflects the overestimation of symptoms in the absence of objective compromise. The Danger Zone (DZ) represents a failure to recognize clinically significant pulmonary compromise. Each participant thus obtains three scores, which are the proportions of their blows falling into each of the three Grid zones. It is important to note when interpreting the Grid scores that they are interdependent in that they sum to 100%. Thus, decrements in a child’s Accurate Zone score will result in increases in Danger Zone and/or Symptom Magnification Zone scores.
Fig. 1.
The Asthma Risk Grid reproduced with permission from Oceanside Publications, Inc., from an article by Klein et al. (2004).
Prior to calculating Asthma Risk Grid scores, cases with fewer than 20 valid blows were excluded from the data set (n = 45). This cut off value was established via data simulations that revealed 20 to be the minimum number of blows needed to produce stable Grid scores. On average, children had forty-five valid subjective–objective data points across the assessment period (range = 21–122).
1.6. Data analysis
Probit transformations were applied to all proportional data, specifically the three Grid scores and the PEF summary variable, to normalize their distributions (Cohen and Cohen, 1983). Untransformed scores were retained for sample description. The analyses were undertaken in two steps. First, sociodemographic and asthma variables were assessed for their associations with symptom perception (AZ, DZ and SMZ) and obesity status (BMI above/below the 95th percentile) using the appropriate parametric (Pearson’s r, analyses of variance) and nonparametric (Chi-square) tests. Next, variables significantly associated with both obesity status and symptom perception were included in factorial analyses of variance (ANOVA) examining the relationship between BMI categories (obese versus nonobese) and each index of symptom perception. Potential interactions between obesity status and demographic variables were also assessed. An alpha level of .05 was used for all statistical tests. Cohen’s d effect size was calculated for differences between two means. Effect sizes for correlations were obtained via r-to-d conversions. Values can be interpreted as small (.2), medium (.5) or large (.8) according to Cohen’s (Cohen, 1988) guidelines. Eta squared – an estimate of variance in the DV accounted for by a given IV – was the index for factorial ANOVAs. The w index was used for chi squared analyses, interpreted as small (.1), medium (.3) or large (.5) (Cohen, 1988).
2. Results
2.1. Symptom perception
Participants averaged 72.5% of blows in the Accurate Zone (3–100%), 8% in the Danger Zone (0–78%), and 19.6% in the Symptom Magnification Zone (0–96.7%), the wide ranges demonstrating significant variation among subjects. Differences in symptom perception emerged by age, ethnic minority status, occupational prestige and asthma severity (Table 1). Specifically, age was positively related to Accurate Zone scores and negatively related to Symptom Magnification Zone scores, both indicating higher levels of perceptual accuracy with increasing age. The children from ethnic minority groups had significantly lower Accurate Zone scores and higher Symptom Magnification Zone scores than the white group. Higher occupational prestige was significantly related to higher Accuracy and lower Symptom Magnification. Proportion of blows below 80% of PEF was negatively related to Accuracy and positively related to Danger Zone scores and Symptom Magnification Zone scores, all indicating that decrements in lung function were related to less accurate symptom assessment. Accurate Zone scores differed across asthma severity levels. The Student-Neuman–Keuls post hoc analysis approached significance (p = .051); and the trend appeared to be that participants with mild persistent and moderate persistent asthma may have been, on average, more accurate than those with severe persistent asthma. Danger Zone and Symptom Magnification Zone scores did not differ by asthma severity level. There were no gender differences in symptom perception scores (Table 1).
Table 1.
Demographic and asthma variables by Grid zone scores†.
| Accurate | Danger | Symptom Magnification | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | F or r | ES†† | Mean (SD) | F or r | ES | Mean (SD) | F or r | ES | |
| Age in years | r = .21** | .43 | r = −.04 | .08 | r = −.23** | .47 | |||
| Child gender | |||||||||
| Female | 71.03 (24.27) | F(1,198)<1.0 | .09 | 8.31 (11.89) | F(1,198)<1.0 | .08 | 20.65 (23.73) | F(1,198)<1.0 | .06 |
| Male | 73.71 (23.30) | 7.65 (10.77) | 18.64 (22.63) | ||||||
| Ethnicity | |||||||||
| White | 77.00 (22.91) | F(1,198) = 19.35*** | .68 | 7.21 (11.28) | F(1,198) = 2.54 | .24 | 15.79 (20.84) | F(1,198) = 15.35*** | |
| Minority | 62.12 (24.65) | 9.68 (11.17) | 28.19 (25.75) | ||||||
| Occupational prestige | r = .24** | .49 | r = −.04 | .08 | r = −.20** | .41 | |||
| Asthma severity | |||||||||
| Mild intermittent | 65.08 (22.94) | F(3,182) = 3.17* | .22 | 13.06 (13.21) | F(3,182) = 2.25 | .19 | 21.86 (26.95) | F(3,182) = 1.44 | .15 |
| Mild persistent | 74.47 (23.71) | 7.35 (11.28) | 18.18 (23.08) | ||||||
| Moderate persistent | 75.69 (21.08) | 5.42 (6.82) | 18.88 (20.60) | ||||||
| Severe persistent | 55.05 (21.35) | 12.47 (18.77) | 32.48 (25.86) | ||||||
| AM2 measurements <80% PEF personal best | – | r = −.46*** | 1.04 | – | r = .36*** | .77 | – | r = .35*** | .75 |
p <.05;
p <.01;
p <.001.
Scores = percentage of total blows in each grid zone.
Effect sizes for correlations are Pearson’s r-to-d conversions. Cohen’s d represents effect sizes for difference between two means, and eta squared for differences among three or more means.
2.2. Obesity status
Thirty-nine children (19.5%) in the sample were categorized as obese. Differences in obesity status were found by age, ethnicity and gender (Table 2). Specifically, children at or above the 95th BMI percentile were significantly younger than children below the cut-off for obesity. Boys were more likely to be classified as obese than were girls. A larger proportion of ethnic minority participants were classified as obese relative to white participants. Obesity status was unrelated to occupational prestige, asthma severity, and proportion of AM2 blows below 80% of PEF personal best.
Table 2.
Demographic and asthma variables by obesity status.
| Obesity status | ||||
|---|---|---|---|---|
| <95th percentile | ≧95th percentile | F or χ2 | ES†† | |
| n† | 161 | 39 | ||
| Age in years, mean (SD) | 12.20 (2.23) | 11.20 (2.15) | F(1,198) = 6.38* | .45 |
| Gender, % | χ2 = 8.47** | .21 | ||
| Female | 89.2 | 10.8 | ||
| Male | 72.9 | 27.1 | ||
| Ethnicity, % | χ2 = 7.58** | .19 | ||
| White | 85.6 | 14.4 | ||
| Minority | 68.9 | 31.1 | ||
| Occupational prestige, mean (SD) | 51.69 (14.87) | 50.12 (14.84) | F(1,183)<1.0 | .11 |
| Asthma severity, % | χ2 = 3.26 | .13 | ||
| Mild intermittent | 84.6 | 15.4 | ||
| Mild persistent | 78.8 | 21.2 | ||
| Moderate persistent | 84.4 | 15.6 | ||
| Severe persistent | 60.0 | 40.0 | ||
| % AM2 measurements ≦ 80% PEF personal best, mean (SD) | 31.33 (27.91) | 39.14 (32.80) | F(1,192) = 2.26 | .27 |
p <.05;
p <.01;
p <.001.
Some analyses involved fewer cases due to missing data on selected variables.
Cohen’s d represents effect size for difference between two means. Effect sizes for χ2 analyses are expressed as w.
2.3. Asthma symptom perception and obesity status
Obesity status, ethnicity and age effects on symptom perception scores were assessed using analyses of variance. A preliminary set of analyses tested the 3-way interaction between age, ethnicity and obesity status, and the 2-way interaction between age and ethnicity. Both of these tests were nonsignificant (data not shown), therefore it was determined that age and ethnicity could be considered independently of each other in subsequent analyses.
To account for age in the analyses, the sample was split along the median age (11.95 years), and the relationship between BMI and Asthma Risk Grid scores (AZ, DZ, SMZ) controlling for ethnicity was examined for each age group, resulting in 6 separate analyses. This strategy rather than to conduct analyses with age as a covariate was adopted because of the close association between age and cognitive level, the latter of which is known to be related to asthma symptom perception accuracy (Koinis Mitchell et al., 2009).
Results appear in Table 3. For the younger age group, none of the Asthma Risk Grid scores differed by obesity status. Ethnicity was the only child characteristic significantly associated with symptom perception scores in this group. Specifically, ethnic minority children had lower Accuracy Zone scores than their white counterparts (means = 59.20 versus 75.38, respectively) as well as higher Symptom Magnification Zone scores (means = 31.16 versus 16.88, respectively). Neither obesity status nor ethnic minority status were related to Danger Zone scores.
Table 3.
Symptom perception by obesity status—participants below the median age.
| Accurate Zone | Danger Zone | Symptom Magnification Zone | ||||
|---|---|---|---|---|---|---|
| Mean (SE) Asthma Risk Grid scores, adjusted for ethnicity | ||||||
| BMI below 95th percentile (n = 75) | 69.76 (2.66) | 7.66 (1.40) | 22.59 (2.61) | |||
| BMI above 95th percentile (n = 25)† | 65.73 (4.76) | 11.69 (2.51) | 22.57 (4.67) | |||
| ANOVA results | ||||||
| BMI category | F(1,96) = 2.09 | ES†† = .02 | F(1,96) = 1.57 | ES = .02 | F(1,96)<1.0 | ES = .00 |
| Ethnic minority status | F(1,96) = 5.65* | ES = .05 | F(1,96)<1.0 | ES = .00 | F(1,96) = 7.29** | ES = .07 |
| BMI category × ethnic minority status | F(1,96) = 1.81 | ES = .02 | F(1,96)<1.0 | ES = .01 | F(1,96)<1.0 | ES = .01 |
p <.05;
p <.01;
p <.001.
Fifty-two percent (13/25) of the obese children in this age group were ethnic minorities.
Effect sizes are expressed as eta squared for the main effects and interactions.
Table 4 presents ANOVA results for the older age group. There was a significant interaction between obesity status and ethnicity for Accurate Zone scores (F(1,96) = 7.21, p < .01). Subsequent examination of mean accuracy scores by obesity status and ethnic group indicated that white children classified as obese had significantly lower Accurate Zone scores (mean = 58.00, SD = 27.96) than nonobese white children (mean = 80.67, SD = 20.19) (t = 2.89, p < .01, d = 1.08). In the ethnic minority group, children above the obesity cut-off did not differ significantly in Accurate Zone scores (mean = 77.74, SD = 19.05) relative to those below the cut-off (mean = 63.16, SD = 25.62) (t = −1.27, p > .05, d = .60). Regardless of ethnic group membership, there was a significant main effect of obesity status on Symptom Magnification Zone scores for children in this age group. Specifically, children classified as obese had greater symptom magnification scores than children classified as nonobese. The interaction between obesity classification and ethnic minority status in this group was a nonsignificant trend (F(1,96) = 3.51, p = .06). No significant results emerged between children in lower and higher weight groups for Danger Zone scores.
Table 4.
Symptom perception by obesity status, covarying ethnicity—participants above the median age.
| Accurate Zone | Danger Zone | Symptom Magnification Zone | ||||
|---|---|---|---|---|---|---|
| Mean (SE) Asthma Risk Grid scores, adjusted for ethnicity | ||||||
| BMI below 95th percentile (n = 86) | 76.64 (2.36) | 7.28 (1.13) | 16.08 (2.40) | |||
| BMI above 95th percentile (n = 14)† | 63.54 (6.27) | 10.21 (3.0) | 27.26 (6.37) | |||
| ANOVA results | ||||||
| BMI category | F(1,96) = 8.54** | ES†† = .08 | F(1,96)<1.0 | ES = .01 | F(1,96) = 4.42* | ES = .04 |
| Ethnic minority status | F(1,96)<1.0 | ES = .00 | F(1,96)<1.0 | ES = .00 | F(1,96)<1.0 | ES = .00 |
| BMI category × ethnic minority status | F(1,96) = 7.21** | ES = .07 | F(1,96)<1.0 | ES = .01 | F(1,96) = 3.51 | ES = .03 |
p <.05;
p <.01;
p <.001.
Forty-three percent (6/14) of the obese children in this age group were ethnic minorities.
Effect sizes are expressed as eta squared for the main effects and interactions.
3. Discussion
The primary purpose of this study was to examine the relationship between perception of asthma symptoms and obesity in children with asthma using a well-established symptom perception methodology. An additional aim was to explore the potential role of sociodemographic and asthma variables in the association between symptom perception and obesity. In preliminary analyses, ethnic minority status and child age were significantly related both to symptom perception and obesity status and were therefore included in subsequent factorial analyses.
We found significantly less optimal symptom perception skills among the older obese white youth in our sample, compared to their white counterparts who were not obese. Specifically, for this group, being classified as obese was associated with lower symptom accuracy and higher symptom magnification scores than their white peers that were not obese. Other researchers have also observed that obese individuals have more difficulties accurately recognizing and responding to symptoms of asthma. For instance, a survey of adult patients presenting to the ED for asthma exacerbations found that obese patients reported the severity of their symptoms as higher, and had taken more doses of quick-relief medications than patients who were not obese, despite having comparable markers of pulmonary function (Thomson et al., 2003). The relationship between obesity and asthma symptom magnification might be explained partially by physical deconditioning associated with obesity. Simply put, if people are “out of shape”, they may experience and/or perceive more breathing difficulty upon exertion, in the absence of an underlying respiratory illness. Clinically speaking, patients with asthma may misinterpret this cardiopulmonary deconditioning as “asthma”, and this leads to the problematic cycle of increasing sedentary time as a maladaptive effort to avoid asthma exacerbation. This effort, in turn, worsens obesity and weakens pulmonary status, thus increasing asthma vulnerability.
In our study, there was an intriguing interaction between obesity and ethnic minority status as a function of child age. For minority children in the older group, weight group classification was not significantly associated with accuracy or symptom magnification scores. Moreover, there was no association between obesity status and perception of asthma symptoms for either ethnic group among the younger group of children in our sample. This encourages further consideration of developmental and cultural factors that may affect the association between obesity and perceptual accuracy in children. For example, in minority groups, cultural and socioeconomic factors may exert stronger influences on asthma symptom perception than physiological processes such as obesity. Fear of asthma exacerbations has been found to be stronger in Hispanics relative to whites (McQuaid, 2008). Given the asthma disparities that exist in the U.S., minority children are more likely to have experienced an episode of severe asthma (their own or another’s) and be sensitized to the problems of getting timely medical care – both of which might lead to heightened symptom magnification scores. Further, poverty and inner-city living experienced by a disproportionately high number of minority families in the U.S. has been associated with worse asthma outcomes (Koinis-Mitchell et al., 2007). Hence, there may be many more competing demands in poorer environments (e.g., financial stability, exposure to violence) that may interfere with children’s ability to accurately gauge their asthma symptoms. In the current study, it is possible that symptom perception in the minority participants, who on average, had lower occupational prestige scores than the whites, was affected in this way.
A number of limitations of this study are noteworthy. Significant differences in Accuracy scores found between the two age groups might have been the result of differences in cognitive development rather than in symptom perception ability. Specifically, the younger age group may have had more difficulty with the abstract task of translating their subjective estimates into numerical values. Of note, we excluded participants with significant cognitive delays as evidenced by special education school placement in order to limit developmental/cognitive variability among our sample. Additionally, we were unable to consistently utilize objective weight measurements, and instead relied on a combination of scale measurement and parent report. While parent-report is a reasonable means of data collection (Goodman et al., 2000), objective measurement is a more appropriate and accurate approach (Sherry et al., 2007). Though some imprecision in parent reported child weight is assumed, in our sample it was likely minimized by our use of obesity categories. Underweight individuals were not included in the analyses, and while most research has focused on obesity and asthma, it appears that underweight children may also be an important area of further study (Kwon et al., 2006; Schachter et al., 2001). A substantially smaller proportion (19.5%) of participants in this study were classified as obese compared to those classified as nonobese. While the obesity rate in our sample was higher than in the general U.S. population, the low number of obese relative to nonobese study participants may have limited statistical power and/or increased variability in analyses stratified by age group, as evidenced by the marginal interaction between obesity status and ethnicity in the examination of symptom magnification in the older age group. Our analyses primarily featured categorical comparisons of minority versus white children. Combining ethnic minority groups into a single cohort diminishes the unique cultural complexities that may be involved in illness processes. Accordingly, further within-group studies of obesity, symptom perception and ethnicity that are prepared to more carefully examine cultural factors are encouraged. Finally, SES and minority status may be inherently confounded. Research enrolling larger numbers of minority participants across a wider range of income levels may be needed to address this issue.
Despite these limitations, the study provides novel data to support the observation that that obesity is related to overestimation of asthma symptoms and that this relationship may be moderated by patient characteristics such as ethnic background and child age. In our sample, obesity was associated with less accurate asthma symptom perception in the older white participants. The association between obesity and perception was not found in the younger children or in minority participants of any age, though the minority group had significantly less accurate perception and a higher rate of obesity than the white participants. This pattern of findings suggests that while weight reduction may be an important component of asthma treatment in overweight patients, the magnitude of improvement may differ by patient characteristics such as age and cultural group, and that other factors also need careful attention. Learning how to accurately monitor and appropriately respond to asthma symptoms is a priority for all patients, and appears to be a particular challenge for overweight individuals with asthma. Optimizing symptom perception skills and minimizing weight-related breathlessness will help overweight patients with asthma in their efforts to engage in physical activity and accomplish weight loss.
Acknowledgement
Funded by the National Heart Lung and Blood Institute, R01-HL45157 (G. Fritz, PI) and U01-HL072438 (G. Fritz & G. Canino, PIs).
Footnotes
Portions of this manuscript were presented at the International Society for the Advancement of Respiratory Psychophysiology 2007 Annual Meeting, Bristol, UK.
References
- Abramson NW, Wamboldt FS, Mansell AL, Carter R, Federico MJ, Wamboldt MZ. Frequency and correlates of overweight status in adolescent asthma. Journal of Asthma. 2008;45(2):135–139. doi: 10.1080/02770900701840246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apter AJ, Affleck G, Reisine ST, Tennen HA, Barrows E, Wells M, Willard A, ZuWallack RL. Perception of airway obstruction in asthma: sequential daily analyses of symptoms, peak expiratory flow rate, and mood. Journal of Allergy & Clinical Immunology. 1997;99(5):605–612. doi: 10.1016/s0091-6749(97)70020-4. [DOI] [PubMed] [Google Scholar]
- Barlow S. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120:S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- Belamarich PF, Luder E, Kattan M. Do obese inner-city children with asthma have more symptoms than nonobese children with asthma? Pediatrics. 2000;106:1436–1441. doi: 10.1542/peds.106.6.1436. [DOI] [PubMed] [Google Scholar]
- Bibi H, Shoseyov D, Feigenbaum D, Genis M, Friger M, Peled R, Sharff S. The relationship between asthma and obesity in children: is it real or a case of over diagnosis? Journal of Asthma. 2004;41(4):403–410. doi: 10.1081/jas-120026097. [DOI] [PubMed] [Google Scholar]
- Calcaterra V, Klersy C, Muratori T, Telli S, Caramagna C, Scaglia F, Cisternino M, Larizza D. Prevalence of metabolic syndrome (MS) in children and adolescents with varying degrees of obesity. Clinical Endocrinology. 2008;68(6):868–872. doi: 10.1111/j.1365-2265.2007.03115.x. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Cohen J, Cohen P. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum; 1983. [Google Scholar]
- Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third national health and nutrition examination survey, 1988–1994. Archives of Pediatrics and Adolescent Medicine. 2003;157(8):821–827. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- Cook S, Auinger P, Li C, Ford E. Metabolic syndrome rates in United States adolescents, from the National Health and Nutrition Examination Survey, 1999–2002. Journal of Pediatrics. 2008;152(2):165–170. doi: 10.1016/j.jpeds.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Davis S, Permutt Z, Permutt S, Naureckas E, Bilderback A, Rand C, Stein B, Krishnan J. Perception of airflow obstruction in patients hospitalized for acute asthma. Annals of Allergy, Asthma and Immunology. 2009;102(6):455–461. doi: 10.1016/S1081-1206(10)60117-2. [DOI] [PubMed] [Google Scholar]
- Dirks JF, Schraa JC. Patient mislabelling of symptoms and rehospitalization in asthma. Journal of Asthma. 1983;20(43) doi: 10.3109/02770908309070912. [DOI] [PubMed] [Google Scholar]
- Feldman JM, McQuaid EL, Klein RB, Kopel SJ, Nassau JH, Mitchell DK, Wamboldt MZ, Fritz GK. Symptom perception and functional morbidity across a 1-year follow-up in pediatric asthma. Pediatric Pulmonology. 2007;42(4):339–347. doi: 10.1002/ppul.20584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherman V, Rutherford GW. A meta-analysis of the effect of high weight on asthma. Archives of Disease in Childhood. 2006;91(4):334–339. doi: 10.1136/adc.2005.080390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288(14):1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- Forrest KY, Leeds MJ. Prevalence and associated factors of overweight among Mexican–American adolescents. Journal of the American Dietetic Association. 2007;107(10):1797–1800. doi: 10.1016/j.jada.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Fritz G, McQuaid E, Spirito A, Klein R. Symptom perception in pediatric asthma: relationship to functional morbidity and psychological factors. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:1033–1041. doi: 10.1097/00004583-199608000-00014. [DOI] [PubMed] [Google Scholar]
- Fritz G, McQuaid E, Kopel S, Seifer R, Klein R, Koinis-Mitchell D, Esteban C, Rodriguez-Santana J, Colon A, Alvarez M, Canino G. Ethnic differences in symptom perception: a factor in pediatric asthma disparities? American Journal of Respiratory and Critical Care Medicine. doi: 10.1164/rccm.200906-0836OC. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennuso J, Epstein L, Paluch R, Cerny F. The relationship between asthma and obesity in urban minority children and adolescents. Archives of Pediatrics and Adolescent Medicine. 1998;152(12):1197–1200. doi: 10.1001/archpedi.152.12.1197. [DOI] [PubMed] [Google Scholar]
- Glazebrook C, McPherson AC, Macdonald IA, Swift JA, Ramsay C, Newbould R, Smyth A. Asthma as a barrier to children’s physical activity: implications for body mass index and mental health. Pediatrics. 2006;118(6):2443–2449. doi: 10.1542/peds.2006-1846. [DOI] [PubMed] [Google Scholar]
- Goodman E, Hinden BR, Khandelwal S. Accuracy of teen and parental reports of obesity and body mass index. Pediatrics. 2000;106(1):52–58. doi: 10.1542/peds.106.1.52. [DOI] [PubMed] [Google Scholar]
- Guerra S, Wright AL, Morgan WJ, Sherrill DL, Holberg CJ, Martinez FD. Persistence of asthma symptoms during adolescence: role of obesity and age at the onset of puberty. American Journal of Resipiratory and Critical Care Medicine. 2004;170:78–85. doi: 10.1164/rccm.200309-1224OC. [DOI] [PubMed] [Google Scholar]
- Guyatt G, Juniper E, Griffith L, Feeny D, Ferrie P. Child and adult perceptions of childhood asthma. Pediatrics. 1997;99(2):165–168. doi: 10.1542/peds.99.2.165. [DOI] [PubMed] [Google Scholar]
- Jacobson J, Mellins R, Garfinkel R, Rundle A, Perzanowski M, Chew G, Andrews H, Goldstein I. Asthma, body mass, gender, and Hispanic national origin among 517 preschool children in New York City. Allergy. 2008;63:87–94. doi: 10.1111/j.1398-9995.2007.01529.x. [DOI] [PubMed] [Google Scholar]
- Klein RB, Walders N, McQuaid EL, Adams S, Yaros D, Fritz GK. The Asthma Risk Grid: clinical interpretation of symptom perception. Allergy and Asthma Proceedings. 2004;25(1):1–6. [PubMed] [Google Scholar]
- Koinis Mitchell D, McQuaid E, Seifer R, Kopel S, Nassau J, Klein R, Feldman J, Wamboldt M, Fritz G. Symptom perception in children with asthma: cognitive and psychological factors. Health Psychology. 2009;28:226–237. doi: 10.1037/a0013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koinis-Mitchell D, McQuaid EL, Seifer R, Kopel SJ, Esteban C, Canino G, Garcia-Coll C, Klein R, Fritz GK. Multiple urban and asthma-related risks and their association with asthma morbidity in children. Journal of Pediatric Psychology. 2007;32(5):582–595. doi: 10.1093/jpepsy/jsl050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs NF, Jacobson MS, American Academy of Pediatrics Committee on Nutrition Preventionof pediatric overweight and obesity. Pediatrics. 2003;112(2):424–430. doi: 10.1542/peds.112.2.424. [DOI] [PubMed] [Google Scholar]
- Kwon HL, Ortiz B, Swaner R, Shoemaker K, Jean-Louis B, Northridge ME, Vaughan RD, Marx T, Goodman A, Borrell LN, Nicholas SW, Harlem Children’s Zone Asthma Initiative Childhood asthma and extreme values of body mass index: the Harlem Children’s Zone Asthma Initiative. Journal of Urban Health. 2006;83(3):421–433. doi: 10.1007/s11524-006-9050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara M, Akinbami L, Flores G, Morgenstern H. Heterogeneity of childhood asthma among Hispanic children: Puerto Rican children bear a disproportionate burden. Pediatrics. 2006;117:43–53. doi: 10.1542/peds.2004-1714. [DOI] [PubMed] [Google Scholar]
- Lara M, Rosenbaum S, Rachelefsky G, Nicholas W, Morton SC, Emont S, Branch M, Genovese B, Vaiana ME, Smith V, Wheeler L, Platts-Mills T, Clark N, Lurie N, Weiss KB. Improving childhood asthma outcomes in the United States: a blueprint for policy action. Pediatrics. 2002;109(5):919–930. doi: 10.1542/peds.109.5.919. [DOI] [PubMed] [Google Scholar]
- Lavoie KL, Bacon SL, Labrecque M, Cartier A, Ditto B. Higher BMI is associated with worse asthma control and quality of life but not asthma severity. Respiratory Medicine. 2006;100(4):648–657. doi: 10.1016/j.rmed.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Magadle R, Berar-Yanay N, Weiner P. The risk of hospitalization and near-fatal asthma in relation to the perception of dyspnea. Chest. 2002;121(2):329. doi: 10.1378/chest.121.2.329. [DOI] [PubMed] [Google Scholar]
- Main J, Moss-Morris R, Booth R, Kaptein AA, Kolbe J. The use of reliever medication in asthma: the role of negative mood and symptom reports. Journal of Asthma. 2003;40(4):357–365. doi: 10.1081/jas-120018635. [DOI] [PubMed] [Google Scholar]
- McQuaid E. Pediatric asthma management: Culture and context; Paper Presented at the 15th Annual Meeting, International Society for the Advancement of Respiratory Psychophysiology; 2008. [Google Scholar]
- McQuaid EL, Koinis Mitchell D, Walders N, Nassau JH, Kopel SJ, Klein RB, Wamboldt MZ, Fritz GK. Pediatric asthma morbidity: the importance of symptom perception and family response to symptoms. Journal of Pediatric Psychology. 2007;32(2):167–177. doi: 10.1093/jpepsy/jsj112. [DOI] [PubMed] [Google Scholar]
- National Asthma Education and Prevention Program. National Heart, Lung, and Blood Institute; 1997. Expert Panel Report 2 Guidelines for the diagnosis and management of asthma. [Google Scholar]
- National Asthma Education and Prevention Program. Bethesda, MD: US Department of Health and Human Services, National Institutes of Health; 2007. Expert Panel Report 3: guidelines for the diagnosis and management of asthma. [Google Scholar]
- Nakao K, Treas J. The 1989 socioeconomic index of occupations: construction from the 1989 occupational prestige scores. GSS Methodological Report. 1992:74. [Google Scholar]
- National Institutes of Health. Update on Selected Topics. Bethesda, MD: National Institutes of Health; 2002. Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma. [Google Scholar]
- Pèrez-Perdomo R, Pèrez-Cardona C, Disdier-Flores O, Cintrón Y. Prevalence and correlates of asthma in the Puerto Rican population: behavior risk factor surveillance system, 2000. Journal of Asthma. 2003;40(5):465–474. doi: 10.1081/jas-120018713. [DOI] [PubMed] [Google Scholar]
- Pianosi PT, Davis HS. Determinants of physical fitness in children with asthma. Pediatrics. 2004;113(3 Pt 1):e225–e229. doi: 10.1542/peds.113.3.e225. [DOI] [PubMed] [Google Scholar]
- Rouse C, Jones G, Jones K. The effect of body composition and gender on cardiac awareness. Psychophysiology. 1988;25(4):400–407. doi: 10.1111/j.1469-8986.1988.tb01876.x. [DOI] [PubMed] [Google Scholar]
- Schachter LM, Salome CM, Peat JK, Woolcock AJ. Obesity is a risk for asthma and wheeze but not airway hyperresponsiveness. Thorax. 2001;56(1):4–8. doi: 10.1136/thorax.56.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter LM, Peat JK, Salome CM. Asthma and atopy in overweight children. Thorax. 2003;58(12):1031–1035. doi: 10.1136/thorax.58.12.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen Y, Kandemir N, Alikasifoglu A, Gonc N, Ozon A. Prevalence and risk factors of metabolic syndrome in obese children and adolescents: the role of the severity of obesity. European Journal of Pediatrics. 2008;167(10):1183–1189. doi: 10.1007/s00431-007-0658-x. [DOI] [PubMed] [Google Scholar]
- Sherry B, Jefferds ME, Grummer-Strawn LM. Accuracy of adolescent self-report of height and weight in assessing overweight status: a literature review. Archives of Pediatrics and Adolescent Medicine. 2007;161(12):1154–1161. doi: 10.1001/archpedi.161.12.1154. [DOI] [PubMed] [Google Scholar]
- Shore SA. Obesity and asthma: possible mechanisms. Journal of Allergy and Clinical Immunology. 2008;121(5):1087–1093. doi: 10.1016/j.jaci.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Sin DD, Jones RL, Man SF. Obesity is a risk factor for dyspnea but not for airflow obstruction. Archives of Internal Medicine. 2002;162(13):1477–1481. doi: 10.1001/archinte.162.13.1477. [DOI] [PubMed] [Google Scholar]
- Spear BA, Barlow SE, Ervin C, Ludwig DS, Saelens BE, Schetzina KE, Taveras EM. Recommendations for treatment of child and adolescent overweight and obesity. Pediatrics. 2007;120 Suppl. 4:S254–S288. doi: 10.1542/peds.2007-2329F. [DOI] [PubMed] [Google Scholar]
- Story RE. Asthma and obesity in children. Current Opinion in Pediatrics. 2007;19(6):680–684. doi: 10.1097/MOP.0b013e3282f1ddfa. [DOI] [PubMed] [Google Scholar]
- Strunk R, Mrazek DA, Wofson, Fuhrmann GS, LaBreque JF. Physiological and psychological characteristics associated with deaths due to asthma in childhood: a case-controlled study. Journal of the American Medical Association. 1985;254:1193–1198. [PubMed] [Google Scholar]
- Sturdy PM, Victor CR, Anderson HR, Bland JM, Butland BK, Harrison BD, Peckitt C, Taylor JC, Mortality and Severe Morbidity Working Group of the National Asthma Task, F Psychological, social and health behaviour risk factors for deaths certified as asthma: a national case–control study. Thorax. 2002;57(12):1034–1039. doi: 10.1136/thorax.57.12.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantisira KG, Litonjua AA, Weiss ST, Fuhlbrigge AL. Association of body mass with pulmonary function in the Childhood Asthma Management Program (CAMP) Thorax. 2003;58:1036–1041. doi: 10.1136/thorax.58.12.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B, Mannino D, Brown C, Crocker D, Twum-Baah N, Holguin F. Body mass index and asthma severity in the National Asthma Survey. Thorax. 2008;63(1):14–20. doi: 10.1136/thx.2007.082784. [DOI] [PubMed] [Google Scholar]
- Thomson CC, Clark S, Camargo CA, Jr, Marc Investigators Body mass index and asthma severity among adults presenting to the emergency department. Chest. 2003;124(3):795–802. doi: 10.1378/chest.124.3.795. [DOI] [PubMed] [Google Scholar]
- von Mutius E. Environmental factors influencing the development and progression of pediatric asthma. Journal of Allergy & Clinical Immunology. 2002;109(6 Suppl.):S525–S532. doi: 10.1067/mai.2002.124565. [DOI] [PubMed] [Google Scholar]
- Weiss S, Shore S. Obesity and asthma. American Journal of Respiratory and Critical Care Medicine. 2004;169:963–968. doi: 10.1164/rccm.200303-403WS. [DOI] [PubMed] [Google Scholar]
- Wickens K, Barry D, Friezema A, Rhodius R, Bone N, Purdie G, Crane J. Obesity and asthma in 11–12 year old New Zealand children in 1989 and 2000. Thorax. 2005;60(1):7–12. doi: 10.1136/thx.2002.001529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoos HL, Kitzman H, McMullen A, Sidora K. Symptom perception in childhood asthma: how accurate are children and their parents? Journal of Asthma. 2003;40(1):27–39. doi: 10.1081/jas-120017204. [DOI] [PubMed] [Google Scholar]