Abstract
In 2007 the Association of Research-Based Pharmaceutical Companies (vfa) published recommendations to improve the quality and transparency of non-interventional studies. These recommendations include quality assurance measures, in particular with respect to transparency as well as for the verification of the data collected in these studies. This publication presents the results of a survey on fees in non-interventional studies which was conducted within the member companies of the vfa in June 2011. These results demonstrate a consistent adherence to the statutory requirements and the implementation of the recommendations concerning the remuneration of the study centers. Depending on the indication, the number of routine doctor/patient contacts is different and associated with that number the documentation efforts vary. Accordingly, the fee varies based on the fee schedule for physicians (German: Gebührenordnung für Ärzte) by taking into account the actual efforts at the study center.
Abstract
Der Verband der forschenden Pharma-Unternehmen (vfa) hat 2007 Empfehlungen zur Verbesserung der Qualität und Transparenz von nichtinterventionellen Studien veröffentlicht. Darin sind Qualitätssicherungsmaßnahmen, insbesondere zur Verifizierung der erhobenen Daten und der Transparenz wesentliche Bestandteile. In dieser Publikation werden die Ergebnisse der Umfrage zur Honorierung bei AWB aus dem Juni 2011 bei den Mitgliedsfirmen des vfa dargestellt. Es zeigt sich eine konsequente Einhaltung der gesetzlichen Vorgaben sowie die Umsetzung der Empfehlungen betreffs der Honorierung der Studienzentren. Je nach Indikation ist die Zahl der routinemäßigen Arzt/Patientenkontakte verschieden; analog ist der Dokumentationsaufwand variabel. Entsprechend variiert das Honorar auf Grundlage der Gebührenordnung für Ärzte und unter Berücksichtigung des tatsächlichen Aufwandes des Studienzentrums.
Introduction
Non-interventional studies (NIS) and “Anwendungsbeobachtungen” (AWB), which are included in this study class, have been a fixed and important component of medical research after the licensing of a medicine for decades. However, AWB are repeatedly the subject of public discussion as the criticism of this tool is almost as old as the AWB itself. The question which is repeatedly asked is whether the AWB really represents necessary healthcare research with licensed medicines or whether these are merely studies without any scientific value. The remuneration of the medical doctors for the work they provide in the scope of an AWB is also often the main focus of discussions: AWB are there to initiate prescriptions in the interests of the pharmaceutical industry and are therefore legalised corruption. “Anwendungsbeobachtungen” should therefore be banned [1], [2], [3], [4].
However, this perspective largely masks out the development in the AWB field and fails to appreciate that an AWB is an important tool for generating knowledge on the pharmacovigilance of a medication and its licensed use. The importance of AWB as a tool for generating this knowledge is confirmed in the documentation of a scientific meeting at the Federal Institute for Drugs and Medical Devices (Bundesinstitut für Arzneimittel und Medizinprodukte; 2006 [5]), in a publication of the Federal Ministry of Health [6] and in a statement on behalf of the Drug Commission of the German Medical Association [Arzneimittelkommission der deutschen Ärzteschaft] [7].
The aim to ensure quality and transparency of AWB was reached with successive statutory changes to Article 67 (6) of the German Drug Law [Arzneimittelgesetz (AMG)] [8]. Beginning in 1989 (important changes enforced in August 2004 and July 2009) all studies, which collect information from the use of licensed or registered drugs, must be notified to the Federal health insurance associations [kassenärztliche Bundesvereinigungen], the National Association of Health Insurance Funds [Spitzenverband Bund der Krankenkassen] as well as the responsible National Competent Authorities. In addition, the location, time, aims and observation plan of the study as well as the doctors participating in the study must be named. In this connection, the German Drug Law also includes: “According to its type and amount, remuneration which is paid to doctors for their participation in examinations according to sentence 1, is to be measured in such a way that there is no incentive to prescribe or recommend certain medicines.” [8]
Nearly at the same time as this topic developed, and partly even before the statutory regulations, the pharmaceutical industry acted independently in this regard. According to the guidelines of the “Freiwillige Selbstkontrolle für die Arzneimittelindustrie e.V.” (FSA) Code of Conduct “Healthcare Professionals” [9], the remuneration for NIS/AWB must be in an appropriate ratio to the services to be provided. In terms of the amount of remuneration, Article 19 (2), No. 7 applies with the proviso that this is to be measured in a way which ensures there is no incentive to prescribe the medication. The remuneration must be measured based on the German Scale of Medical Fees [Gebührenordnung für Ärzte (GOÄ)] [10] and the execution of the study may not influence the therapy, prescription and procurement decisions.
In 2007, the Association of Research-Based Pharmaceutical Companies (vfa) published its recommendations to improve further the quality and transparency of non-interventional studies [11]. These recommendations include quality assurance measures, particularly on the verification of the data collected and the transparency.
In two surveys carried out amongst the member companies of the vfa, the implementation of the vfa recommendations in 2008 and 2010 was investigated and the results were compared [12], [13]. Regular, internal quality control measures are applied in the planning, implementation and analysis phase [14], [15]. Almost 1/3 of the companies carried out quality controls on site.
The results of the current survey amongst the member companies of the vfa on remuneration for AWB in June 2011 also show the consistent compliance with statutory guidelines as well as the implementation of the recommendations with regard to the payment of the study centres. Depending on the indication, the number of doctor/patient contacts varies; the documentation required also varies in line with this. The remuneration is calculated based on the German Scale of Medical Fees and the actual work provided by the study centre.
Background
The term “Anwendungsbeobachtung” (AWB) was established in 1986 by the then newly introduced para. 6 in Article 67 of the German Drug Law and since then, examinations with varying objectives using licensed medicines have been assigned the term AWB. The term was explicitly used in official regulations in 1989 in section 5.1 of the Drug Testing Guidelines [Arzneimittelprüfrichtlinien] [16] when counting the possible forms of scientific findings in the official evaluation of the effectiveness and harmlessness of drugs with a known agent. In comparison to this, AWB have been explicitly excluded from the regulatory scope of the Directive 2001/20/EC of the European Parliament and the Council from 4th April 2001 on clinical trials [17] and therefore do not present any form of human clinical study pursuant to Article 4, para. 23, sentence 1 of the German Drug Law [8]. However, the German Drug Law does use the term non-interventional examination in Article 4, para. 23. This definition clearly demonstrates that AWB is to be considered as a subset of NIS in accordance with the respective definition in Directive 2001/20/EC Article 2 (c) (“non-interventional trial”).
The purpose of an AWB is to gain information and experiences on the use of a certain, licensed medicine in everyday conditions, i.e. on risks and side effects and on the effectiveness of a medicinal product, which ultimately guarantees patient safety.
In addition to this, a product monitoring obligation exists for the pharmaceutical entrepreneur as a manufacturer of drugs (“Public safety obligation”; see Article 84 of German Drug Law [8], Article 1 of the Product Liability Act [18] and Article 823 of the German Civil Code [19]). This means an obligation to observe the circumstances of the use as well as the users (doctor and patient) of the drugs. AWB are particularly important as regards exercising this obligation to observe the product and consumer behaviour.
Furthermore, in recent years the licensing authorities have been increasingly promoting “Post-Authorisation Safety Studies” (PASS) [20] or “Post-Authorisation Efficacy Studies” (PAES) [21]. Both licensing requirements can often also be met with NIS/AWB.
According to Article 67 (6) of the German Drug Law [8], the National Association of Statutory Health Insurance Physicians, the National Association of Statutory Health Insurance Funds as well as the responsible National Competent Authorities are to be informed about all studies which collect information from the use of licensed or registered medicines, i.e. all AWB. In addition, the location, time, objective and observation plan of the AWB are to be indicated as well as the participating doctors. According to its type and amount, remuneration, which is paid to doctors for their participation in the studies according to sentence 1, is measured in such a way that there is no incentive to prescribe or recommend certain drugs. Should participating doctors provide services at the expense of statutory health insurance, the statements according to sentence 1 must also indicate the type and amount of remuneration paid to them as well as a copy of the contracts concluded with them. The statutory regulation therefore creates maximum transparency vis-a-vis the authorities and service providers in the statutory health insurance, including the amount of remuneration paid.
Alongside the legislator, the “Freiwillige Selbstkontrolle für die Arzneimittelindustrie e.V.” (FSA), which was founded in 2004 and to which all regular member companies of the vfa belong, has been dealing with the topic of remunerating AWB from the very beginning of its work. According to the FSA Code of Conduct “Healthcare Professionals” [9], the remuneration for AWB must be in an appropriate ratio to the services to be provided. As regards the amount of remuneration, Article 19, para. 2, No. 7 applies with the proviso that the remuneration is measured in such a way to ensure there is no incentive to prescribe a medicine. Therefore, AWB may not influence medical prescribing behaviour. Given that conducting an AWB is associated with additional work (e.g. separate documentation; patient clarification about the use of the health data collected), appropriate remuneration is justified and authorised. The legislator does not mention concrete figures. The payment must be based on the “extent of the respective work”. This regulation should exclude AWB being abused to influence therapy, regulation and procurement decisions [22]. Varying amounts of remuneration can be explained by different requirements for the participating study centres according to the field of indication and the associated documentation work.
The FSA made an important decision with regards to the amount of remuneration for AWB:
-
Resolution from 09.02.2009 on Ref. No.: FS II 5/08/2007.12-217 [23] [In line with German Scale of Medical Fees] – “An hourly rate of € 75.00 (according to point 85 attachment to the German Scale of Medical Fees, per working hour started) is, in principle, appropriate for calculating the remuneration of medical activities in an AWB in accordance with Article 19 para. 5 in connection with Article 18, para. 3 (FSA Code of Conduct 2006) and Article 19 para. 2 No. 7 in connection with Article 18, para. 1 No. 6 (FSA Code of Conduct Health Professionals, 2008). An hourly rate of € 150.00 is, without a doubt, unreasonably high.
Breaks, which take into account the average work of the doctors, are practical and conform with the code of conduct. The agreed breaks, however, must be in an appropriate ratio to the doctors’ services (to be) provided. When reviewing, an average time for the individual services is to be assumed and this afterwards multiplied with the suitable hourly rate.
According to the FSA Code of Conduct, when reviewing the agreed remuneration, including an hourly rate subject to a flat-rate fee, the Scale of Medical Fees offers a reference point for doctors, even if this is outdated and requires amendment. In particular, lump-sum amounts which are within the scope of the Scale of Medical Fees conform with the Code of Conduct. As, according to the FSA Code of Conduct, appropriate hourly rates can also be agreed in order to take the time worked into account, flat-rate fees are correspondingly in line with Code of Conduct if they are based on suitable hourly rates.”
“Generally, the remuneration is stipulated under application of point 85 of the Scale of Medical Fees. According to this, the single fee per working hour started is € 29.14 for an expert statement in writing which requires work exceeding the standard (more than 20 minutes). The level of difficulty of the activity shall also be taken into account in the form of a corresponding multiplier, according to Article 5, para. 2 of the German Scale of Medical Fees [10]. Based on a rate which is 2–3 times the hourly rate, one hour of work could therefore be paid € 67.00 in the scope of an AWB. The arbitration board of the FSA deemed an hourly payment of € 75.00 to be appropriate.”
Two other decisions by the FSA are also important in this context:
Resolution from 07.05.2007 on Ref. No.: 2005.9-92 [Remuneration only in cash] – “Competitive practices are unfair if, by remunerating the participation in an AWB, a non-member promises the participating doctor payments in kind or other benefits completely at the discretion of the doctor. When reviewing the question as to whether a certain competitive practice is unfair pursuant to Article 4, No. 11 of the Law against Unfair Competition (UWG), the Code of Conduct is important in as much as it represents an index for which competitive behaviour is deemed as unfair according to the opinion of the persons involved.”
Resolution from 17.02.2006 on Ref. No.: 2005.8-87 [No influence on prescriptions] – “Conducting an AWB unauthorised if the project documentation already mentions that discontinuing or changing to another preparation is necessary as a requirement for participation. The principles of the German Scale of Medical Fees are to be consulted when reviewing the suitability for remuneration when conducting an AWB. In this respect, the amount of remuneration is not based on the general market standard for remuneration.”
Methods
In June 2011, the vfa companies were asked in a comprehensive survey how they paid AWB between 2008 and 2010, which standards were set and what other aspects were considered for the remuneration. The questions related to the indication, the number of doctors involved (not differentiated by activity in practice, hospital or specialty), the time spent and documentation work for a complete patient documentation, the number of doctor/patient meetings, the duration of the AWB per patient and the remuneration paid.
Survey
By mid July 2011, responses from 18 of the 44 member companies had been received; the 8 extraordinary member companies do not yet have any medication on the market and therefore could not have conducted an AWB. This corresponds to a return rate of 50% (18 out of 36 companies able to conduct an AWB). 17 out of these 18 companies returned completed questionnaires.
One company declared remuneration with an hourly rate of € 75.00 (according to point 85 attachment to German Scale of Medical Fees [17]) per working hour started without the use of the questionnaire. The time required for the documentation is said to be assessed and determined in advance. This evaluation is carried out by means of a test run with doctors and nonmedical staff in potential study centres. Remuneration which is higher than that in the test runs is therefore excluded.
Based on the 17 completed questionnaires, three companies indicated that they had not conducted any AWB in the time frame concerned. The following illustration is therefore based on the statements of 14 companies which conducted AWB in Germany between 2008 and 2010. This gives a return rate of 47%.
The survey collected data on 126 AWB from the years 2008, 2009 and 2010.
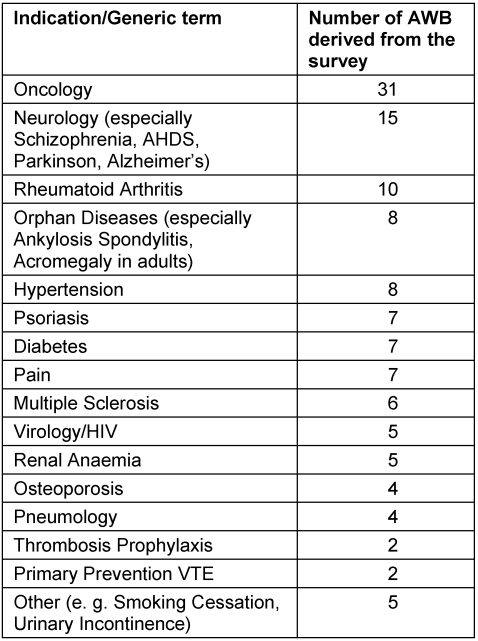
The AWB were conducted primarily in oncology (31), neurology (15) and rheumatoid arthritis (10), but also AWB were performed e.g. in multiple sclerosis, hypertension and in the field of virology (HIV) – see Table 1 (Tab. 1) for details.
Table 1. Illustration of the indications/diseases that were considered in the recorded survey of the AWB.
At the end of December 2010, 196 AWB were recorded on the website of the vfa register (http://www.vfa.de/nisdb) [24]. Furthermore, at the same time, 141 AWB in Germany were registered by vfa companies under http://www.clinicaltrials.gov/. The survey therefore reflected 38% of the AWB carried out placed by vfa member companies in a publically accessible register according to the transparency guidelines of the vfa voluntary self-regulation obligation.
Remuneration
The recommendations of the FSA/vfa as well as the German Drug Law stipulate that “remuneration which is paid to doctors for their participation in examinations is to be measured in such a way that ensures there is no incentive to prescribe or recommend a certain drug” [8], [9]. All companies stated that they used the Scale of Medical Fees from 2002 [10], in accordance with the FSA Code of Conduct, as a basis for calculating the remuneration.
The results of the survey show that the amount of remuneration is based on the actual service provided by the study centre, which comprises the work of the doctor and non-medical staff:
Documentation work with the help of the record sheets;
Time spent informing/notifying the participating patient or the patient addressed about possible participation, particularly about data protection aspects with regard to the transfer or processing of data collected by the client;
Explanations, for example, of the patient surveys, collecting these and checking to ensure they are complete by the client;
Time spent carrying out a quality control of the data collected by the client, in cases in which a quality control is actually carried out.
None of the companies provided for remuneration without considering the documentation and informative work actually provided. In all cases, the remuneration was paid in accordance with the fully documented visits and/or complete documentation surveys, whereby the remuneration was dependent on the duration of the AWB and the complexity of the documentation.
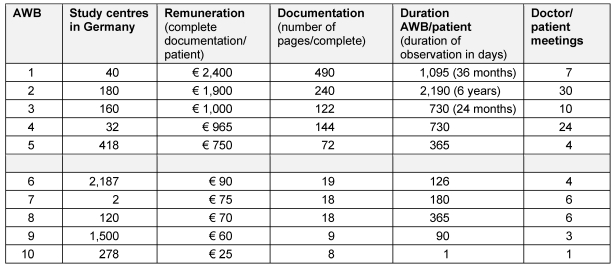
The survey recorded AWB with documentation requirements of between 8 and 490 DIN A4 pages per complete patient documentation. This range shows the very different approaches (and the differing level of work required for the doctor/the study centre as a result) between the individual AWB. Accordingly the remuneration was also different; from € 25 in an AWB with a documentation requirement of 8 pages with one doctor/patient contact and € 2,400 in an AWB with 490 pages of complete documentation with seven visits in 36 months. This AWB not only examined the use of the drug but also the resources necessary and the specific costs for the standard therapy (therapies) in the indication examined in Germany. This involved approx. 70 pages of documentation per doctor/patient contact and also contained questionnaires for patients and involved relatives.
The observation period in AWB is also very different. The range recorded varied between one doctor/patient contact (visit), right through to examinations which monitor patients with a chronic illness over 15 years. In some answers (5), it was highlighted that in case of incomplete documentation, no remuneration for the doctor/study centre is stipulated, i.e. the remuneration is only paid with complete patient documentation. Table 2 (Tab. 2) presents 5 AWB with the highest and lowest remuneration.
Table 2. Illustration of five AWB with the highest and lowest remuneration.
Overall on average for 126 AWB and complete documentation of a patient a reward of € 339 was paid, this documentation expenditure encompasses 41 pages of documentation and 4 doctor-patient contacts in general.
Discussion
The remuneration cannot be viewed independently of suitable quality assurance measures as these contribute substantially to the work at a study centre. Therefore, in this context recommendations which focus on the quality [11], [25], [26], [27], [28], [29] and transparency [11], [30] in NIS and AWB must be highlighted. Already in 1998, the Federal Institute for Drugs and Medical Devices published recommendations for planning, implementing and analysing AWB [25], which were to be considered as state-of-the-art at this time. These recommendations were revised together with the Paul Ehrlich Institute and published in July 2010 [26]. They also refer to applicable conventional quality requirements for epidemiological studies. These include the guidelines and recommendations for safeguarding Good Epidemiological Practice (GEP) [27] published in April 2004 by the Working Group of Epidemiological Methods of the German Association of Epidemiology [Deutsche Arbeitsgemeinschaft für Epidemiologie] (DAE). They document fundamental aspects on establishing quality standards in order to achieve valid research results. According to these recommendations quality assurance of all relevant tools and procedures must be an essential component of any epidemiological study and thus also of all AWB. Furthermore the handbook “Registries for Evaluating Patient Outcomes” of the AHRQ (Agency for Healthcare Research and Quality – one of 11 operative units of the American Health Ministry) [28] recommends quality assurance measures also at study centres.
Quality assurance measures are also essential components of the self-regulation obligation of the vfa published in May 2007 [11]. In line with this, the Federal Association of the Pharmaceutical Industry [Bundesverband der Pharmazeutischen Industrie] (BPI) presented its “Points to Consider” for AWB [29] in 2009.
In vfa surveys as well as in peer reviewed articles [12], [13] quality control and quality assurance measures are described, for example when verifying the data collected, during data entry and data acquisition as well as for the statistical analysis [14], [31]. These articles and publications indicate that a high degree of trust in the validity of the data recorded in NIS/AWB and the results obtained from NIS/AWB is justified [15]. In addition, publishing information on the conduct of NIS/AWB together with the study results in publicly accessible internet portals serves to ensure transparency in this research sector. Study results are to be presented in accordance with the recommendations of the STROBE statement (Strengthening the Reporting of Observational Studies in Epidemiology) [30].
The return rate in this survey was at 47% and included a vast majority of major pharmaceutical companies within the vfa. However, the survey results deem to be representative based on the number of AWB conducted by these companies.
The survey results show that the remuneration of AWB in the member companies of the vfa is generally based on the time required at the study centre and that it applies to the standards of the Scale of Medical Fees [10]. In accordance with no. 85 of the attachment of the Scale, all companies are calculating with a fee of € 75.00 per working hour. The parameters evaluated within this survey, i.e. compensation for a full set of patient documentation (in particular the number of case report sheets together with the number of doctor-patient contacts), there is no evidence for any deviation from this standard Higher documentation requirements may be caused and in most cases justified by the routine medical behaviour in a particular indication and associated with the number of doctor/patient contacts This is true in particular in indications such as oncology, neurodegenerative and mental illnesses and infectious diseases such as HIV.
Overall, the results of the survey confirm that the amount of remuneration is based on the actual work provided by the study centre or the medical doctor. Differences in the remuneration may result from partly more extensive documentation requirements, the informed consent process as well as participation in quality assurance measures such as source data verification at the study centre or the participation in study meetings.
Notes
Acknowledgement
We would like to thank the member companies of the vfa which have participated in this survey for their input, dedication and openness when discussing the questionnaires.
Conflicts of interest
Dr. Thorsten Ruppert is an employee of the vfa. Michael Hahn is an employee of Pfizer Pharma GmbH. Dr. Ferdinand Hundt is an employee of Sanofi-Aventis Deutschland GmbH.
References
- 1.Transparency International Deutschland e.V. Transparency International Deutschland fordert gesetzliches Verbot von "Anwendungsbeobachtungen". Nov 03, 2010. Available from: http://www.transparency.de/fileadmin/pdfs/Themen/Gesundheitswesen/Positionspapier_Anwendungsbeobachtungen_10-11-03.pdf.
- 2.Rücker D. Anwendungsbeobachtungen – Wissenschaft oder Marketing? Pharmazeutische Zeitung. 2009;41 Available from: http://www.pharmazeutische-zeitung.de/index.php?id=31248. [Google Scholar]
- 3.Lieb K, Brandtönies S. Eine Befragung niedergelassener Fachärzte zum Umgang mit Pharmavertretern. [A survey of german physicians in private practice about contacts with pharmaceutical sales representatives]. Dtsch Arztebl Int. 2010 Jun;107(22):392–398. doi: 10.3238/arztebl.2010.0392. (Ger). Available from: http://dx.doi.org/10.3238/arztebl.2010.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bausch J. Small gifts sustain sales. Dtsch Arztebl Int. 2010 Jun;107(22):390–391. doi: 10.3238/arztebl.2010.0390. Available from: http://dx.doi.org/10.3238/arztebl.2010.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bundesinstitut für Arzneimittel und Medizinprodukte. Die Bedeutung nicht interventioneller Studien für die Bewertung der Wirksamkeit und der Sicherheit von Arzneimitteln. BfArM im Dialog; 29.08.2006; Available from: http://www.bfarm.de/DE/BfArM/Termine-und-Veranstaltungen/Dialog_und_Sonstige/2006/060829-Dialog.html. [Google Scholar]
- 6.Federal Ministry of Health [Bundesgesundheitsministerium] Anwendungsbeobachtung: Transparenz herstellen, Missbrauch abstellen. Oct 2, 2009. (Press release; no. 103 [Pressemitteilung; Nr. 103). Available from: http://www.bmg.bund.de/fileadmin/redaktion/pdf_pressemeldungen/2009/091002-PM-anwendungsbeobachtung.pdf.
- 7.Wink K. Anwendungsbeobachtung in der ärztlichen Praxis. Stellungnahme im Auftrag der Arzneimittelkommission der deutschen Ärzteschaft. 2. Aufl. Berlin: 2010. Available from: http://www.akdae.de/Stellungnahmen/Weitere/20101218.pdf. [Google Scholar]
- 8.Arzneimittelgesetz in der Fassung der Bekanntmachung vom 12. Dezember 2005 (BGBl. I S. 3394), das zuletzt durch Artikel 1 der Verordnung vom 19. Juli 2011 (BGBl. I S. 1398) geändert worden ist. Available from: http://www.gesetze-im-internet.de/bundesrecht/amg_1976/gesamt.pdf.
- 9.FS Arzneimittelindustrie e.V. Kodex zur Zusammenarbeit von Pharmaunternehmen und medizinischen Fachkreisen des FSA ("Freiwillige Selbstkontrolle für die Arzneimittelindustrie e.V.") Available from: http://www.fs-arzneimittelindustrie.de/en/presse/pressemitteilungen/31032010-neuauflage-fsa-kodex-fachkreise/ [Google Scholar]
- 10.The German Scale of Medical Fees [Gebührenordnung für Ärzte (GOÄ) vom 9. Februar 1996]. Stand: 01.01.2002. Available from: http://www.aerztekammer-bw.de/10aerzte/05kammern/10laekbw/10service/30goae/volltext.pdf.
- 11.Verband der forschenden Pharma-Unternehmen e.V. VFA-Empfehlungen zur Verbesserung der Qualität und Transparenz von nicht-interventionellen Studien. Jan 31, 2007. Available from: http://www.vfa.de/embed/vfa-empfehlungen-zu-nis.pdf. [Google Scholar]
- 12.Hahn M, Bethke TD, Hecht A, Henn D, Ruppert T, Hundt F. Qualitätssichernde Maßnahmen in nicht-interventionellen Studien: Ergebnisse einer Umfrage unter den Mitgliedsunternehmen des Verbandes Forschender Arzneimittelhersteller. [Quality assurance measures in non-interventional studies: Results of a survey among the members of the Association of Research-Based Pharmaceutical Companies]. GMS Ger Med Sci. 2008;6:Doc12. (Ger). Available from: http://www.egms.de/en/journals/gms/2008-6/000057.shtml. [Google Scholar]
- 13.Hahn M, Ruppert T, Bethke TH, Hundt F. Results of a survey on applied quality standards in non-interventional studies among the members of the German Association of Research-based Pharmaceutical Companies. GMS Ger Med Sci. 2010;8:Doc29. doi: 10.3205/000118. Available from: http://dx.doi.org/10.3205/000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Theobald K, Capan M, Herbold M, Schinzel S, Hundt F. Quality assurance in non-interventional studies. GMS Ger Med Sci. 2009;7:Doc29. doi: 10.3205/000088. Available from: http://dx.doi.org/10.3205/000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wörz K, Hundt F. Results of a quality control on non-interventional studies. GMS Ger Med Sci. 2011;9:Doc21. doi: 10.3205/000144. Available from: http://dx.doi.org/10.3205/000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bekanntmachung der Neufassung der Allgemeinen Verwaltungsvorschrift zur Anwendung der Arzneimittelprüfrichtlinien vom 5. Mai 1995. Bundesanzeiger. 1995 May 20;96a [Google Scholar]
- 17.Richtlinie 2001/20/EG des Europäischen Parlaments und des Rates vom 4. April 2001 zur Angleichung der Rechts- und Verwaltungsvorschriften der Mitgliedstaaten über die Anwendung der guten klinischen Praxis bei der Durchführung von klinischen Prüfungen mit Humanarzneimitteln. Official Journal of the European Communities. 2001 May 1;L 121:34–44. Available from: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32001L0020:DE:NOT. [Google Scholar]
- 18.Gesetz über die Haftung für fehlerhafte Produkte (Produkthaftungsgesetz – ProdHaftG) Available from: Dec 15, 1989. Available from: http://www.gesetze-im-internet.de/bundesrecht/prodhaftg/gesamt.pdf. [Google Scholar]
- 19.Bürgerliches Gesetzbuch in der Fassung der Bekanntmachung vom 2. Januar 2002 (BGBl. I S. 42, 2909; 2003 I S. 738), das zuletzt durch Artikel 1 des Gesetzes vom 27. Juli 2011 (BGBl. I S. 1600) geändert worden ist. As of: Neugefasst durch Bek. v. 2.1.2002 I 42, 2909; 2003, 738; zuletzt geändert durch Art. 1 G v. 27.7.2011 I 1600. Available from: http://www.gesetze-im-internet.de/bundesrecht/bgb/gesamt.pdf.
- 20.Regulation (EU) No. 1235/2010 of the European Parliament and of the Council of 15 December 2010 amending, as regards pharmacovigilance of medicinal products for human use, Regulation (EC) No 726/2004 laying down Community procedures for the authorisation and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency, and Regulation (EC) No 1394/2007 on advanced therapy medicinal products. Official Journal of the European Union. 2010 Dec 31;L 348:1–16. Available from: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:348:0001:0016:EN:PDF. [Google Scholar]
- 21.Directive 2010/84/EU of the European Parliament and of the Council. of 15 December 2010 amending, as regards pharmacovigilance, Directive 2001/83/EC on the Community code relating to medicinal products for human use. Official Journal of the European Union. 2010;L 348:74–99. Available from: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:348:0074:0099:EN:PDF. [Google Scholar]
- 22.Schneider H, Strauß E. Zukunft der Anwendungsbeobachtung. Rechtssichere Grenzen zwischen Korruption und zulässiger Kooperation angesichts der aktuellen Vorlagebeschlüsse des 3. und 5. Strafsenats des Bundesgerichtshofs. HRRS. 2011;8:333ff. Available from: http://www.hrr-strafrecht.de/hrr/archiv/11-08/index.php?sz=7. [Google Scholar]
- 23.FS Arzneimittelindustrie e.V. The decisions of the FSA Code of Conduct "Health Professionals" [FSA-Kodex "Fachkreise". Available from: http://www.fs-arzneimittelindustrie.de/index.php?id=26&no_cache=1&tx_berichterstattung_pi1[sorting]=D&tx_berichterstattung_pi1[showUid]=26.
- 24.vfa – Die forschenden Pharma-Unternehmen. Register nicht-interventioneller Studien. [The register for non-interventional studies]. (Ger). Available from: http://www.vfa.de/de/arzneimittel-forschung/datenbanken-zu-arzneimitteln/nisdb. [Google Scholar]
- 25.Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM) Empfehlungen zur Planung, Durchführung und Auswertung von Anwendungsbeobachtungen, 12. November 1998. BAnz. 1998;229:16884. [Google Scholar]
- 26.Paul-Ehrlich-Institut. Empfehlungen des Bundesinstituts für Arzneimittel und Medizinprodukte und des Paul-Ehrlich-Instituts zur Planung, Durchführung und Auswertung von Anwendungsbeobachtungen vom 7. Juli 2010. Available from: http://www.pei.de/cln_170/nn_160648/DE/infos/pu/02-klinische-pruefung/klin-pruef-awb/klin-pruef-awb-node.html?__nnn=true. [Google Scholar]
- 27.Arbeitsgruppe Epidemiologische Methoden der Deutschen Arbeitsgemeinschaft für Epidemiologie (DAE) Leitlinien und Empfehlungen zur Sicherung von Guter Epidemiologischer Praxis (GEP) 2004. Available from: http://www.gmds.de/publikationen/1b_LeitlinienUndEmpfehlungen_April2004.pdf. [Google Scholar]
- 28.Gliklich RE, Dreyer NA, editors. Registries for Evaluating Patient Outcomes: A User's Guide. 2nd ed. Rockville, MD: Agency for Healthcare Research and Quality Publication; Sep, 2010. (AHRQ Publication; No.10-EHC049). Available from: http://www.effectivehealthcare.ahrq.gov/ehc/products/74/531/Registries%202nd%20ed%20final%20to%20Eisenberg%209-15-10.pdf. [PubMed] [Google Scholar]
- 29.Sickmüller B, Breitkopf S. "Points to Consider" zu Anwendungsbeobachtungen. Pharm Ind. 2009;71(5):764–769. Available from: http://www.kori-lindner.de/pharma-themen/arzneimittelpruefung/anwendungsbeobachtungen/doc_download/745-anwendungsbeobachtungen-points-to-consider-empfehlungen-des-bpi-sickmueller-etal-pharmind-7-2009. [Google Scholar]
- 30.STROBE-Statement (STrengthening the Reporting of OBservational studies in Epidemiology) Bern: ISPM; 2009. Available from: http://www.strobe-statement.org/ [Google Scholar]
- 31.Schinzel S, Hundt F, Theobald KH, Theis F, Buchmann A, Herbold M. CRO Precontract Audits. Applied Clinical Trials online. 2009 Jun 2; Available from: http://appliedclinicaltrialsonline.findpharma.com/appliedclinicaltrials/Online+Extras/CRO-Precontract-Audits/ArticleStandard/Article/detail/600000. [Google Scholar]