Abstract
Purpose of Review
Acute Kidney Injury (AKI) is a major clinical problem without effective therapy. Development of AKI among hospitalized patients drastically increases mortality, and morbidity. With increases in complex surgical procedures together with a growing elderly population, the incidence of AKI is rising. Renal adenosine receptor (AR) manipulation may have great therapeutic potential in mitigating AKI. In this review, we discuss renal AR biology and potential clinical therapies for AKI.
Recent Findings
The 4 AR subtypes (A1AR, A2AAR, A2BAR and A3AR) have diverse effects on the kidney. The pathophysiology of AKI may dictate the specific AR subtype activation needed to produce renal protection. The A1AR activation in renal tubules and endothelial cells produces beneficial effects against ischemia and reperfusion (IR) injury by modulating metabolic demand, decreasing necrosis, apoptosis and inflammation. The A2AAR protects against AKI by modulating leukocyte-mediated renal and systemic inflammation whereas the A2BAR activation protects by direct activation of renal parenchymal ARs. In contrast, the A1AR antagonism may play a protective role in nephrotoxic AKI and radiocontrast induced nephropathy by reversing vascular constriction and inducing naturesis and diuresis. Furthermore, as the A3AR-activation exacerbates apoptosis and tissue damage due to renal IR, selective A3AR antagonism may hold promise to attenuate renal IR injury. Finally, renal A1AR activation also protects against renal endothelial dysfunction caused by hepatic IR injury.
Summary
Despite the current lack of therapies for the treatment and prevention of AKI, recent research suggests that modulation of renal ARs holds promise in treating AKI and extrarenal injury.
Keywords: Apoptosis, inflammation, ischemia and reperfusion injury, necrosis
Introduction
Acute Kidney Injury (AKI) is a common problem in hospitalized patients and dramatically increases in mortality [1]. AKI costs more than $10 billion per year in the United States and no effective treatment exists [1]. Clinical outcomes of AKI are poor and have not improved over the past 50 years [2]. The incidence of AKI in Intensive Care Units (ICU) ranges from 1 to 25% in the United States, with mortality rates ranging between 15 and 60% [3]. With rapid increases in surgical and radiological procedures performed coupled with a growing elderly population, the incidence of AKI has risen over the last 10–15 years [4–6]. AKI commonly progresses to chronic kidney disease and is frequently associated with other life-threatening complications including sepsis and multiorgan failure [2,4,5]. Approximately 14% of surviving patients will go on to require renal replacement therapy, however the prognosis remains poor: mortality in patients treated with dialysis is 50–60% [2–6]. Unfortunately, there are no drugs that are FDA-approved to treat or prevent AKI.
Consequently, novel therapeutic and preventative measures for AKI are under intense investigation. Research on adenosine signaling in the kidney is one area with significant clinical therapeutic potential. This brief review will focus on renal adenosine signaling, the action of renal adenosine receptors (ARs) and their therapeutic potential in AKI and extrarenal injury.
Definitions and Causes of Acute Kidney Injury
AKI is defined as a rapid loss of kidney function (hours to days), resulting in the retention of metabolic waste products and oftentimes oliguria. Stages of kidney failure are defined clinically according to either the RIFLE or AKIN criteria [3]. The RIFLE acronym describes the increasing severity classes Risk, Injury, Failure, defined by rising serum creatinine and decreased urine output, and the two outcome classes Loss and End stage kidney disease, defined by the duration of loss of kidney function, 4 weeks and 3 months respectively [3]. However, the concern over conservative serum creatinine definitions in the RIFLE-classification system, when increases as little as 0.3 mg/dL could be indicative of early stages of AKI and with more than 50% increase in mortality [1], led to the AKI-Network (AKIN) staging system [3].
Renal ischemia and reperfusion (IR) injury, along with sepsis and nephrotoxin injury, are the leading causes of AKI for patients undergoing surgery involving the kidney, liver or aorta with the incidence of renal dysfunction in high-risk patients approaching 70–80% [4,7]. Of these, ischemic AKI is the best studied with highly reproducible experimental models. The basic mechanisms of ischemic AKI involve renal tubular and endothelial cell necrosis, apoptosis and inflammation [8]. Other leading causes of AKI include sepsis and nephrotoxins [4,9]. Drugs in the kidney tubular lumen are concentrated by reabsorption and have a direct toxic effect on the tubules. Radiocontrast dyes, antibiotics, non-steroidal anti-inflammatory drugs, chemotherapeutics and heavy metals are among the more common nephrotoxic agents. AKI occurs in 20% of patients with sepsis and in over 50% of patients with septic shock [4]. AKI also frequently co-manifests with injuries of other organs including the heart, liver, and lungs [9,10]. These extra-renal systemic complications secondary to AKI are the leading causes of mortality in the ICU [11]. Indeed, clinical studies show that patients with AKI complicated by extra-renal organ dysfunction have worsened prognosis compared to patients with isolated AKI [12].
Adenosine Generation in the Kidney
Adenosine is produced by all mammalian cells and regulates a wide variety of physiological activities [8,13]. In the kidney, adenosine regulates renin release, glomerular filtration rate (GFR) and renal vascular tone [13]. Adenosine is also a critical regulator of tubular glomerular feedback (TGF) [13,14]. Adenosine levels are enhanced during states of negative energy balance when the rate of adenosine triphosphate (ATP) hydrolysis is increased with respect to the rate of ATP synthesis. Hence, increased renal ATP consumption, impaired renal perfusion and hypoxia rapidly enhance adenosine formation within the kidney. Adenosine therefore accumulates during pathological insults to the kidney.
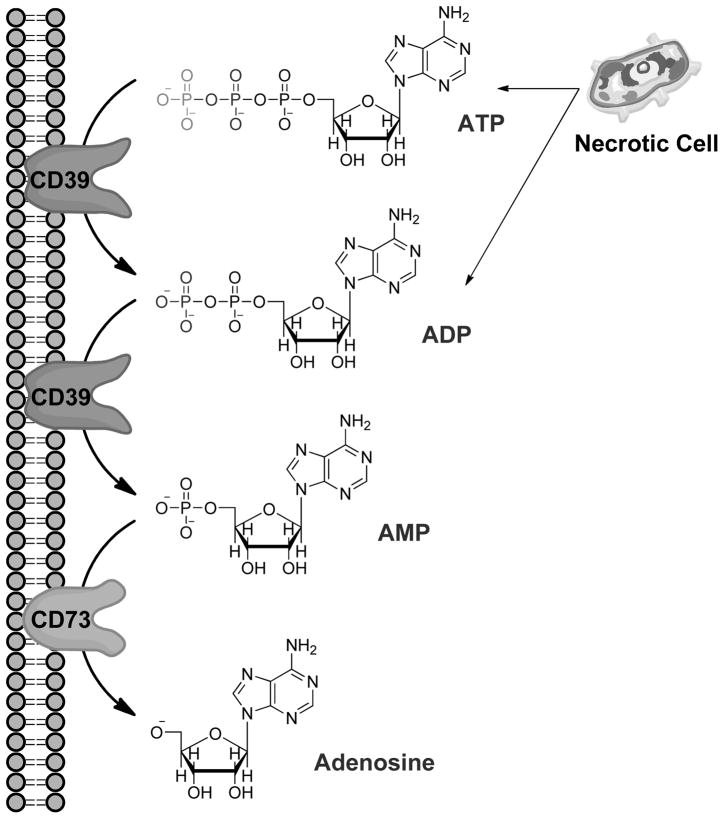
Extracellular adenosine is primarily derived from enzymatic phosphohydrolysis of ATP in the extracellular space. High levels of intracellular ATP (>5mmol/L) may be released into the extracellular space during hypoxic conditions, inflammation or acute injury by destabilizing apoptotic/necrotic cellular membranes [8,15]. Adenosine precursors may also be transported into the extracellular space via nucleotide release mechanisms, such as the release of ADP by granular release from activated platelets or inflammatory cells [8,16]. ATP and ADP are enzymatically phosphohydrolyzed by ectonucleoside-triphosphate-diphosphohydrolase-1 (also known as ectopyrase, CD39), yielding AMP [17,18]. AMP is then converted to adenosine by the surface enzyme ecto-5′-nucleotidase (CD73) (Figure 1) [16,19]. In addition, degradation of AMP to adenosine by CD73 activation decreases the availability of extracellular ATP, a recently recognized danger signal that promotes tissue injury and cell death [15,20,21]. In the extracellular space, ATP acts to attract leukocytes to the site of tissue injury and serve as a strong pro-inflammatory stimulus [22]. Therefore, stimulation of CD73 may serve the dual protective role of utilizing/removing cytotoxic extracellular ATP for the generation of cytoprotective adenosine.
Figure 1.
Cell death by apoptosis and necrosis releases ATP into the extracellular space. Adenosine is then generated from cleavage of ATP and ADP into AMP by the surface enzyme ecto-nucleoside-triphosphate-disphosphohydrolase1 (E-NTPDase1 or CD39) highly expressed in the kidneys. AMP is then dephosphorylated to adenosine by ecto-5′-nucleotidase (CD73). Phosphohydrolysis of AMP by CD73 is the rate-limiting step in this pathway.
Adenosine Receptors and AKI
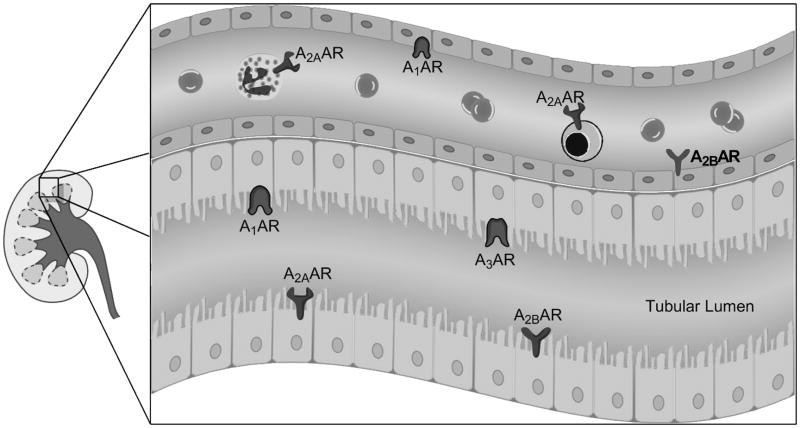
The extracellular adenosine generated by CD39 and CD73 phosphohydrolysis mediates a variety of cellular effects through G-protein coupled purinergic receptors (A1AR, A2AAR, A2BAR and A3AR, Fig. 1) [8,13]. The high-affinity receptors, A1AR, A2AAR, and A3AR, are activated by physiological levels of adenosine (10–100nM) whereas the A2BAR is a low affinity receptor, activating at concentrations above 1μM [13,23]. Such high levels of adenosine are seen only during pathological conditions [24]. While the expression levels of AR subtypes vary in cell types and locations in the kidney (Table 1, Figure 3), expression levels also have been known to change during ischemic, hypoxic or inflammatory conditions [8]. Renal adenosine generation and manipulating ARs have the potential to mitigate AKI.
Figure 3.
Location of adenosine receptors (ARs) in the kidney mediating cytoprotection. Endothelial and tubular A1AR activation produces cytoprotection. A2AARs are found in leukocytes (e.g., neutrophils and lymphocytes) and protect against renal injury by reducing inflammation. Renal tubular and endothelial A2BARs may also produce renal protection. A3ARs appear to be expressed in many cell types (e.g., epithelial and endothelial cells) in the kidney. Selective A3AR antagonist produces renal protection against ischemia and reperfusion injury.
1) A1 Adenosine Receptors
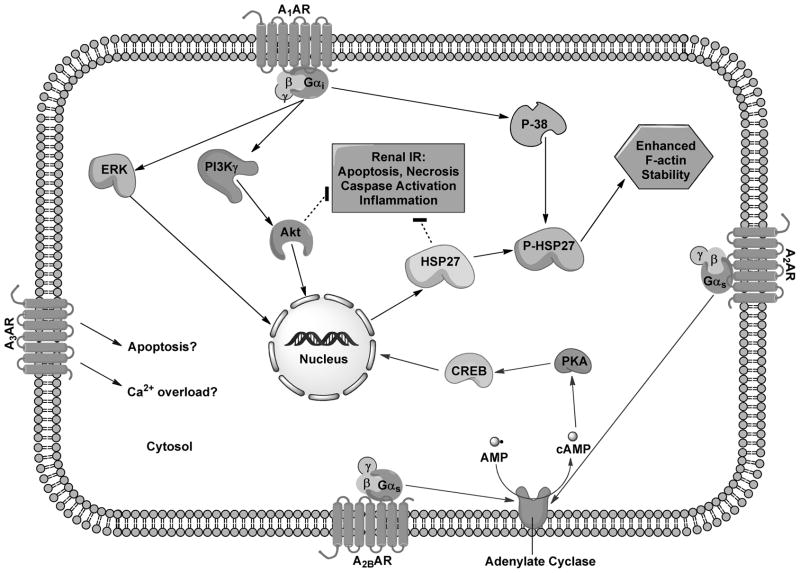
The A1AR is widely expressed in the kidney, especially in the distal afferent arterioles, mesangial cells, proximal convoluted tubules, medullary collecting ducts, and papillary surface epithelia [8] (Figure 3). The A1AR regulates renal vascular tone, TGF and renin secretion [13,14]. Endogenous or exogenous adenosine via A1AR causes renal arteriolar vasoconstriction, thus lowering GFR and stimulates NaCl, HCO3-, phosphate and fluid reabsorption. The A1AR signaling is mediated by pertussis toxin-sensitive G-protein transduced coupling to protein kinase C, extracellular signal-regulated protein kinase mitogen-activated protein kinase (ERK MAPK) and Akt (Figure 2) [25].
Figure 2.
Mechanisms of action of adenosine receptor (AR) subtype activation modulating renal cytoprotection. A1AR-activation results in pertussis toxin-sensitive Gi-mediated activation of mitogen-activated protein kinases (ERK, P-38) and phosphoinositide 3-kinases (PI3K) resulting in Heat Shock Protein 27 (HSP27) phosphorylation and induction leading to reduced apoptosis and inflammation. A2AAR and A2BAR couple with Gs and stimulates adenylate cyclase, raising cAMP and activating Protein Kinase A (PKA). PKA then causes nuclear translocation of cAMP Response-Element Binding (CREB) protein to produce cytoprotection. Mechanism of A3AR activation is still unknown. A3AR activation appears to stimulate apoptosis and calcium overload leading to enhanced renal injury after ischemia and reperfusion.
In addition to its renal hemodynamic effects and critical role in TGF, manipulation of A1AR has significant therapeutic potential in protection against AKI. Clinical benefit of activation or blockade of the A1AR is dictated by the etiology and pathophysiology of the AKI. Selective A1AR activation protects against renal IR injury and septic AKI in mice by reducing inflammation, necrosis and apoptosis [26–30].
As activation of renal A1ARs reduces GFR and afferent cortical blood flow through mediation of TGF, some investigators have implicated A1AR activation in the reduction of renal function due to nephrotoxic AKI, and perhaps due to ischemic and septic AKI [13]. These experimental results and interpretations may be conditioned by whether the outcomes tested are changes in GFR or indicators of tubular damage. However, decrease in GFR, renin, sympathetic outflow and active solute transport associated with A1AR activation would, in theory, reduce renal oxygen consumption in the setting of ischemic and nephrotoxic renal injury. The metabolic effects may differ between different models of AKI as a lower GFR might protect in certain models (e.g., ischemic AKI) and inhibition of transport may provide more protection in other experimental models (e.g., nephroxin induced AKI).
Indeed, we demonstrated that A1AR agonist produced powerful renal protection against ischemic AKI in mice [29,30]. Conversely, mice deficient in A1AR or wild type mice treated with an A1AR antagonist had increased renal dysfunction after ischemic- or septic-AKI [28,30]. We also demonstrated that transient activation of renal A1AR led to acute as well as delayed protective effects against renal IR injury via distinct signaling pathways [25]. In the acute phase, A1AR activation led to phosphorylation of ERK MAPK, Akt and heat shock protein 27 (HSP27), whereas the delayed protective effects observed several hours after A1AR activation may be the result of a dramatic induction of HSP27.
In contrast to the powerful renal protection against ischemic AKI with selective A1AR agonists, selective A1AR antagonists may protect against nephrotoxin-induced AKI and radiocontrast nephropathy [13,31]. A selective A1AR antagonist (DPCPX) or genetic deletion of A1ARs protected against radiocontrast nephropathy in mice [31]. Selective A1AR antagonists also promote natriuresis without kaliuresis and may also have a therapeutic potential as a diuretic in patients with congestive heart failure [32,33]. However, despite theoretical benefits for cardiorenal syndrome, recent clinical trials have shown that A1AR antagonists increased renal dysfunction rather than improving it [34]. Selective and non-selective A1AR antagonists prevented renal injury due to other nephrotoxins including glycerol, uranyl nitrate, cisplatin and gentamicin [13,14,35]. Meta-analysis of clinical trial data concluded that theophylline may reduce the incidence of radiocontrast media-induced nephropathy [13,35,36]. In mitigating radiocontrast induced renal injury, saline hydration and AR antagonists are effective, though the benefits are not additive. AR antagonists such as theophylline may be advantageous in conditions of poor renal blood flow when additional hydration may be deleterious (i.e. congestive heart failure, chronic renal insufficiency [13,35].
2) A2A Adenosine Receptors
In the kidney, the A2AAR receptor is located predominantly in the glomerular epithelium and adjacent vasculature [8] (Figure 3). In contrast to the A1AR-receptor, the A2AAR-receptor activation vasodilates deep cortical glomerular vessels and increases blood flow in the renal medulla [37,38]. A2AAR-activation has also been shown to increase renin release (Table 1) [13]. A2AAR-coupled Gs-mediated stimulation of adenylate cyclase and protein kinase A results in CREB-mediated cytoprotection against AKI (Figure 2) [37,39,40].
The A2AAR activation leads to increased medullary blood flow and oxygenation, and lowers medullary transport activity [13]. Consistent with these effects, treatment with A2AAR agonists has been shown to improve medullary hypoxia or hypoperfusion after renal IR injury [38,41]. The A2AARs are also well known for their ability to regulate hyperactive inflammatory cascade associated with AKI. A2AAR produces immuno-modulatory effects, notably on macrophages and neutrophils, that limit tissue damage [37,41,42]. In IR injury, renal protection by A2AAR-activation is independent of macrophage activation [42]. However in glomerulonephritis, A2AAR-agonists reduce inflammation by diminishing macrophage-derived pro-inflammatory cytokine release including TNF-α, IL-6 and IL-8 [43]. The A2AAR-activation also reduces neutrophil adhesion, infiltration and myeloperoxidasese activity and release of reactive oxygen metabolites likely through increased cAMP and activation of PKA in neutrophils [37,42].
3) A2B Adenosine Receptors
The A2BAR receptors are found predominantly in the renal vasculature with scant expression in renal epithelia under normal physiologic conditions [44,45] (Table 1, Figure 3). Similar to the A2AARs, the A2BARs cause vascular dilatation, increased renin secretion, increased NO production and reduced tissue inflammation through Gs and cAMP signaling pathways (Figure 2) [13]. Grenz et al. demonstrated in a murine model of renal IR injury that kidney ischemic preconditioning was absent in A2BAR deficient mice [46]. In contrast, ischemic preconditioning was produced in mice with specific deletion of A1AR, A2aAR or A3AR. Consistent with these findings, they also showed that wild type mice treated with a selective A2BAR agonist (BAY 60–6583) were protected against ischemic AKI. In addition, an A2BAR selective antagonist (PSB1115) blocked the renal protective effects of kidney ischemic preconditioning. They also found that renal A2BARs rather than leukocyte A2BARs conferred renal protection against IR injury using A2BAR bone-marrow chimera model. Therefore, unlike the A2AARs that regulate infiltrating pro-inflammatory leukocytes, the A2BARs target renal parenchymal (endothelial and/or tubular epithelia) cells to attenuate ischemic AKI.
4) A3 Adenosine Receptors
The A3AR is the least characterized AR subtype in the kidney [47]. The specific location of A3ARs in the kidney is still unclear, as are the mechanisms of A3AR signal transduction [13]. Under normal physiological conditions, A3AR does not affect TGF, GFR or solute excretion [48]. Both pro- and anti-inflammatory effects have been attributed to A3AR activation [49–51]. We have determined that mice genetically deficient in A3ARs or blocking A3ARs in wild-type mice resulted in significant renal protection from ischemic or myoglobinuric renal failure [50]. Moreover, we demonstrated in rats that selective A3AR activation or inhibition worsened or protected, respectively, against ischemic AKI [52]. In contrast, A3AR-activation diminishes inflammation and attenuates mortality and renal and hepatic injury in mice subjected to septic AKI [53]. Therefore, similar to A1AR, A3AR differentially modulates renal function depending on the type of renal injury.
The mechanism(s) by which the A3AR activation or inhibition exacerbates or protects against, respectively, ischemic AKI remains to be determined. The A3AR activation degranulates resident mast cells, which results in the release of stored inflammatory mediators including histamine and proteolytic enzymes [54,55]. We also demonstrated that the A3AR agonist IB-MECA profoundly increases plasma histamine levels in C57 mice (~45 fold increase) [50]. In addition, A3AR agonists cause apoptosis and calcium overload in multiple cell lines including cardiomyocytes, human leukemia cell lines and human proximal tubule (HK-2) cells [56–58]. Chronic A3AR activation or overexpression is detrimental to cell survival [59]. Moreover, overexpression of A3AR is embryologically lethal in mice with prominent fragmentation of DNA.
Remote Organ Injury Induced AKI, AKI-Induced Extrarenal Injury and Modulation by Adenosine Receptors
A host of changes occur during AKI that may cause distant injury to the brain, lungs, pancreas, liver, intestine, heart and vasculature. Leukocyte activation and trafficking, inflammation, oxidative stress, and changes to expression levels of cytokines, chemokines, sodium and water channels all lead to AKI-induced injury to distant organs, including the brain, lungs, intestines, liver, heart and circulation [10,60]. Inflammatory cytokines including TNF-α, IL-6 and IL-17A are released after ischemic AKI from small intestine and liver leading to additional renal, intestinal and liver injury [61]. A1AR activation protects against AKI and also reduces liver and intestinal injury after renal IR injury [62]. We recently demonstrated that severe hepatic IR causes AKI with rapid renal endothelial apoptosis and leukocyte infiltration [63,64]. Endogenous and exogenous activation of renal A1ARs protect against liver and kidney injury after in vivo liver IR via pathways involving Akt activation [62,63]. Therefore, protecting the kidney reduces liver IR injury and selective overexpression of cytoprotective A1ARs in the kidney leads to protection of both liver and kidney after hepatic IR.
Allosteric Manipulation of Adenosine Receptors
The ubiquitous expression of ARs may limit selective activation of renal ARs. One promising therapy has been the use of allosteric activators with endogenous adenosine [65,66]. During AKI, adenosine levels dramatically increase in the kidney, and allosteric drugs may locally protect the kidney from AKI by potentiating the activation of desired ARs [65]. Potential side effects of selective AR agonists can be mitigated by application of AR allosteric enhancers. An AR allosteric enhancer selectively increases the efficacy of endogenous adenosine in tissues (e.g., ischemic kidney producing increased localized adenosine) thereby avoiding potential systemic side effects of AR agonists. At present, AR allosteric modulators (e.g., T-62 for chronic pain and migraine headache) are in various stages of human clinical trials [65].
Conclusions
Manipulation of AR activation has therapeutic potential in mitigating AKI and AKI-induced extrarenal injury. The pathophysiology of the AKI dictates whether activation or inactivation of a particular receptor subtype is beneficial. Modulating AR activation in AKI may also protect against AKI-induced extrarenal injury. While the AR agonists and antagonists may have pharmacological benefit, allosteric-binding drugs may offer the most targeted effects with limited side effects. Therapeutics involving ARs are increasing in scope and value, and will certainly play a role in clinical innovations for treating AKI and other conditions.
Key Points.
Each of the AR subtypes (A1AR, A2AAR, A2BAR or A3AR) produces different effects on the kidney when activated. Modulating ARs in treatment of AKI should be based on the pathophysiology of renal injury.
Under hypoxic or ischemia conditions, activating the A1AR, A2AAR or A2BAR receptors is beneficial: this reduces metabolic demand and inflammation, and increases renal perfusion. Under nephrotoxin-induced AKI, A1AR-antagonism appears to be therapeutic.
A1AR and A2BAR protect against AKI by directly targeting kidney parenchymal cells. A2AAR activation produces immunomodulatory effects on circulating and infiltrating leukocytes. A3AR-activation may exacerbate apoptosis and tissue damage during ischemic AKI.
Mitigating AKI reduces the risk and severity of extrarenal injury, and may also be accomplished through AR manipulation.
Acknowledgments
Funding: This work was supported by National Institute of Health Grant R01 DK-058547.
The authors thank Mr. Jimmy Y. Hu for preparing artwork during manuscript preparation.
Abbreviations
- ADP
Adenosine Diphosphate
- AKI
Acute Kidney Injury
- AMP
Adenosine Monophosphate
- AR
Adenosine Receptor
- ATN
Acute Tubular Necrosis
- ATP
Adenosine Triphosphate
- CD39
Ectonucleoside Triphosphate Diphosphohydrolase 1
- CD73
Ecto-5′-nucleotidase
- ERK MAPK
Extracellular Signal-Regulated Protein Kinase Mitogen Activated Protein Kinase
- GFR
Glomerular Filtration Rate
- ICU
Intensive Care Unit
- IR
Ischemia and Reperfusion
- TGF
Tubular Glomerular Feedback
Footnotes
Conflict of Interest Statement: No financial conflict of interest exists for each author.
References
- 1.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 2.Jones DR, Lee HT. Perioperative renal protection. Best Pract Res Clin Anaesthesiol. 2008;22:193–208. doi: 10.1016/j.bpa.2007.08.005. [DOI] [PubMed] [Google Scholar]
- **3.Srisawat N, Hoste EE, Kellum JA. Modern classification of acute kidney injury. Blood Purif. 2010;29:300–307. doi: 10.1159/000280099. (Review on clinical definitions of acute kidney injury, stages of severity, and epidemiology) [DOI] [PubMed] [Google Scholar]
- **4.Hoste EA, Kellum JA, Katz NM, Rosner MH, Haase M, Ronco C. Epidemiology of acute kidney injury. Contrib Nephrol. 2010;165:1–8. doi: 10.1159/000313737. (Review of epidemiological studies on acute kidney injury) [DOI] [PubMed] [Google Scholar]
- 5.Kellum JA, Hoste EA. Acute kidney injury: epidemiology and assessment. Scand J Clin Lab Invest Suppl. 2008;241:6–11. doi: 10.1080/00365510802144813. [DOI] [PubMed] [Google Scholar]
- 6.Hoste EA, Kellum JA. Incidence, classification, and outcomes of acute kidney injury. Contrib Nephrol. 2007;156:32–38. doi: 10.1159/000102013. [DOI] [PubMed] [Google Scholar]
- 7.Sheridan AM, Bonventre JV. Cell biology and molecular mechanisms of injury in ischemic acute renal failure. Curr Opin Nephrol Hypertens. 2000;9:427–434. doi: 10.1097/00041552-200007000-00015. [DOI] [PubMed] [Google Scholar]
- **8.Bauerle JD, Grenz A, Kim JH, Lee HT, Eltzschig HK. Adenosine generation and signaling during acute kidney injury. J Am Soc Nephrol. 2011;22:14–20. doi: 10.1681/ASN.2009121217. (Review on renal adenosine receptor locations, extracellular adenosine production, and adenosine effects in the kidney) [DOI] [PubMed] [Google Scholar]
- **9.Okusa MD. The changing pattern of acute kidney injury: from one to multiple organ failure. Contrib Nephrol. 2010;165:153–158. doi: 10.1159/000313754. (Review of acute kidney injury induced remote organ injury and clinical significance) [DOI] [PubMed] [Google Scholar]
- 10.Paladino JD, Hotchkiss JR, Rabb H. Acute kidney injury and lung dysfunction: a paradigm for remote organ effects of kidney disease? Microvasc Res. 2009;77:8–12. doi: 10.1016/j.mvr.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faubel S. Pulmonary complications after acute kidney injury. Adv Chronic Kidney Dis. 2008;15:284–296. doi: 10.1053/j.ackd.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Bagshaw SM, Laupland KB, Doig CJ, Mortis G, Fick GH, Mucenski M, Godinez-Luna T, Svenson LW, Rosenal T. Prognosis for long-term survival and renal recovery in critically ill patients with severe acute renal failure: a population-based study. Crit Care. 2005;9:R700–R709. doi: 10.1186/cc3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vallon V, Osswald H. Adenosine receptors and the kidney. Handb Exp Pharmacol. 2009:443–470. doi: 10.1007/978-3-540-89615-9_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vallon V, Muhlbauer B, Osswald H. Adenosine and kidney function. Physiol Rev. 2006;86:901–940. doi: 10.1152/physrev.00031.2005. [DOI] [PubMed] [Google Scholar]
- *15.McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. (This study demonstrated that ATP released from necrotic cells initiated sterile inflammation and directly attracted neutrophils to the site of injury) [DOI] [PubMed] [Google Scholar]
- 16.Thompson LF, Eltzschig HK, Ibla JC, Van De Wiele CJ, Resta R, Morote-Garcia JC, Colgan SP. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eltzschig HK, Kohler D, Eckle T, Kong T, Robson SC, Colgan SP. Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood. 2009;113:224–232. doi: 10.1182/blood-2008-06-165746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grenz A, Zhang H, Hermes M, Eckle T, Klingel K, Huang DY, Muller CE, Robson SC, Osswald H, Eltzschig HK. Contribution of E-NTPDase1 (CD39) to renal protection from ischemia-reperfusion injury. FASEB J. 2007 doi: 10.1096/fj.06-7947com. [DOI] [PubMed] [Google Scholar]
- 19.Grenz A, Zhang H, Eckle T, Mittelbronn M, Wehrmann M, Kohle C, Kloor D, Thompson LF, Osswald H, Eltzschig HK. Protective role of ecto-5′-nucleotidase (CD73) in renal ischemia. J Am Soc Nephrol. 2007;18:833–845. doi: 10.1681/ASN.2006101141. [DOI] [PubMed] [Google Scholar]
- 20.Trautmann A. Extracellular ATP in the immune system: more than just a "danger signal". Sci Signal. 2009;2:e6. doi: 10.1126/scisignal.256pe6. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- *22.Stagg J, Smyth MJ. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene. 2010;29:5346–5358. doi: 10.1038/onc.2010.292. (Review on extracellular adenosine signalling on leukocytes, including the immunosuppressive effects of A2AR receptor activation and the therapeutic potential of modulating adenosine in cancer treatment) [DOI] [PubMed] [Google Scholar]
- 23.Fredholm BB, Irenius E, Kull B, Schulte G. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem Pharmacol. 2001;61:443–448. doi: 10.1016/s0006-2952(00)00570-0. [DOI] [PubMed] [Google Scholar]
- 24.Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14:1315–1323. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- 25.Joo JD, Kim M, Horst P, Kim J, D’Agati VD, Emala CW, Sr, Lee HT. Acute and delayed renal protection against renal ischemia and reperfusion injury with A1 adenosine receptors. Am J Physiol Renal Physiol. 2007;293:F1847–F1857. doi: 10.1152/ajprenal.00336.2007. [DOI] [PubMed] [Google Scholar]
- 26.Kim M, Chen SW, Park SW, Kim M, D’Agati VD, Yang J, Lee HT. Kidney-specific reconstitution of the A1 adenosine receptor in A1 adenosine receptor knockout mice reduces renal ischemia-reperfusion injury. Kidney Int. 2009;75:809–823. doi: 10.1038/ki.2008.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee HT, Kim M, Jan M, Penn RB, Emala CW. Renal tubule necrosis and apoptosis modulation by A1 adenosine receptor expression. Kidney Int. 2007;71(12):1249–1261. doi: 10.1038/sj.ki.5002227. [DOI] [PubMed] [Google Scholar]
- 28.Gallos G, Ruyle TD, Emala CW, Lee HT. A1 adenosine receptor knockout mice exhibit increased mortality, renal dysfunction, and hepatic injury in murine septic peritonitis. Am J Physiol Renal Physiol. 2005;289:F369–F376. doi: 10.1152/ajprenal.00470.2004. [DOI] [PubMed] [Google Scholar]
- 29.Lee HT, Gallos G, Nasr SH, Emala CW. A1 adenosine receptor activation inhibits inflammation, necrosis, and apoptosis after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol. 2004;15:102–111. doi: 10.1097/01.asn.0000102474.68613.ae. [DOI] [PubMed] [Google Scholar]
- 30.Lee HT, Xu H, Nasr SH, Schnermann J, Emala CW. A1 adenosine receptor knockout mice exhibit increased renal injury following ischemia and reperfusion. Am J Physiol Renal Physiol. 2004;286:F298–F306. doi: 10.1152/ajprenal.00185.2003. [DOI] [PubMed] [Google Scholar]
- 31.Lee HT, Jan M, Bae SC, Joo JD, Goubaeva FR, Yang J, Kim M. A1 adenosine receptor knockout mice are protected against acute radiocontrast nephropathy in vivo. Am J Physiol Renal Physiol. 2006;290:F1367–F1375. doi: 10.1152/ajprenal.00347.2005. [DOI] [PubMed] [Google Scholar]
- 32.Gottlieb SS. Adenosine A1 antagonists and the cardiorenal syndrome. Curr Heart Fail Rep. 2008;5:105–109. doi: 10.1007/s11897-008-0017-x. [DOI] [PubMed] [Google Scholar]
- 33.Gottlieb SS, Brater DC, Thomas I, Havranek E, Bourge R, Goldman S, Dyer F, Gomez M, Bennett D, Ticho B, Beckman E, Abraham WT. BG9719 (CVT-124), an A1 adenosine receptor antagonist, protects against the decline in renal function observed with diuretic therapy. Circulation. 2002;105:1348–1353. doi: 10.1161/hc1102.105264. [DOI] [PubMed] [Google Scholar]
- *34.Voors AA, Dittrich HC, Massie BM, DeLucca P, Mansoor GA, Metra M, Cotter G, Weatherley BD, Ponikowski P, Teerlink JR, Cleland JG, O’Connor CM, Givertz MM. Effects of the adenosine A1 receptor antagonist rolofylline on renal function in patients with acute heart failure and renal dysfunction: results from PROTECT (Placebo-Controlled Randomized Study of the Selective Adenosine A1 Receptor Antagonist Rolofylline for Patients Hospitalized with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function) J Am Coll Cardiol. 2011;57:1899–1907. doi: 10.1016/j.jacc.2010.11.057. (Results from A1AR antagonist clinical trial as a diuretic to treat heart failure. Patients had no significant improvements in renal function.) [DOI] [PubMed] [Google Scholar]
- *35.Osswald H, Schnermann J. Methylxanthines and the kidney. Handb Exp Pharmacol. 2011:391–412. doi: 10.1007/978-3-642-13443-2_15. (A review of adenosine receptor biology and adenosine rceeptor antagonist physiology in the kidney) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asif A, Epstein M. Prevention of radiocontrast-induced nephropathy. Am J Kidney Dis. 2004;44:12–24. doi: 10.1053/j.ajkd.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Okusa MD. A(2A) adenosine receptor: a novel therapeutic target in renal disease. Am J Physiol Renal Physiol. 2002;282:F10–F18. doi: 10.1152/ajprenal.2002.282.1.F10. [DOI] [PubMed] [Google Scholar]
- 38.Okusa MD, Linden J, Huang L, Rieger JM, Macdonald TL, Huynh LP. A(2A) adenosine receptor-mediated inhibition of renal injury and neutrophil adhesion. Am J Physiol Renal Physiol. 2000;279:F809–F818. doi: 10.1152/ajprenal.2000.279.5.F809. [DOI] [PubMed] [Google Scholar]
- 39.Lee HT, Emala CW. Adenosine attenuates oxidant injury in human kidney proximal tubular cells via A1 and A2a adenosine receptor activation. Am J Physiol Renal Physiol. 2002;282(5):F844–F852. doi: 10.1152/ajprenal.00195.2001. [DOI] [PubMed] [Google Scholar]
- 40.Lee HT, Emala CW. Systemic adenosine given after ischemia protects renal function via A2a adenosine receptor activation. Am J Kidney Dis. 2001;38:610–618. doi: 10.1053/ajkd.2001.26888. [DOI] [PubMed] [Google Scholar]
- 41.Day YJ, Huang L, McDuffie MJ, Rosin DL, Ye H, Chen JF, Schwarzschild MA, Fink JS, Linden J, Okusa MD. Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells. J Clin Invest. 2003;112:883–891. doi: 10.1172/JCI15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Day YJ, Huang L, Ye H, Linden J, Okusa MD. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: role of macrophages. Am J Physiol Renal Physiol. 2005;288:F722–F731. doi: 10.1152/ajprenal.00378.2004. [DOI] [PubMed] [Google Scholar]
- 43.Garcia GE, Truong LD, Li P, Zhang P, Du J, Chen JF, Feng L. Adenosine A2A receptor activation and macrophage-mediated experimental glomerulonephritis. FASEB J. 2008;22:445–454. doi: 10.1096/fj.07-8430com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood. 2008;111:2024–2035. doi: 10.1182/blood-2007-10-117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Linden J. New insights into the regulation of inflammation by adenosine. J Clin Invest. 2006;116:1835–1837. doi: 10.1172/JCI29125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grenz A, Osswald H, Eckle T, Yang D, Zhang H, Tran ZV, Klingel K, Ravid K, Eltzschig HK. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med. 2008;5:e137. doi: 10.1371/journal.pmed.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol. 2001;41:775–787. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- 48.Mozaffari MS, Abebe W, Warren BK. Renal adenosine A3 receptors in the rat: assessment of functional role. Can J Physiol Pharmacol. 2000;78:428–432. [PubMed] [Google Scholar]
- 49.Young HW, Molina JG, Dimina D, Zhong H, Jacobson M, Chan LN, Chan TS, Lee JJ, Blackburn MR. A3 adenosine receptor signaling contributes to airway inflammation and mucus production in adenosine deaminase-deficient mice. J Immunol. 2004;173:1380–1389. doi: 10.4049/jimmunol.173.2.1380. [DOI] [PubMed] [Google Scholar]
- 50.Lee HT, Ota-Setlik A, Xu H, D’Agati VD, Jacobson MA, Emala CW. A3 adenosine receptor knockout mice are protected against ischemia- and myoglobinuria-induced renal failure. Am J Physiol Renal Physiol. 2003;284:F267–F273. doi: 10.1152/ajprenal.00271.2002. [DOI] [PubMed] [Google Scholar]
- 51.Mabley J, Soriano F, Pacher P, Hasko G, Marton A, Wallace R, Salzman A, Szabo C. The adenosine A3 receptor agonist, N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide, is protective in two murine models of colitis. Eur J Pharmacol. 2003;466:323–329. doi: 10.1016/s0014-2999(03)01570-x. [DOI] [PubMed] [Google Scholar]
- 52.Lee HT, Emala CW. Protective effects of renal ischemic preconditioning and adenosine pretreatment: role of A(1) and A(3) receptors. Am J Physiol Renal Physiol. 2000;278:F380–F387. doi: 10.1152/ajprenal.2000.278.3.F380. [DOI] [PubMed] [Google Scholar]
- 53.Lee HT, Kim M, Joo JD, Gallos G, Chen JF, Emala CW. A3 adenosine receptor activation decreases mortality and renal and hepatic injury in murine septic peritonitis. Am J Physiol Regul Integr Comp Physiol. 2006;291:R959–R969. doi: 10.1152/ajpregu.00034.2006. [DOI] [PubMed] [Google Scholar]
- 54.Reeves JJ, Jones CA, Sheehan MJ, Vardey CJ, Whelan CJ. Adenosine A3 receptors promote degranulation of rat mast cells both in vitro and in vivo. Inflamm Res. 1997;46:180–184. doi: 10.1007/s000110050169. [DOI] [PubMed] [Google Scholar]
- 55.Fozard JR, Pfannkuche HJ, Schuurman HJ. Mast cell degranulation following adenosine A3 receptor activation in rats. Eur J Pharmacol. 1996;298:293–297. doi: 10.1016/0014-2999(95)00822-5. [DOI] [PubMed] [Google Scholar]
- 56.Shneyvays V, Nawrath H, Jacobson KA, Shainberg A. Induction of apoptosis in cardiac myocytes by an A3 adenosine receptor agonist. Exp Cell Res. 1998;243:383–397. doi: 10.1006/excr.1998.4134. [DOI] [PubMed] [Google Scholar]
- 57.Jacobson KA. Adenosine A3 receptors: novel ligands and paradoxical effects. Trends Pharmacol Sci. 1998;19:184–191. doi: 10.1016/s0165-6147(98)01203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kohno Y, Sei Y, Koshiba M, Kim HO, Jacobson KA. Induction of apoptosis in HL-60 human promyelocytic leukemia cells by adenosine A3 receptor agonists. Biochem Biophys Res Comm. 1996;232:904–910. doi: 10.1006/bbrc.1996.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao Z, Yaar R, Ladd D, Cataldo LM, Ravid K. Overexpression of A3 adenosine receptors in smooth, cardiac, and skeletal muscle is lethal to embryos. Microvasc Res. 2002;63:61–69. doi: 10.1006/mvre.2001.2366. [DOI] [PubMed] [Google Scholar]
- 60.Levy RM, Mollen KP, Prince JM, Kaczorowski DJ, Vallabhaneni R, Liu S, Tracey KJ, Lotze MT, Hackam DJ, Fink MP, Vodovotz Y, Billiar TR. Systemic inflammation and remote organ injury following trauma require HMGB1. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1538–R1544. doi: 10.1152/ajpregu.00272.2007. [DOI] [PubMed] [Google Scholar]
- **61.Park SW, Chen SW, Kim M, Brown KM, Kolls JK, D’Agati VD, Lee HT. Cytokines induce small intestine and liver injury after renal ischemia or nephrectomy. Lab Invest. 2011;91:63–84. doi: 10.1038/labinvest.2010.151. (Investigators demonstrated that IL-17A derived from small intestinal cells, along with TNF-α and IL-6 following AKI exacerbates AKI-induced liver and small intestine injury) [DOI] [PMC free article] [PubMed] [Google Scholar]
- *62.Park SW, Chen SW, Kim M, Brown KM, D’Agati VD, Lee HT. Protection against acute kidney injury via A(1) adenosine receptor-mediated Akt activation reduces liver injury after liver ischemia and reperfusion in mice. J Pharmacol Exp Ther. 2010;333:736–747. doi: 10.1124/jpet.110.166884. (Following liver ischemia-reperfusion injury, investigators demonstrated using A1AR-knockout mice and A1AR-antagonists that exogenous and endogenous A1AR-activation protects against AKI and liver injury via Akt-activation) [DOI] [PMC free article] [PubMed] [Google Scholar]
- *63.Park SW, Chen SW, Kim M, D’Agati VD, Lee HT. Selective intrarenal human A1 adenosine receptor overexpression reduces acute liver and kidney injury after hepatic ischemia reperfusion in mice. Lab Invest. 2010;90:476–495. doi: 10.1038/labinvest.2009.143. (Investigators demonstrated that cytoprotective A1AR activation in the kidney protects against AKI, and in doing so, also protects against liver injury) [DOI] [PubMed] [Google Scholar]
- 64.Lee HT, Park SW, Kim M, D’Agati VD. Acute kidney injury after hepatic ischemia and reperfusion injury in mice. Lab Invest. 2009;89:196–208. doi: 10.1038/labinvest.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kiesman WF, Elzein E, Zablocki J. A1 adenosine receptor antagonists, agonists, and allosteric enhancers. Handb Exp Pharmacol. 2009:25–58. doi: 10.1007/978-3-540-89615-9_2. [DOI] [PubMed] [Google Scholar]
- 66.Baraldi PG, Iaconinoto MA, Moorman AR, Carrion MD, Cara CL, Preti D, Lopez OC, Fruttarolo F, Tabrizi MA, Romagnoli R. Allosteric enhancers for A1 adenosine receptor. Mini Rev Med Chem. 2007;7:559–569. doi: 10.2174/138955707780859459. [DOI] [PubMed] [Google Scholar]