Abstract
Objectives. To evaluate changes in medical, pharmacy, and nurse practitioner students’ drug-drug interaction (DDI) knowledge after attending an educational program.
Design. A DDI knowledge assessment containing 15 different drug pairs was administered to participants before and after a 45-minute educational session.
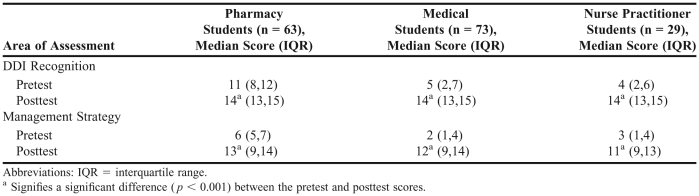
Evaluation. Pharmacy, medical, and nursing students scored significantly higher on the posttest assessment for DDI recognition (median change 3, 9, and 8, respectively) and management strategy (median change 5, 9, 8, respectively), indicating a significant improvement in DDI knowledge as a result of the educational session. Pharmacy students scored significantly higher on the pretest; however, no difference was observed between the students’ posttest scores. Posttest scores for all student groups were significantly greater than their respective pretest scores (p < 0.001).
Conclusions. Significant improvement in healthcare professional students’ DDI knowledge was observed following participation in the educational session.
Keywords: drug-drug interaction, drug interaction knowledge, medical education, pharmacy education, nurse practitioner education
INTRODUCTION
As guardians of patient health and safety, healthcare professionals possess a responsibility to identify and prevent adverse drug events (ADEs),1 which are defined as a serious injury due to a medication error.2 Healthcare providers who prescribe or dispense medications must be educated about DDIs, their potential to produce ADEs, and subsequent negative patient-related outcomes.
Clinically significant DDIs are potentially life threatening, and in some instances, fatal.3-5 While not all ADEs are predictable, exposure to a clinically significant DDI is a preventable medical mistake.6 The prevalence of DDIs identified in the literature varies widely due to differences in research methods used in the studies.3,7-11 Qato and colleagues conducted a study using in-home interviews of a nationally representative sample of 3,005 community-residing older adults and found that 4% of individuals were at potential risk for a major DDI.10
Physicians, nurse practitioners, and pharmacists constitute the group of providers in closest proximity to patients receiving medications. Thus, understanding the degree to which these providers can recognize an interaction and identify a proper management strategy is vital to developing new methods to reduce DDIs. The limited data available suggest that DDI knowledge of practicing physicians, nurse practitioners, and pharmacists is poor.12-16
Incorporating DDI-specific educational programs into healthcare professional student curricula is one way to ensure that these future professionals receive adequate DDI training. Currently, DDI curricular content varies; some colleges offer a single educational session, while others offer year-long courses dedicated to drug interactions.17,18 Identifying potentially harmful DDIs and strategies to manage possible interactions is an important and complex issue. Therefore, healthcare students must receive formal education in this area. The objective of this study was to evaluate change in DDI knowledge among medical, pharmacy, and nurse practitioner students after attending an educational program.
DESIGN
This was a cross-sectional prospective pretest-posttest study that used subjects recruited from the colleges of medicine, nursing, and pharmacy at the University of Arizona. To be eligible to participate, students had to be enrolled in their final year of academic study. The researchers were specifically interested in medical, pharmacy, and nursing students, based on the importance of these students’ future professional roles in rational medication use.
Three separate training sessions were held; one training session was held for each health profession (pharmacy, medical, and nursing students) in a required class. At the beginning of each training session, research staff members provided an overview of study procedures, answered questions, and distributed the disclosure form. Students who agreed to participate were then given a knowledge assessment test containing 15 drug pairs; the assessment included 11 DDIs and 4 non-interacting pairs. The DDI assessment was administered prior to and immediately following the educational session; pre- and post assessments were identical. The 11 DDIs selected for use in this assessment were deemed clinically significant based on previous research.19 The medications included are commonly used cardiovascular medications and those with potentially significant adverse effects (eg, warfarin).9 An evidence-based summary was developed for each DDI pair using a process similar to that used in previous research.19
For each drug pair, both the generic and brand names of the drugs were provided. All drugs were listed alphabetically according to object drug (the drug affected by the interaction) and precipitant drug (the drug causing the interaction). The format of the assessment scales was based on a modified version of the Operational Classification (ORCA) system for drug interactions.20 This model was chosen because DDIs are assigned to categories based on management of the interaction.
First, students assessed each drug pair and determined whether it was an interacting or non-interacting combination. Then students selected a corresponding appropriate action; options included “avoid combination,” “usually avoid combination,” “take precautions,” and “no special precautions.” An additional option of “not sure” was offered to discourage guessing. A previous Rasch analysis validated the instrument for use with a health professional student population.21
DDI Educational Program
Following the pretest knowledge assessment, students attended a 45-minute educational program during which all 15 drug pairs were addressed. The session was specifically designed for integration into healthcare professional curricula; it included a PowerPoint presentation, case-based evidence summaries, and a supplementary DDI primer. Each drug pair was presented individually in a case-based format. Students shared and discussed their choices for DDI recognition and management strategy before the presenter disclosed the correct answers. The educational program addressed the following: DDI terminology and definitions; interaction mechanism explanations; various DDI management strategies; and an overview of selected, clinically important DDIs. Interaction mechanisms, potential clinical consequences, and management strategies also were presented for all DDIs on the assessment. Immediately following the presentation, students completed the posttest.
EVALUATION AND ASSESSMENT
Two analyses were conducted: 1 on DDI recognition and 1 on management strategy. For scoring of DDI recognition analysis, students received credit for correctly identifying a potential DDI. For example, if a drug pair interacted, students received credit for any response that indicated an interaction including: “avoid combination,” “usually avoid combination,” or “take precautions.” A response of “not sure” or nonresponse to an item was scored as incorrect.
For the management strategy analysis, students received credit only if they selected the correct corresponding management strategy for the interacting pairs. If no interaction existed, credit was given only for the “no special precautions” response. A selection of “not sure” or a nonresponse to an item was scored as incorrect.
The distributions for the DDI recognition and management strategy analyses aggregated scores were skewed, so nonparametric Freidman tests were used to compare pretest and posttest scores for student groups (pharmacy, medical, and nurse practitioner). If Freidman test results were significant, post-hoc Wilcoxon rank sum tests were performed to determine whether significant differences existed between the pretest and posttest assessment scores for student groups. A Kruskal-Wallis analysis was performed for both analyses to determine whether significant differences existed between the posttest scores. The alpha level was set at 0.05 a priori. SPSS was used for all analyses (PASW Statistics, Chicago, IL, Version 17.0). The University Institutional Review Board approved the project.
DDI knowledge assessment participation rates varied by student group; 61% (n = 73) participation for medical, 82% (n = 63) for pharmacy, and 100% (n = 29) for nurse practitioner students. Pharmacy and medical student nonparticipants were absent during the class period or elected not to complete the assessment. The participant mean age was 26.7 ± 6.6 years, 26.7 ± 4.3 years, and 37.7 ± 10.4 years for pharmacy, medical, and nurse practitioner students, respectively. Across all groups, more than 50% of the students were female; 93% of the nurse practitioner students were female.
The DDI recognition and management strategy scores for each group of students are shown in Table 1. For both DDI recognition and management strategy, significant differences were observed between pretest and posttest scores for all 3 groups (p < 0.001). In contrast, analyses indicated no significant difference between the groups’ posttest scores for DDI recognition (p = 0.81) or management strategy (p = 0.55).
Table 1.
Median Pretest and Posttest Scores for DDI Recognition and Management Strategy
DISCUSSION
This study found that pharmacy, medical, and nurse practitioner students performed significantly better on a DDI knowledge assessment administered immediately following an educational session. Pharmacy students at the University of Arizona have more medication-related content incorporated into the curriculum than do medical or nurse practitioner students. The Accreditation Council for Pharmacy Education requires DDI education for pharmacy students, while equivalent accreditation organizations for medical and nurse practitioner programs do not explicitly require providing DDI educational material.22-24 While pharmacy students in this sample scored significantly higher on both DDI recognition and management strategy items at pretest, this difference was not observed at posttest, implying that the educational session was successful at improving DDI knowledge.18 Posttest scores between the groups also were similar due to a ceiling effect inherent in the assessment tool. Future research may require inclusion of more difficult assessment items.
These study findings parallel other studies that assessed the effect of educational programs on healthcare provider DDI knowledge.17,25,26 Saverno and colleagues found that DDI educational sessions improved pharmacy students’ short-term knowledge.25 Trujillo and colleagues offered a 2-semester drug interaction elective course to third-year pharmacy students; students completing the course showed improved DDI recognition scores and increased confidence in identifying interactions in comparison to their peers who opted out of the course.17 Tskuruoka and colleagues evaluated 2 physician groups from the same undergraduate medical school who had been in clinical practice for 3 to 7 years.26 One group had participated in a predoctoral clinical pharmacology course while the other group graduated prior to the course offering. Practicing physicians who had taken the course as medical students were significantly more knowledgeable about drug interactions and adverse effects than their counterparts.
For this study, the DDI educational session may have been particularly effective because the teaching technique allowed students to apply their prior medication knowledge during the educational session and to supplement their knowledge with assessment answers and reasoning.18 This teaching method has been used successfully in other educational program assessments, allowing students to combine their current knowledge with the new information.27
There are several limitations inherent in this study. First, there is typically a longer time interval between healthcare providers’ participation in a DDI educational session and their need for recollection and application of DDI content. Valdez and colleagues found knowledge retention declined after a 4-month period,28 indicating that future research is necessary to assess student knowledge retention. Second, the study is not reflective of a real-world situation in that most healthcare providers have access to drug-information resources such as software or compendia but the students in this study were not permitted to use reference material during the evaluation. However, DDI information aids do not replace the need for healthcare providers to have a basic understanding of important drug interactions.25 This study tested and retested the same pharmacy, medical, and nursing students with the same test instrument pre- and posttraining, potentially introducing testing bias. The threat is especially relevant given that the posttest was administered immediately following the educational session. Instrument reliability and validity were not addressed in this paper; a Rasch analysis performed for this particular assessment was previously published.21
CONCLUSION
A DDI-specific educational program improved the short-term DDI knowledge of medical, nurse practitioner, and pharmacy students. Drug interactions are both identifiable and preventable if a healthcare professional is familiar with the adverse drug events associated with the interaction. Therefore, focused DDI education may better prepare healthcare professional students for their future clinical roles and has the potential to improve the quality and safety of healthcare.
AKNOWLEDGEMENTS
This project was funded under contract/grant number U18 HS017001-01 from the Agency of Healthcare Research and Quality (AHRQ), US Department of Health and Human Services. The opinions expressed in this document are those of the authors and do not reflect the official position of AHRQ or the US Department of Health and Human Services.
REFERENCES
- 1.Sears EL, Generali JA. Adverse drug reaction and medication error reporting by pharmacy students. Ann Pharmacother. 2005;39(3):452–459. doi: 10.1345/aph.1E369. [DOI] [PubMed] [Google Scholar]
- 2.Aspden P, Wolcott J, Bootman JL, Cronenwett LR. Preventing Medication Errors: Quality Chasm Series. Washington, DC: National Academies Press; 2007. [Google Scholar]
- 3.Hamilton R, Briceland L, Andritz M. Frequency of hospitalization after exposure to known drug-drug interactions in a Medicaid population. Pharmacotherapy. 1998;18(5):1112. [PubMed] [Google Scholar]
- 4.Juurlink DN, Mamdani M, Kopp A, Laupacis A, Redelmeier DA. Drug-drug interactions among elderly patients hospitalized for drug toxicity. JAMA. 2003;289(13):1652. doi: 10.1001/jama.289.13.1652. [DOI] [PubMed] [Google Scholar]
- 5.Kelly CM, Juurlink DN, Gomes T, et al. Selective serotonin reuptake inhibitors and breast cancer mortality in women receiving tamoxifen: a population based cohort study. Br Med J. 2010;340:c693. doi: 10.1136/bmj.c693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peterson JF, Bates DW. Preventable medication errors: identifying and eliminating serious drug interactions. J Am Pharm Assoc (Wash) 2001;41(2):159–160. doi: 10.1016/s1086-5802(16)31243-8. [DOI] [PubMed] [Google Scholar]
- 7.Gurwitz JH, Field TS, Harrold LR, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA. 2003;289(9):1107. doi: 10.1001/jama.289.9.1107. [DOI] [PubMed] [Google Scholar]
- 8.Lafata JE, Schultz L, Simpkins J, et al. Potential drug-drug interactions in the outpatient setting. Med Care. 2006;44(6):534. doi: 10.1097/01.mlr.0000215807.91798.25. [DOI] [PubMed] [Google Scholar]
- 9.Malone DC, Hutchins DS, Haupert H, et al. Assessment of potential drug-drug interactions with a prescription claims database. Am J Health-Syst Pharm. 2005;62(19):1983–1991. doi: 10.2146/ajhp040567. [DOI] [PubMed] [Google Scholar]
- 10.Qato DM, Alexander GC, Conti RM, Johnson M, Schumm P, Lindau ST. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA. 2008;300(24):2867. doi: 10.1001/jama.2008.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solberg LI, Hurley JS, Roberts MH, et al. Measuring patient safety in ambulatory care: potential for identifying medical group drug-drug interaction rates using claims data. Am J Manag Care. 2004;10(11 Pt 1):753–759. [PubMed] [Google Scholar]
- 12.Blix H, Viktil K, Moger T, Reikvam A. Identification of drug interactions in hospitals–computerized screening vs. bedside recording. J Clin Pharm Ther. 2008;33(2):131–139. doi: 10.1111/j.1365-2710.2007.00893.x. [DOI] [PubMed] [Google Scholar]
- 13.Cavuto NJ, Woosley RL, Sale M. Pharmacies and prevention of potentially fatal drug interactions. JAMA. 1996;275(14):1086–1087. [PubMed] [Google Scholar]
- 14.Glassman PA, Simon B, Belperio P, Lanto A. Improving recognition of drug interactions: benefits and barriers to using automated drug alerts. Med Care. 2002;40(12):1161–1171. doi: 10.1097/00005650-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Ko Y, Malone DC, Skrepnek GH, et al. Prescribers’ knowledge of and sources of information for potential drug-drug interactions: a postal survey of US prescribers. Drug Saf. 2008;31(6):525–536. doi: 10.2165/00002018-200831060-00007. [DOI] [PubMed] [Google Scholar]
- 16.Weideman RA, Bernstein IH, McKinney WP. Pharmacist recognition of potential drug interactions. Am J Health-Syst Pharm. 1999;56(15):1524. doi: 10.1093/ajhp/56.15.1524. [DOI] [PubMed] [Google Scholar]
- 17.Trujillo J. A drug interactions elective course. Am J Pharm Educ. 2009;73(4):Article 72. doi: 10.5688/aj730472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warholak TL, Hines LE, Song MC, et al. Medical, nursing, and pharmacy students’ ability to recognize potential drug-drug interactions: a comparison of healthcare professional students. J Am Acad Nurse Pract. 2011;23(4):216–221. doi: 10.1111/j.1745-7599.2011.00599.x. [DOI] [PubMed] [Google Scholar]
- 19.Malone DC, Abarca J, Hansten PD, et al. Identification of serious drug-drug interactions: Results of the partnership to prevent drug-drug interactions. Am J Geriatr Pharmacother. 2005;3(2):65–76. doi: 10.1331/154434504773062591. [DOI] [PubMed] [Google Scholar]
- 20.Hansten PD, Horn JR, Hazlet TK. ORCA: OpeRational ClassificAtion of drug interactions. J Am Pharm Assoc (Wash) 2001;41(2):161–165. doi: 10.1016/s1086-5802(16)31244-x. [DOI] [PubMed] [Google Scholar]
- 21.Warholak TL, Menke JM, Hines LE, Murphy JE, Reel S, Malone DC. A drug-drug interaction knowledge assessment instrument for health professional students: a Rasch analysis of validity evidence. Res Soc Admin Pharm. 2011;7(1):16–26. doi: 10.1016/j.sapharm.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Learning objectives for medical student education–guidelines for medical schools: report I of the Medical School Objectives Project. Acad Med. 1999;74(1):13–18. doi: 10.1097/00001888-199901000-00010. [DOI] [PubMed] [Google Scholar]
- 23.National League for Nursing Accreditation Commission. Accreditation Manual. 2011(2008):104. http://www.nlnac.org/manuals/NLNACManual2008.pdf. Accessed October 3, 2011. [Google Scholar]
- 24.Accreditation Council for Pharmacy Education. Accreditation standards and guidelines for the professional program in pharmacy leading to the doctor of pharmacy degree. 2011(2.0):1–54. http://www.acpe-accredit.org/pdf/S2007Guidelines2.0_ChangesIdentifiedInRed.pdf. Accessed October 3, 2011. [Google Scholar]
- 25.Saverno KR, Malone DC, Kurowsky J. Pharmacy students’ ability to identify potential drug-drug interactions. Am J Pharm Educ. 2009;73(2):Article 27. doi: 10.5688/aj730227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsuruoka S, Kawaguchi A, Harada K, Fujimura A. Favorable effect on postgraduate clinical practice of a drug-interaction exercise for undergraduate students. Eur J Clin Pharmacol. 2006;62(7):571–576. doi: 10.1007/s00228-006-0142-y. [DOI] [PubMed] [Google Scholar]
- 27.Whiting B, Holford NHG, Begg EJ. Clinical pharmacology: principles and practice of drug therapy in medical education. Br J Clin Pharmacol. 2002;54(1):1–2. doi: 10.1046/j.1365-2125.2002.01586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valdez CA, Thompson D, Ulrich H, Bi H, Paulsen S. A comparison of pharmacy students’ confidence and test performance. Am J Pharm Educ. 2006;70(4):Article 76. doi: 10.5688/aj700476. [DOI] [PMC free article] [PubMed] [Google Scholar]