Summary
Growth Hormone (GH) and Insulin-like Growth Factor (IGF-1) stimulate proliferation, differentiation and extracellular matrix production in osteoblastic cells. GH and IGF-1 also stimulate recruitment and bone resorption activity in osteoclastic cells. A chronic systemic GH and IGF-1 excess produces an increased bone turn over in acromegalic patients (pts). Osteoporosis, joint alterations and bone deformities have a great clinical relevance in acromegalic pts and favour mortality and morbility. In the present study we evaluate the still unclear GH/IGF-1 activity on bone, Bone Mineral Density (BMD) and risk of osteoporotic Vertebral Fractures (VF), in relation to gender and gonadal status in acromegalic pts.
Twenty acromegalic pts (12 F, 8 M) ranging 26–64 years were studied. Four pts were hypogonadic (1 F, 3 M), seven women were in post-menopause (PM) and four women eugonadic. The disease was active in twelve pts and inactive in eight pts. Serum and urinary 24/hrs calcium and phosphate and serum PTH, bone formation (P1NP) and resorption (beta-CTX) markers were assayed. BMD was measured using dual energy X ray absorptiometry (DXA) at the lumbar spine and femoral neck and bone quantitative ultrasonography (QUS) at phalanges. Osteoporotic VF were assessed by antero-posterior and lateral x-ray examinations of the thoracic and lumbar spine.
Serum IGF-1, calcium and phosphate and 24-hours urinary calcium were significantly higher in pts with active disease in respect to pts with inactive disease. BMD was reduced in more of 50% of pts, in each skeletal sites measured. Z-score values were lower in males than in females. VF prevalence was 39% (43% in women, 57% in men). Fractured and non-fractured pts were not significantly different for BMD, T-score and Z-score.
In conclusion, VF are frequent in acromegaly and, even mild and asymptomatic, play an important role on life quality and survival, already decreased in acromegalic pts. DXA and QUS methods are not sufficient for identifying pts at risk for fracture, due to the many possible interferences (bone deformities, osteoarthritis, joint rigidity and soft tissue tickening), since BMD is just one determinant of bone fracture. In the screening of acromegalic complications, it is necessary to perform a radiographic study of the spine at the time of diagnosis and during follow up.
Keywords: acromegaly, osteoporosis
Introduction
Growth Hormone (GH) and its peripheral mediator, Insulin-like Growth Factor-1 (IGF-1), play a significant role in the regulation of bone metabolism.
The anabolic actions of GH on many organ systems, including bone, are well documented. During the pre-pubertal period GH stimulates longitudinal bone growth. During the adolescence and early adulthood it stimulates skeletal maturation till the achievement of peak bone mass. In adult age GH is important in the maintenance of bone mass through the regulation of bone turn-over. Serum GH levels decline with increasing age (GH secretion reduces by approximately 14% for each decade of adult life after puberty) and a dysfunctional GH axis may thus play a role in the pathogenesis of post-menopausal and senile osteoporosis. In fact, it has been hypothesized that the ageing process may be also due to a relative GH deficiency state (1).
GH and its interactions with IGFs (insulin-like growth factors) and IGFBPs (insulin-like growth factors binding proteins) and locally produced IGFs and IGFBPs, acting in autocrine and paracrine ways, stimulate proliferation, differentiation and extra-cellular matrix production in osteoblastic like-cell lines and finally bone formation. GH also stimulates recruitment and bone resorption activity in osteoclastic like cells (2–4).
While GH deficiency (GHD) has been shown to be involved in determining bone loss and osteoporosis, the consequences of GH excess on bone are not clear. In fact, traditionally, acromegaly is considered as one cause of secondary osteoporosis; early studies showed hypercalciuria and negative calcium balance. However, bone mineral density (BMD) is not unequivocally reported to be decreased in acromegaly. Some studies showed normal or increased bone mass in patients with acromegaly because of the anabolic effects of GH on bone; although BMD measurements in the axial skeleton may be overestimated in patients with acromegaly because of the structural modifications of the spine occurring in these patients (5). Other studies showed that effect of GH on bone mass could be different in relation to sites investigated: GH excess has a different effect on the axial (70% trabecular bone) and appendicular skeleton (90% cortical bone) with unchanged or reduced vertebral density and increased forearm bone density (5–7). Reduction of bone mass in acromegaly, particularly in trabecular bone, could be also a consequence of many factors; one of the most important is hypogonadism. It is relatively common in patients with acromegaly because of tumor enlargement, associated hyperprolactinemia, surgery, radiation. Sex steroids deficiency leads to an increased rate of bone remodeling, shifting the balance toward bone resorption. It is possible that trabecular bone, with its more intimate contact with the circulation, is influenced by sex steroids to a greater extent (2, 6, 8, 9).
In the present study we evaluated the still unclear GH-IGF-1 activity on bone and risk of osteoporotic fractures, related to the gender, gonadal status, disease activity and BMD.
Subjects and methods
Subjects
Twenty patients with acromegaly (12 females, 8 males) routinely followed at the Clinical Operative Unit of Endocrinology of the Garibaldi – Nesima Hospital (Catania, Italy) were studied (Table 1). The mean age of the patients was 47 years (range 24–64), with a different activity of the disease.
Table 1.
Baseline characteristics and clinical data of acromegalic patients.
| Tot. (20) | F (12) | M (8) | |
|---|---|---|---|
| Age (years) | 47 ± 12.7 | 51.5 ± 10.6 | 40.2 ± 13.3 |
| BMI (kg/m2) | 30.7 ± 6.6 | 30.4 ± 8.0 | 31.0 ± 4.2 |
| Serum GH values (ng/ml) | 18.3 ± 36.3 | 20.7 ± 39.9 | 6.1 ± 6.8 |
| Serum IGF-1 values (ng/ml) | 537.6 ± 556.4 | 464.2 ± 7.4 | 647.6 ± 595.3 |
| Activity | 12 | 7 | 5 |
| Disease duration (years) | 11± 9 | 14 ± 9 | 6 ± 6 |
| Smokers | 17 | 9 | 8 |
| Suppressive therapy with LT4 | 3 | 2 | 1 |
| Drugs | 2 | 1 | 1 |
| Familiarity | 0 | 0 | 0 |
| Menopause | 5 | 5 | |
| Hypogonadism | 4 | 1 | 3 |
| Glucorticoid deficiency | 2 | 1 | 1 |
| Hypothiroidism | 4 | 2 | 2 |
Acromegaly has been defined active when circulating IGF-1 values were elevated relating to an age-sex adjusted normal range and when serum GH concentrations failed to suppress less than 1 ng/ml after a 75-g oral glucose load. On these criteria, twelve patients had active disease.
The duration of previous untreated hypogonadism was not easy to calculate. Four patients (1 female and 3 males) had secondary hypogonadism but only one of them (1 male) was on adequate replacement therapy with androgens. Seven females were in post-menopause.
Two patients had glucocorticoid deficiency but only one of them was adequately replaced at the time of study. Four patients had hypothyroidism (because of thyroidectomy for thyroid tumors or multinodular goiter) and only three were adequately replaced at the time of study.
We looked for other risk factors for osteoporosis. Seventeen patients were smokers without any significant difference between males and females neither between other groups.
Two patients were in therapy with anti-depressant medications and oral anticoagulants.
None had a history of bone diseases or other conditions known to cause osteopenia.
Measurement of BMD and Ad-Sos and quantitative morphometrical assessment of vertebral fractures
All subjects performed DXA; sites measured were the lumbar spine (L1–L4) and the femoral neck (Unigamma Plus – l’acn). Furthermore, QUS (DBM Sonic Bone Profiler – IGEA) and antero-posterior and lateral x-ray examinations of the spine with semi-quantitative method and morphometric analysis to detect osteoporotic vertebral fractures were performed. BMD measured by DXA was expressed as T-score and Z-score and the WHO consensus definitions were used for the diagnosis of osteoporosis (T-score < −2.5 DS) and osteopenia (T-score between −2.5 and −1 DS); results of QUS were expressed as Ad-Sos, T-score and Z-score: values of T-score < − 3.2 and between − 3.2 and − 1 DS were used for diagnosis of osteoporosis and osteopenia respectively. Osteoporotic vertebral fractures were graded in mild, moderate and severe.
Biochemical measurements
GH and IGF-1 were assayed by IRMA; the sensibility of test was 0.04 ng/ml for GH and 0.80 ng/ml for IGF-1 and intra- and inter-assay coefficient of variations was < 5% for GH and 3.4% and 8.2% respectively for IGF-1. Serum calcium and phosphate and urinary 24-hours calcium and phosphate concentrations were assayed by standard colorimetric method; calcium was corrected for albumin concentrations. Markers of bone turn over were also assayed; markers of bone formation (amino-propeptides of type 1 collagene, P1NP) and markers of bone resorption (carboxyterminal-propeptides of type 1 collagene, beta-CTX) were assayed by ECLIA, with sensibility of tests of 5 ng/ml and 0.01 ng/ml respectively.
Statistiscs
Statistical analysis was performed with the software StatView. Descriptive data were expressed as mean +/− SD. Fisher’s test was used to compare categorical variables. Student’s t test for unpaired data was used to compare data with parametric distribution. Data with non-parametric distribution and differences between groups were analyzed with the Mann-Whitney U-test. Relationships between variables were tested by Spearman correlation analysis. A p value of < 0.05 was considered significant.
Results
BMD was reduced in more of 50% of patients in each skeletal sites measured. Osteoporosis/osteopenia were observed in 58% at lumbar spine, in 74% at femoral neck and in 70% by phalanges QUS (Table 2).
Table 2.
Frequency of osteopenia/osteoporosis in acromegalic patients.
| DXA L1-L4 | Tot. (19)* | F (12) | M (7)* |
|---|---|---|---|
| - Osteoporosis | 5 (26%) | 3 (25%) | 2 (29%) |
| - Osteopenia | 6 (32%) | 3 (25%) | 3 (42%) |
| DXA femoral neck | Tot. (19)** | F (11)** | M (8) |
| - Osteoporosis | 6 (32%) | 1 (9%) | 5 (63%) |
| - Osteopenia | 8 (42%) | 6 (55%) | 2 (25%) |
| Bone QUS phalanges | Tot. (20) | F (12) | M (8) |
| - Osteoporosis | 3 (15%) | 1 (8%) | 2 (25%) |
| - Osteopenia | 11 (55%) | 7 (59%) | 4 (50%) |
a man did not perform DXA at lumbar spine
a woman did not perform DXA at femoral neck.
No significant difference was found between women and men in densitometric and ultra-sonographic parameters except for parameters measured by femoral DXA. In fact, men showed a significantly lower femoral T-score and especially Z-score than women (Table 3), regardless of the gonadal status (Table 4).
Table 3.
Mean BMD, T-score and Z-score in acromegalic patients related to gender.
| N. | Tot. (20) | F (12) | M (8) | p values |
|---|---|---|---|---|
| DXA L1-L4 | ||||
| • BMD | 0.95 ± 0.15 | 0.96 ± 0.17 | 0.93 ± 0.11 | 0.72 |
| • T-score | −1.05 ± 1.48 | −0.82 ± 1.70 | −1.43 ± 1.02 | 0.40 |
| • Z-score | −0.50 ± 1.49 | −0.06 ± 1.60 | −1.27 ± 0.95 | 0.09 |
| DXA femoral neck | ||||
| • BMD | 0.79 ± 0.12 | 0.83 ± 0.09 | 0.74 ± 0.15 | 0.12 |
| • T-score | −1.88 ± 1.39 | −1.24 ± 0.98 | −2.76 ± 1.44 | 0.014 |
| • Z-score | −1.23 ± 1.41 | −0.36 ± 0.86 | −2.42 ± 1.13 | 0.0003 |
| QUS phalanges | ||||
| • AdSoS | 2005.6 ± 108.7 | 2003.3 ± 111.2 | 2009.0 ± 112.4 | 0.91 |
| • T-score | −1.69 ± 1.55 | −1.72 ± 1.59 | −1.64 ± 1.60 | 0.91 |
| • Z-score | −0.94 ± 1.46 | −0.68 ± 1.51 | −1.32 ± 1.36 | 0.35 |
Table 4.
Mean BMD, T-score and Z-score in acromegalic men related to gonadal status.
| M tot. | Eugonadism (5) | Hypogonadism (3) | p values | |
|---|---|---|---|---|
| DXA L1-L4 | ||||
| • BMD | 0.93 ± 0.11 | 0.97 ± 0.13 | 0.88 ± 0.06 | 0.28 |
| • T-score | −1.43 ± 1.02 | −1.04 ± 1.20 | −1.95 ± 0.51 | 0.28 |
| • Z-score | −1.27 ± 0.95 | −1.04 ± 1.23 | −1.57 ± 0.43 | 0.51 |
| DXA femoral neck | ||||
| • BMD | 0.74 ± 0.15 | 0.78 ± 0.17 | 0.67 ± 0.08 | 0.35 |
| • T-score | −2.76 ± 1.44 | −2.36 ± 1.68 | −3.44 ± 0.77 | 0.34 |
| • Z-score | −2.42 ± 1.13 | −2.29 ± 1.41 | −2.63 ± 0.60 | 0.71 |
| QUS phalanges | ||||
| • AdSoS | 2009.0 ± 112.4 | 2057.6 ± 97.6 | 1928.0 ± 97.1 | 0.12 |
| • T-score | −1.64 ± 1.60 | −0.95 ± 1.40 | −2.80 ± 1.39 | 0.12 |
| • Z-score | −1.32 ± 1.36 | −0.81 ± 1.42 | −2.18 ± 0.85 | 0.19 |
No significant difference was found between hypogonadal men and eugonadal men in incidence of osteoporosis and densitometric and ultra-sonographic parameters (Table 4). Post-menopausal women had a lower BMD than pre-menopausal women, but not in a significant way, except for Ad-Sos and T-score measured by QUS of phalanges (Table 5).
Table 5.
BMD, T-score and Z-score in acromegalic women related to gonadal status.
| F tot. | Pre-menopause (4) | Post-menopause (7) | p values | |
|---|---|---|---|---|
| DXA L1-L4 | ||||
| • BMD | 0.96 ± 0.17 | 1.07 ± 0.19 | 0.90 ± 0.16 | 0.088 |
| • T-score | −0.82 ± 1.70 | 0.31 ± 1.79 | −1.44 ± 1.54 | 0.089 |
| • Z-score | −0.06 ± 1.60 | 0.53 ± 1.84 | −0.28 ± 1.62 | 0.47 |
| DXA femoral neck | ||||
| • BMD | 0.83 ± 0.09 | 0.84 ± 0.07 | 0.78 ± 0.07 | 0.087 |
| • T-score | −1.24 ± 0.98 | −1.04 ± 0.70 | −1.70 ± 0.77 | 0.088 |
| • Z-score | −0.36 ± 0.86 | −0.75 ± 0.74 | −0.35 ± 0.77 | 0.29 |
| Bone QUS phalanges | ||||
| • AdSoS | 2003.3 ± 111.2 | 2125.5 ± 45.9 | 1965.3 ± 41.5 | 0.0002 |
| • T-score | −1.72 ± 1.59 | 0.02 ± 0.65 | −2.27 ± 0.59 | 0.0002 |
| • Z-score | −0.68 ± 1.51 | 0.15 ± 0.52 | −0.55 ± 0.80 | 0.15 |
No significant difference was found between patients with or without active disease respect DXA and QUS parameters in each examinated sites.
Vertebral fractures prevalence was 39% (43% of observed fractures was in women and 57% in men). Fractures are often asymptomatic and they are largely under-diagnosed. Fractured men often showed two or more fractures and more severe fractures than women (Table 6). Fractures do not correlate with age; in fact, some patients with fractures are young (two men with fractures are < 35 yrs). Fractured and non-fractured patients did not show significant difference in BMD, T-score and Z-score (Table 7).
Table 6.
Frequency and type of fractures in acromegalic patients.
| Tot. (18)* | F (10)* | M (8) | |
|---|---|---|---|
| Pt with fractures | 7 (39%) | 3 (30%) | 4 (50%) |
| Kind of fracture: | |||
| ➢ single | 2 (29%) | 2 (67%) | -- |
| ➢ multiple | 5 (71%) | 1 (33%) | 4 (100%) |
| ➢ mild | 2 (29%) | 1 (33%) | 1 (25%) |
| ➢ moderate | 3 (42%) | 2 (67%) | 1 (25%) |
| ➢ severe | 2 (29%) | -- | 2 (50%) |
2 women did not perform x-ray examinations of the spine.
Table 7.
Mean BMD, T-score and Z-score in acromegalic patients with fractures and in patients without fractures.
| N. (%) | Fracture 7 (39%) | No fracture 11 (61%) |
|---|---|---|
| DXA L1-L4 | ||
| • BMD | 0.97 ± 0.12 | 0.94 ± 0.17 |
| • T-score | −0.92 ± 1.31 | −1.17 ± 1.61 |
| • Z-score | −0.36 ± 1.54 | −0.80 ± 1.32 |
| DXA femoral neck | ||
| • BMD | 0.76 ± 0.10 | 0.81 ± 0.15 |
| • T-score | −2.23 ± 1.29 | −1.62 ± 1.61 |
| • Z-score | −1.42 ± 1.47 | −1.23 ± 1.54 |
| QUS phalanges | ||
| • AdSoS | 1981.1 ± 84.8 | 2029.2 ± 129.3 |
| • T-score | −2.04 ± 1.21 | −1.35 ± 1.85 |
| • Z-score | −1.26 ± 1.32 | −0.82 ± 1.68 |
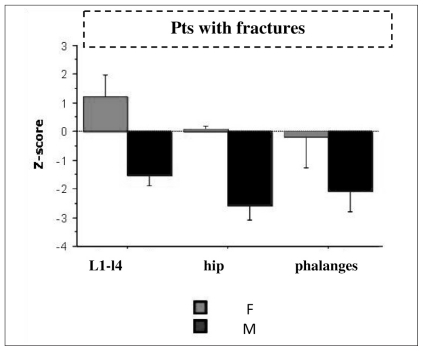
Fractured men showed significantly lower Z-score values than fractured women, at lumbar site (p<0.002), femoral neck (p<0.0003) and by QUS at phalanges (p<0.04) (Figure 1 and Table 8). However, there are not significant difference between men with fractures and men without fractures in DXA and QUS parameters (Table 9).
Figure 1.
Mean Z-score in acromegalic men and women with fractures.
Table 8.
BMD, T-score and Z-score in acromegalic patients with fractures related to gender.
| Pts with fractures | |||
|---|---|---|---|
| F (3) | M (4) | P values | |
| DXA L1-L4 | |||
| • BMD | 1.06 ± 0.11 | 0.89 ± 0.06 | 0.04 |
| • T-score | 0.25 ± 1.06 | −1.80 ± 0.52 | 0.02 |
| • Z-score | 1.20 ± 0.77 | −1.52 ± 0.36 | 0.002 |
| DXA femoral neck | |||
| • BMD | 0.85 ± 0.02 | 0.70 ± 0.09 | 0.04 |
| • T-score | −1.03 ± 0.21 | −3.13 ± 0.88 | 0.01 |
| • Z-score | 0.10 ± 0.09 | −2.56 ± 0.51 | 0.0003 |
| QUS phalanges | |||
| • AdSoS | 2028.7 ± 66.2 | 1945.5 ± 86.7 | 0.23 |
| • T-score | −1.36 ± 0.94 | −2.55 ± 1.24 | 0.23 |
| • Z-score | −0.16 ± 1.12 | −2.08 ± 0.72 | 0.04 |
Table 9.
Mean BMD, T-score and Z-score in acromegalic men with fractures and in men without fractures.
| Males (8) | ||
|---|---|---|
| With fractures 4 (50%) | Without fractures 4 (50%) | |
| DXA L1-L4 | ||
| • BMD | 0.89 ± 0.06 | 0.99 ± 0.16 |
| • T-score | −1.80 ± 0.52 | −0.94 ± 1.45 |
| • Z-score | −1.52 ± 0.36 | −0.92 ± 1.48 |
| DXA femoral neck | ||
| • BMD | 0.70 ± 0.09 | 0.78 ± 0.20 |
| • T-score | −3.13 ± 0.88 | −2.40 ± 1.93 |
| • Z-score | −2.56 ± 0.51 | −2.27 ± 1.62 |
| QUS phalanges | ||
| • AdSoS | 1945.5 ± 86.7 | 2072.5 ± 106.0 |
| • T-score | −2.55 ± 1.24 | −0.73 ± 1.51 |
| • Z-score | −2.08 ± 0.72 | −0.56 ± 1.51 |
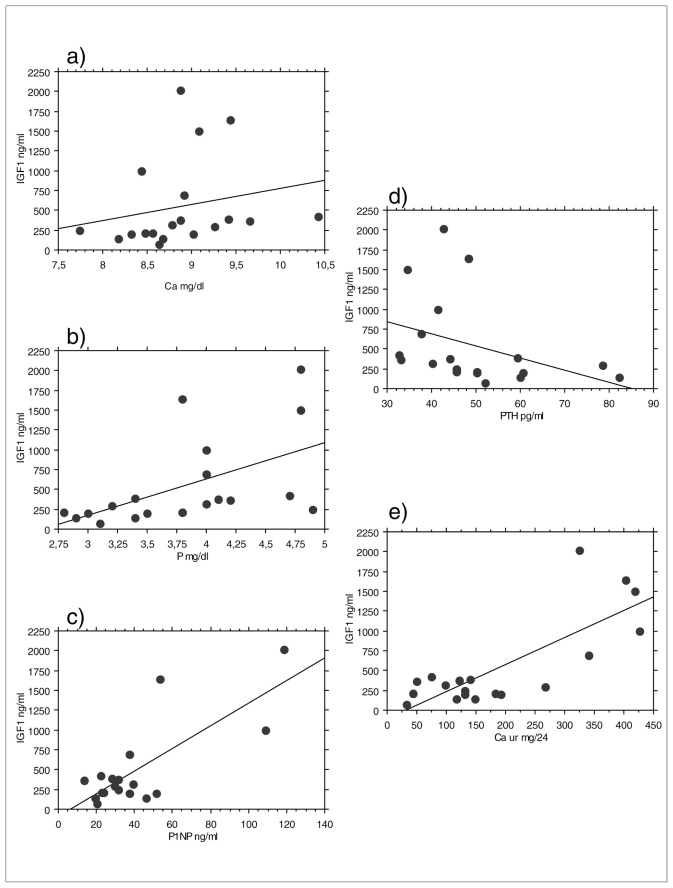
Calcium and phosphate values and 24-hours urinary calcium levels were significantly higher in patients with active disease than in patients with inactive disease (Table 10). There was a significant positive correlation between IGF-1 values and serum calcium (p<0.04), serum phosphate (p<0.006), urinary 24-hours calcium (p<0.02) and serum P1NP (p<0.04). There was a significant negative correlation between IGF-1 values and PTH (p<0.007) (Figure 2). No significant difference between men and women, hypogonadal and eugonadal patients in biochemical markers of bone metabolism was found.
Table 10.
Serum IGF-1, calcium and phosphate and urinary 24-hours calcium in acromegalic patients with active disease respect to patients with inactive disease.
| Total (20) | Active disease (12) | Inactive disease (8) | P values | |
|---|---|---|---|---|
| IGF-1 (ng/ml) | 537.6 ± 556.4 | 777.4 ± 613.2 | 178.0 ± 56.0 | 0.0002 |
| Serum calcium (mg/dl) | 8.9 ± 0.6 | 9.2 ± 0.5 | 8.4 ± 0.4 | 0.004 |
| Phosphate (mg/dl) | 3.8 ± 0.7 | 1.1 ± 0.5 | 3.4 ± 0.7 | 0.03 |
| Urinary calcium (mg/24 ore) | 191.8 ± 130.8 | 242.4 ± 147.5 | 122.4 ± 58.1 | 0.04 |
Figure 2.
Correlation between IGF-1 values and biochemical parameters of bone metabolism. Panel a) serum calcium (Ca); b) serum phosphate (P); c) P1NP; d) PTH; e) urinary calcium.
Discussion
These data show that the effects of GH and IGF-1 on bone mass, bone quality and bone mineral metabolism and therefore on risk of osteoporosis and osteoporotic fractures in patients with acromegaly, are complex and not yet clearly defined.
Osteoporosis/osteopenia were observed in 58–74% of our patients by DXA and QUS. No significant difference between women and men in densitometric and ultra-sonographic parameters except for femoral DXA was found. In fact, men showed a significantly lower femoral T-score and especially Z-score than women, regardless of the disease activity and gonadal status.
Seeman et al. reported that BMD was increased in the lumbar spine in acromegaly and suggested that osteopenia was probably rare in acromegaly not complicated by hypogonadism (10). On the other hand, in their study, Ueland et al. did not report significant differences between hypogonadal and eugonadal patients with acromegaly in bone mass at any sites, although hypogonadal patients generally showed lower scores. Furthermore, they reported a decreased total body BMD in women, not men, with active acromegaly than non-acromegalic post-menopausal patients, regardless of gonadal status (11).
The absence of relationship between gonadal status and BMD in our series cannot clearly be established, not only because of the small number of patients observed, but also because of the great heterogeneity of variables such as severity and duration of hypogonadism, the presence of other anterior pituitary deficiencies, duration of GH excess, age.
Disease activity is an important factor which influences the bone mass. Scillitani et al. described increased BMD, especially at femoral level, related to serum IGF-1 levels in patients with active disease (12). Other studies reported long-term maintenance of the anabolic effects of GH on the skeleton and BMD in successfully treated patients with acromegaly and therefore after remission of acromegaly (11, 13). In our study we did not observe significant difference between patients with or without active disease.
Only few studies have reported increased fracture frequency in patients with acromegaly (14, 15). Vertebral fractures are often asymptomatic and not diagnosed or detected and, even mild and asymptomatic, they have a significant prognostic impact. Vertebral fractures do not correlate with BMD, they are also found in patients with normal or minimally decreased BMD (3, 14). In fact, in many forms of secondary osteoporosis, BMD is not such good predictor of fracture risk as it is in post-menopausal osteoporosis. Therefore, the radiological morphometric approach is necessary for an accurate detection of vertebral fractures (16). They occurr more frequently in patients with active acromegaly as compared with patients whose disease is controlled by somatostatin analogs (15).
We observed vertebral fractures even in patients with normal T-score and other densitometric and ultra-sonographic parameters. Therefore, DXA and QUS measurements may not reflect the bone health status; they are just one determinant of bone fractures and are not sufficient for identifying patients at risk for fracture. This finding is in agreement with previous reports (3, 14). Moreover, BMD measurements at the lumbar spine may be strongly influenced by the structural modifications of this site (bone deformities, osteoarthritis, joint rigidity) and in the same way QUS measurements by soft tissue tickening occurring in patients with acromegaly. Osteoporotic fractures reported in acromegalic patients could be correlated with an insufficient quality of bone, one of the most important determinant of bone health.
We did not observe any significant correlation between fractures and activity of disease or gonadal status, neither a significant difference between men and women in prevalence of vertebral fractures; men often showed multiple and more severe fractures than women (maybe for a different sex steroids-modulated sensitivity of tissues, including bone, to the GH action).
A chronic GH and IGF-1 excess produces an increased bone turnover, reflected in an increase of bone formation and resorption markers; bone resorption markers are disproportionately increased respect bone formation markers and their increase could reflect the degree of bone loss often observed.
As in previous, in our study biochemical markers as PTH, serum calcium and phosphate, urinary calcium and bone turn-over markers correlate with circulating GH and IGF-1 levels; therapy-induced IGF-1 normalization in patients with acromegaly returns these elevated markers to normal (2, 3, 5, 17, 18).
In fact, serum IGF-1, calcium and phosphate values and 24-hours urinary calcium were significantly higher in patients with active disease than patients with inactive disease. Rise in serum calcium levels may be a consequence of increased intestinal calcium absorption (GH-mediated action through vit. D), reflected by an elevation of urinary/24 hours calcium. Rise in serum phosphate levels may be due to GH-mediated increased absorption at intestinal and renal level.
Some studies reported higher PTH concentrations in patients with active acromegaly as a result of GH induced parathyroid gland hyperstimulation (19), but in our study we did not observe a significant difference between patients with active and inactive acromegaly regard PTH levels. In fact, increase of PTH levels in patients with lower IGF-1 levels could be a consequences of hypocalcemia, probably secondary to therapy of acromegaly; it could be attributed to a reduction of calcium intestinal absorption observed in patients treated with somatostatin analogs (SSA) (19, 20).
Previous studies reported an increase of PTH levels but a reduction in its target organ sensitivity because of abnormalities in PTH circadian rhythmicity in GHD patients and also in successfully treated patients (19) because of GH deficiency due to the therapy (13). In fact, GH seems to be an important regulator of PTH secretion and target organ sensitivity.
In conclusion, our data suggest that acromegaly is a risk factor for osteoporotic fractures per se, especially in men, generally considered at low risk of osteoporosis.
Since vertebral fractures, even mild and asymptomatic, play an important role on life quality, already decreased in acromegalic patients, it is necessary to perform a radiographic study of the spine at the time of diagnosis and during follow up. A specific anti-osteoporotic treatment is necessary in presence of osteoporosis and, moreover, of fractures. In fact osteoporosis, joint alterations and bone deformities have a great clinical relevancy in acromegalic patients and favour morbility and mortality and they should be prevented or early detected and treated.
References
- 1.White HD, Ahmad AM, Durham BH, Patwala A, Whittingham P, Fraser WD, Vora JP. Growth Hormone Replacement is important for the Restoration of Parathyroid Hormone sensitivity and Improvement in Bone Metabolism in Older Adult Growth Hormone-Deficient Patients. JCEM. 2005;90( 6):3371–3380. doi: 10.1210/jc.2004-1650. [DOI] [PubMed] [Google Scholar]
- 2.Andreassen TT, Oxlund H. The effect of GH on cortical and cancellous bone. J Muscoloskel Neuron Interact. 2001;2( 1):49–58. [PubMed] [Google Scholar]
- 3.Giustina A, Mazziotti G, Canalis E. Growth hormone, insulin-like growth factors and the skeleton. Endocr Rev. 2008;29:535–559. doi: 10.1210/er.2007-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ueland T. GH/IGF-1 and bone resorption in vivo and in vitro. European Journal of Endocrinology. 2005;152:327–332. doi: 10.1530/eje.1.01874. [DOI] [PubMed] [Google Scholar]
- 5.Colao A, Ferone D, Marzullo P, Lombardi G. Systemic complications of acromegaly: epidemiology, pathogenesis and management. Endocr Rev. 2004;25:102–52. doi: 10.1210/er.2002-0022. [DOI] [PubMed] [Google Scholar]
- 6.Jean Ho P, Lorraine M, Barkan A, Shapiro B. Bone mineral density of the Axial Skeleton in Acromegaly. J Nucl Med. 1992;33:1608–1612. [PubMed] [Google Scholar]
- 7.Biermasz NR, Hamdy NA, Pereira AM, Romijn JA, Roelfsema F. Long-term maintenance of the anabolic effects of GH on the skeleton in successfully treated patients with acromegaly. Eur J Endocrinol. 2005;152:53–60. doi: 10.1530/eje.1.01820. [DOI] [PubMed] [Google Scholar]
- 8.Bonadonna S, Mazziotti G, Nuzzo M, et al. Increased prevalence of radiological spinal deformities in active acromegaly: a cross-sectional study in post-menopausal women. J Bone Miner Res. 2005;20:1837–44. doi: 10.1359/JBMR.050603. [DOI] [PubMed] [Google Scholar]
- 9.Battista C, Chiodini I, Muscarella S, Guglielmi G, Mascia ML, Carnevale V, Scillitani A. Spinal volumetric trabecular bone mass in acromegalic patients: a longitudinal study. Clin Endocrinol (Oxf) 2009 Mar;70(3):378–82. doi: 10.1111/j.1365-2265.2008.03322.x. [DOI] [PubMed] [Google Scholar]
- 10.Seeman E, Wahner HW, Offord KP, Kumar R, Johnson WJ, Riggs BL. Differential effects of endocrine dysfunction on the axial and the appendicular skeleton. J Clin Invest. 1982;69(6):1302–9. doi: 10.1172/JCI110570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ueland T, Fougner SL, Godang K, Schreiner T, Bollerslev J. Serum GH and IGF1 are significant determinants of bone turnover but not bone mineral density in active acromegaly: a prospective study of more than 70 consecutive patients. Eur J Endocrinol. 2006;155:709–715. doi: 10.1530/eje.1.02285. [DOI] [PubMed] [Google Scholar]
- 12.Scillitani A, Battista C, Chiodini I, et al. Bone mineral density in acromegaly: the effect of gender, disease activity and gonadal status. Clin Endocrinol. 2003;58:725–731. doi: 10.1046/j.1365-2265.2003.01777.x. [DOI] [PubMed] [Google Scholar]
- 13.Biermasz NR, Hamdy NA, Pereira AM, Romijn JA, Roelfsema F. Long-term maintenance of the anabolic effects of GH on the skeleton in successfully treated patients with acromegaly. Eur J Endocrinol. 2005;152:53–60. doi: 10.1530/eje.1.01820. [DOI] [PubMed] [Google Scholar]
- 14.Mazziotti G, Bianchi A, Bonadonna S, Cimino V, Patelli I, Fusco A, Pontecorvi A, De Marinis L, Giustina A. Prevalence of vertebral fractures in men with acromegaly. JCEM. 2008;93( 12):4649–4655. doi: 10.1210/jc.2008-0791. [DOI] [PubMed] [Google Scholar]
- 15.Bonadonna S, Mazziotti G, Nuzzo M, et al. Increased prevalence of radiological spinal deformities in active acromegaly: a cross-sectional study in post-menopausal women. J Bone Miner Res. 2005;20:1837–44. doi: 10.1359/JBMR.050603. [DOI] [PubMed] [Google Scholar]
- 16.Genant H, et al. J Bone Miner Res. 1993;8:137–42. [Google Scholar]
- 17.Ezzat, et al. Biochemical Assessment of Bone Formation and Resorption in Acromegaly. JCEM. 1993;78:1552–1457. doi: 10.1210/jcem.76.6.8501150. [DOI] [PubMed] [Google Scholar]
- 18.Parkinson C, Kassem M, Heickendorff L, Flyvbjerg A, Trainer PJ. Pegvisomant-induced serum insulin-like growth factor-1 normalization in patients with acromegaly returns elevated markers of bone turnover to normal. J Clin Endocrinol Metab. 2003;88:5650–5655. doi: 10.1210/jc.2003-030772. [DOI] [PubMed] [Google Scholar]
- 19.White, et al. Effect of Active Acromegaly and Its treatment on Parathyroid Circadian Rhythmicity and Parathyroid Target-Organ Sensitivity. JCEM. 2005;91( 3):913–919. doi: 10.1210/jc.2005-1602. [DOI] [PubMed] [Google Scholar]
- 20.Cappelli C, Gandossi E, Agosti B, Cerudelli B, Cumetti D, Castellano M, Pirola I, De Martino E, Rosei E. Long term treatment of acromegaly with Lanreotide: evidence of increased serum parathormone concentration. Endocr J. 2004;51(6):517–520. doi: 10.1507/endocrj.51.517. [DOI] [PubMed] [Google Scholar]