Summary
Cardiovascular involvement is relatively rare in osteogenesis imperfecta and has a predilection for left-sided cardiac valves. We report a 5 years old female child affected by osteogenesis imperfecta type I in which an asymptomatic mild form of Ebstein’s anomaly, a congenital tricuspid malformation, was diagnosed during routinely investigation. The association of these two relatively rare entities could provide new insight to better understand the pathogenesis of cardiac involvement in osteogenesis imperfecta.
Keywords: Ebstein’s anomaly, osteogenesis imperfect, valvular heart disease, collagen type I
Introduction
Ebstein’s anomaly (EA) is an uncommon congenital heart defect occurring in about 1 per 20.000 live births and accounting for 0.5–1% of all congenital heart disease. The malformation is characterized by downward displacement of the septal and posterior leaflets of the tricuspid valve with adherence to the underlying myocardium. The degree of displacement varies from minimal to very severe. The proximal portion of the right ventricle in continuity with the right atrium is thin and dysplastic and defined as “atrialized”. EA shows a spectrum of phenotypic expressions, mainly due to hemodynamic effects, ranging from severe forms incompatible with neonatal life to mild form that may come to clinical attention late in life, if at all. It may present at any age and variable clinical courses are correlated with the age of presentation (1).
Osteogenesis Imperfecta (OI) is an inherited disorder that most often involves a mutation in one of the two genes (COL1A1, COL1A2) for type I collagen, a major structural component of musculo-skeletal and cardiovascular connettive tissue, occurring in 1 out of 20,000 to 30,000 live births. To categorize OI patients according their different clinical presentation in 1979 Sillence et al. proposed a classification based on 4 different groups (I–IV) subsequently updated to the current VIII (2). Multiple fractures and skeletal deformity are the hallmark of OI but clinical presentation could also involve different apparatus like eyes, dentine, middle and inner ear, integument, tendons, ligaments and cardiovascular structures. Cardiovascular involvement is relatively rare in OI and has a predilection for left-sided cardiac valves (3).
Herein is reported the case of a five-years old child affected by OI type I associated to a mild form of EA, a congenital right- sided cardiac disease.
Case report
The female proband was the first child of healthy, unrelated and phenotipically normal parents. She was born at term by spontaneous delivery after an uneventful pregnancy. Starting from 20 days of life multiple bone fractures associated with low bone density in the entire skeleton were detected. No further evaluation has been performed until she was referred to the Pediatric Department of the University of Rome at the age of five to confirm the diagnosis. Clinical investigations revealed normal body weight and height referred to age and sex. Faintly bleu sclerae, normal dentinogenesis and dysmorfic triangular face with frontal bossing, hollow-eyes, hypotelorism, micrognathia were also present. Ligamentous laxity, generalized hypotonia and hypotrofia, legs eterometria, bowlegs, mild dorsal iper-kyphosis, bilateral coxa valga, flat valgus feet were also detected at clinical examination. Cardiac evaluation revealed normal perfusion and pulses, normal first and second heart sound without murmurs. Her blood pressure was always within the normal range and her heart rate was regular. The patient did not experience arrhythmias, exercise intolerance or other symptoms associated with cardiac failure. Electrocardiogram and chest X-radiography were normal. A transthoracic echocardiography, performed as routinely investigation for OI, showed a mild displacement of the septal leaflet of the tricuspid valve towards the apex of the right ventricle with a moderate regurgitation (Figure 1). A small atrial septal defect ostium II type with left-to-right shunt was associated. The systolic pulmonary arterial pressure was about 30 mmHg. On the basis of these features a diagnosis of EA was made. Mutation analysis, based on DHPLC screening, revealed a nonsense mutation [c.199A>T; p.Lys67X] in exon 2 of COL1A1 gene consistent with OI type I diagnosis. The patient has been treated with cyclical infusion of neridronate for 18 months and a removal of Rush nails with intramedullary fixation with Ten nail was performed. The pharmacological and surgical treatment resulted in slight improvement of her clinical condition. The patient was lost after two years of follow-up.
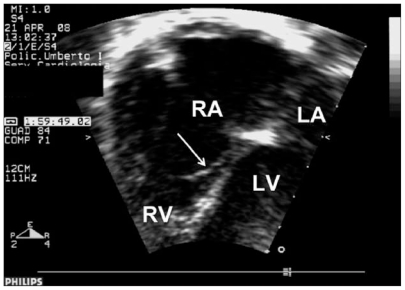
Figure 1.
Two-dimensional echocardiography. Apical 4-chamber view shows a mild displacement of the septal leaflet of the tricuspid valve (arrow). The anterior leaflet is normally mobile. RA, right atrium; LA, left atrium; RV, right ventricle; LV, left ventricle.
Discussion
We report a child affected by OI type I with a mild form of Ebstein’s anomaly. During follow-up the patient did not present arrhythmias, exercise intolerance or other symptoms associated with cardiac failure. It is not surprising considering that many patients with mild forms of EA remain asymptomatic until adult age and in our case the phenotypic expression of cardiac symptoms could be prevented by OI-induced skeletal deformities causing reduced functional capacity to undertake exercise.
Unlike the other more common connective tissue disorders, cardiovascular involvement is relatively rare in OI, and the degree of cardiovascular involvement does not correlate with the severity of skeletal disease. The disease usually affects left-sided cardiac structure being aortic root dilation the most commonly reported cardiovascular abnormality with a prevalence ranging from 12% to 28% in different series (3). The most common left-side valvular abnormality is aortic regurgitation, followed by mitral regurgitation and mitral valve prolapse. Other less common cardiac manifestations include aortic dissection, sinus of Valsalva aneurysm, and coronary artery dissection. These abnormalities tend to occur late in the disease reflecting connective tissue abnormalities of valvular apparatus represented by cystic medial necrosis, myxoid degeneration, decreased and disorganized collagen architecture. Animal models of OI, suggested that increased hemodynamic stress on the left side of the heart may play a role in the preponderance of left cardiac pathology (4).
To date only three patients affected by OI have been reported with right-side cardiac involvement. Khashu et al. (5) described a neonate with a severely dysplastic tricuspid valve and right ventricular hypertrophy that was difficult to differentiate from tricuspid dysfunction secondary to intrauterine circulatory abnormalities leading to postnatal pulmonary hypertension. In the other two cases a diagnosis of EA was made (6,7). The first concerns a 46-years-old woman that presented in addition prolapse of the mitral and aortic valves. The second report refers to the autopsy findings of a neonate affected by a severe form of EA without alteration of the left side of the heart including the aortic and mitral valves. Authors suggested that the development of EA may reflect a systemic expression of a defect in collagen synthesis. The involvement of genes codifying collagen synthesis in the pathogenesis of EA seems to be supported by the occurrence of this rare cardiac abnormality in other connective tissue genetic disorder that include Marfan’s syndrome and Ehlers-Danlos syndrome. Of interest are the results of genetic studies performed on canine animal model affected by tricuspide valve malformation, the equivalent of EA in dogs. The authors identified a group of candidate genes located in a critical interval that is homologous to a gene rich region of human chromosome 17q 12 to 17q 23, including COL 1A1 (8). In this view, it is likely that in OI patients with this rare congenital heart disease, the genetic mutation of collagen type I could alter the valvular morphogenesis in the prenatal period. Therefore, mutations of COL1A1 gene seems to be at the same time an etiologic factor for the congenital right sided malformation as well as for the acquired stress-induced and late onset cardiac anomalies in the left side described in adults.
In conclusion, our case reinforces the importance of collagen type I in valvular morphogenesis suggesting a pathogenetic role of mutational events of COL1A1 in the development of EA. The association of these two relatively rare entities could provide new insight to better understand the pathogenesis of cardiac involvement in OI.
References
- 1.Paranon S, Acar P. Ebstein's anomaly of the tricuspid valve: from fetus to adult: congenital heart disease. Heart. 2008;94:237–43. doi: 10.1136/hrt.2006.105262. [DOI] [PubMed] [Google Scholar]
- 2.Cheung MS, Glorieux FH. Osteogenesis Imperfecta: update on presentation and management. Rev Endocr Metab Disord. 2008;9:153–60. doi: 10.1007/s11154-008-9074-4. [DOI] [PubMed] [Google Scholar]
- 3.Hortop J, Tsipouras P, Hanley JA, Maron BJ, Shapiro JR. Cardiovascular involvement in osteogenesis imperfecta. Circulation. 1986;73:54–61. doi: 10.1161/01.cir.73.1.54. [DOI] [PubMed] [Google Scholar]
- 4.Weis SM, Emery JL, Becker KD, McBride DJ, Omens JH, McCulloch AD. Myocardial mechanics and collagen structure in the osteogenesis imperfecta murine (oim) Circ Res. 2000;87:663–9. doi: 10.1161/01.res.87.8.663. [DOI] [PubMed] [Google Scholar]
- 5.Khashu M, Pelligra G, Sandor GGS, Singh AJ. Right-Sided cardiac involvement in osteogenesis imperfecta. J Heart Valve Dis. 2006;15:588–90. [PubMed] [Google Scholar]
- 6.Kawano T, Oki T, Tominaga T, Ohkushi H, Uchida T, Iuchi A, Fukuda N, Kawano K, Okumoto T, Mori H. Van der Hoeve's syndrome with Ebstein's anomaly, and prolapse of the mitral and aortic valves: a case report. J Cardiol. 1988;18:1173–82. [PubMed] [Google Scholar]
- 7.Warshaver Y, Bearer C, Belchis DA. Osteogenesis imperfecta and Ebstein's anomaly: a case report with autopsy findings. Pediatr Pathol. 1992;12:425–31. doi: 10.3109/15513819209023321. [DOI] [PubMed] [Google Scholar]
- 8.Andelfinger G, Wright KN, Lee HS, Siemens LM, Benson DW. Canine tricuspid valve malformation, a model of human Ebstein anomaly, maps to dog chromosome 9. J Med Genet. 2003;40:320–4. doi: 10.1136/jmg.40.5.320. [DOI] [PMC free article] [PubMed] [Google Scholar]