Summary
Hypercalcaemia is most commonly caused by primary hyperparathyroidism or malignancy. Vitamin D intoxication, also a cause of hypercalcaemia, is mostly caused by excessive administration of vitamin D-containing medications and excessive intake of foods fortified with vitamin D. We present a young cricketer, with recurrent vomiting due to hypercalcaemia and hypervitaminosis D, who used to drink large volumes of soup prepared by boiling long beef bones, for many months. This case presentation highlights the importance of in-depth dietary history for arriving at proper diagnosis.
Keywords: hypercalcaemia, hypervitaminosis D, dietary history
Introduction
Among many causes, hypercalcaemia is an important metabolic cause of recurrent vomiting, anorexia and dyspepsia (1). In majority of the patients routine history, physical examination and investigations help to find out the cause and source of the hypercalcaemia. We present a young man, who had hypercalcaemia due to hypervitaminosis D, in whom only in-depth and repeated dietary history helped to find out source of excess vitamin D and calcium.
Case report
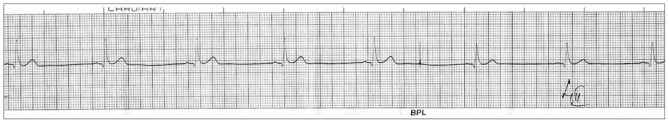
A 29-year-old man presented to us in July, 2009 with 2 months history of postprandial abdominal fullness, excessive thirst, nocturia and generalised weakness. For the past one month he was having recurrent vomiting, anorexia and constipation. He had no history of fever, headache, visual blurring, vertigo, colic, blood in stools, breathlessness, cough or jaundice. He was a smoker, non-alcoholic, and was not taking any prescribed medications or illicit drugs prior to onset of symptoms. Noncontrast computerised tomography (CT) scan of head, barium study of upper gut and oesophagogastroduodenoscopy tests, which had been done prior to presentation to us, were unremarkable. His physical examination was remarkable only for dehydration. His blood urea was 98mg/dL (reference range RR: 10–40 mg/dL) and serum creatinine was 3.1 mg/dL (RR: 0.6–1.2 mg/dL). Ultrasound of abdomen revealed bilateral increased renal cortical echogenicity. Complete blood count, plasma glucose, serum cortisol, thyroid function tests, serum sodium, potassium and phosphate levels, and liver function tests including serum albumin and alkaline phosphatase were within reference range. Arterial blood gas analysis sample revealed no abnormality, except an increase ionised calcium concentration of 1.7 mmol/L (RR:1.13–1.32 mmol/L). His tests for hepatitis B and C viruses were nonreactive. Electrocardiogram showed shortened corrected QT interval (Figure 1). After adequate rehydration with normal saline, we took appropriate serum samples for assays of parathormone (Intact), 25(OH)D3 and 1,25(OH)2D3. These were 9.6 pg/mL (RR:15–65 pg/mL), 1146 ng/mL (RR:16–70 ng/mL) and 103.7 pg/mL (RR:19.6–54.3) respectively. We administered intravenous hydrocortisone 200mg daily for 4 days. He improved remarkably. When his serum creatinine improved to 1.6mg/dL, we did CT scan of his abdomen and chest to rule out granulomatous disease, as the source of his excess vitamin D. He denied use of medications containing vitamin D and also excess intake of its usual dietary sources. He used to play cricket for long hours in sunshine. After repeated enquiry about his diet he revealed that in the belief of improving his stamina, he used to drink 1–2 litres of soup, prepared by prolonged boiling of long beef bones, at least 3 days every week, for 6 months prior to falling ill.
Figure 1.
ECG of the patient with hypercalcaemia due to hypervitaminosis D showing shortening of QT interval.
Discussion
Majority of patients with hypercalcaemia will be found to have primary hyperparathyroidism or malignancy. Long list of other causes constitutes fewer than 10% of hypercalcaemia patients (2). Two categories of hypercalcaemia are a) parathyroid dependent hypercalcaemia, in which excess parathormone is not appropriately suppressed by increased serum calcium and b) parathyroid independent hypercalcaemia, in which parathormone production is suppressed by excess serum calcium (2). Our patient belonged to the latter category, as his parathormone level was low. Vitamin D intoxication is one of the causes of parathyroid independent hypercalcaemia. Sources of vitamin D in the circulation are photochemical synthesis in the skin, its pathological production by various granulomas (3) and its exogenous intake. Alternative photoisomerisation of vitamin D to biologically inert products prevents its excessive production, with prolonged sun exposure (4). Therefore playing in sunshine for long hours cannot be a cause of hypervitaminosis D in our patient. Granulomatous diseases as a source of excess vitamin D in our patient was ruled out by normal CT scan chest and abdomen, much higher levels of 25(OH)D3 than 1,25(OH)2D3 which goes against the diagnosis of granulomatous disease. Although vitamin D is present in many food sources, both vegetable and animal, major dietary sources of vitamin D are fortified dairy products, egg yolk, fish oils and fortified cereal products. Injectable and oral preparations of vitamin D are also its important exogenous sources (4, 5). Our patient denied use of any vitamin D containing medications, or ingestion of excessive amounts of usual dietary sources of vitamin D. His dietary history was remarkable for daily ingestion of large volumes of soup prepared from beef bones. Since vitamin D is stored in fat (4), source of excessive vitamin D in our patient seems to be this “toxic” beef bone soup, with vitamin D coming from fatty marrow. In conclusion, this case report underscores the importance of in-depth dietary history for arriving at diagnosis particularly in young adults and adolescents in whom food fads are very common.
References
- 1.Cho KC, Fukagawa M, Kurokawa K. Fluid and electrolyte disorders. In: McPhee SJ, Papadakis MA, editors. P. Current medical diagnosis and treatment. 48th edn. New York: McGraw Hill; 2009. pp. 766–93. [Google Scholar]
- 2.Jacobs Thomas P, Bilezikian John P. Rare causes of hypercalcaemia. J Clin Endocrinol Metab. 2005;90:6316–22. doi: 10.1210/jc.2005-0675. [DOI] [PubMed] [Google Scholar]
- 3.Kallas M, Green F, Hewison M, White C, Kline Gregory. Rare Causes of Calcitriol-Mediated Hypercalcemia: A Case Report and Literature Review. J Clin Endocrinol Metab. 2010 Jul;95(7):3111–3117. doi: 10.1210/jc.2009-2673. [DOI] [PubMed] [Google Scholar]
- 4.Bringhurst FR, Demay MB, Kronenberg HM. Hormones and disorders of mineral metabolism. In: Larsen PR, Kronenberg HM, Melmed S, Polonsky KS, editors. Williams textbook of endocrinology. 10th edn. Philadelphia: Saunders; 2003. pp. 1303–61. [Google Scholar]
- 5.Baron RB. Nutritional disorders. In: McPhee SJ, Papadakis MA, editors. Current medical diagnosis and treatment. 48th edn. New York: McGraw Hill; 2009. pp. 1107–25. [Google Scholar]