Abstract
Objective:
Leopard syndrome is an acronym (multiple Lentigines, electrocardiographic conduction abnormalities, ocular hypertelorism, pulmonic stenosis, abnormal genitalia, retardation of growth, and sensorineural deafness) describing an autosomal dominant disease due to mutations in the raS-MapK pathway.
Methods:
Here, we describe a family (mother and daughter) with clinical and molecular diagnosis of Leopard syndrome 1 and HCM, and we report the prenatal diagnosis of HCM in a fetus at risk for Leopard syndrome.
Results:
An echocardiography was conducted showing a significant hypertrophy of both ventricles (left and right ventricular wall thickness 9mm and 3 mm). After a multidisciplinary counseling the couple opted for the termination of pregnancy
Conclusion:
Further genotype-phenotype studies are warranted to fully elucidate the impact of the genotype on the natural history of patients with LS and LVH
Keywords: prenatal diagnosis, LEOPARD syndrome, hypertrophic cardiomyopathy, natural history
Case Report
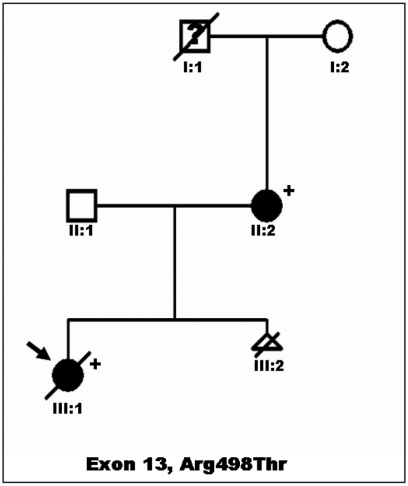
The family pedigree is showed in Figure 1. A 32-year-old woman with LEOPARD syndrome with multiple lentigines, facial dysmorphia, short stature, and mild, non obstructive HCM (asymmetric left ventricular hypertrophy, with maximal wall thickness of 14 mm at the mid-portion of the posterior interventricular septum, in absence of outflow tract obstruction) was referred to our Department for foetal echocardiography and genetic counselling during her second pregnancy. Her first pregnancy resulted in a daughter (the proband) who was born with a severe form of obstructive HCM, and pulmonary valvar and subvalvar stenosis. She had cafè au lait spots on her trunk, hypertelorism, broad nasal bridge, pterigium colli, pectus excavatum and short stature. She was administered with beta blockers (7 mg/kg/day) for her cardiomyopathy, but she died suddenly at 2 years of age. Clinical diagnosis of LEOPARD syndrome was confirmed by genetic analysis showing a mutation in exon 13 of the PTPN11gene (codon 498), in both mother and daughter.
Figure 1.
Family Pedigree: an Italian kindred with LEOPARD syndrome and hypertrophic cardiomyopathy. Proband is indicated by arrow. Circles indicate females; squares, males; triangle, sex unknown; open symbols, unaffected individuals; filled symbols, individuals affected (by LEOPARD syndrome); symbols with slash are deceased individuals; question mark, unknown clinical status; plus sign, presence of mutation (i.e., PTPN11 mutation); minus sign, absence of mutation.
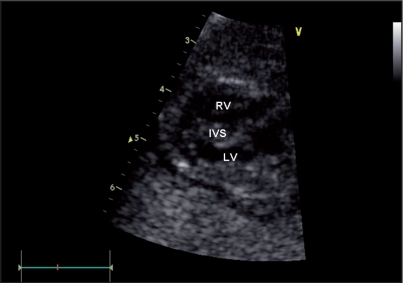
During the second pregnancy, prenatal scan at 22 weeks of gestation evidenced fetal growth restriction (about 3 weeks), and fetal echocardiography showed a significant hypertrophy of both ventricles (left and right ventricular wall thickness 9mm and 3 mm, respectively) (Figure 2). Systolic function was slightly depressed (ejection fraction measured by biplane Simpson method was 45%), and diastolic function was abnormal.
Figure 2.
Fetal echocardiography showing hypertrophic cardiomyopathy.
The parents were counselled by a team of physicians (gynaecologists, paediatric cardiologists, psychologists, and geneticists) regarding the clinical manifestations and outcome of LEOPARD syndrome in general and HCM associated with this condition in particular and decided to terminate the pregnancy.
Discussion
HCM is an etiologically heterogeneous condition with intrafamilial and interfamilial variability in the clinical manifestations (8). Natural history of early onset HCM is extremely variable (9, 10). Pedra et al. described prenatal characteristic and postnatal outcome of 33 foetuses with hypertrophic cardiomyopathy (11). Two termination of pregnancy (1 Noonan, 1 sarcomeric hypertrophic cardiomyopathy) were reported. Of note, one patient with severe, biventricular hypertrophy survived the perinatal period, and cardiac hypertrophy resolved by 3 months of age.
HCM is the most common defect in patients with LEOPARD syndrome (about 70% of the cases) (6). Long-term prognosis seems benign in LEOPARD syndrome patients with only mild cardiac abnormalities (6, 7). On the other hand, patients with HCM may develop arrhythmias and other life-threatening complications (6, 7). The phenotype (a severe, obstructive left ventricular hypertrophy) may represent a risk factor for adverse clinical outcome (sudden death; as in the present case) in patients with LEOPARD syndrome and HCM. In addition, the genotype may represent a potential risk factor of adverse event in selected patients. We have recently reported 2 patients with an early onset obstructive HCM, associated with a high risk of heart failure and cardiac events. We showed a specific mutation in exon 13 of the PTPN11 gene (codon 510) (7). Of note, in the present report both the mother and her daughter had a mutation in exon 13 of the PTPN11 gene (codon 498). However, intrafamilial variability is clear in the present report (since the mother had a mild form of HCM, while the first daughter and the foetus showed a severe, early onset hypertrophic disease), representing a strong limitation to the potential use of the genotype (i.e. mutations in exon 13) as clinical predictor of malignant events in patients with HCM and LEOPARD syndrome.
In conclusion, HCM significantly worsen the prognosis in LEOPARD syndrome. However, lacking large population studies on HCM associated to the rare LEOPARD syndrome, the clinical and genetic heterogeneity of the disease warrant particular attention, especially in prenatal counselling, which should involve a multidisciplinary and experienced team (gynecologist, cardiologist, geneticists, and psycologist).
Footnotes
No conflict of interest declared.
References
- 1.Voron DA, Hatfield HH, Kalkhoff RK. Multiple lentigines syndrome. Case report and review of the literature. Am J Med. 1976;60:447–456. doi: 10.1016/0002-9343(76)90764-6. [DOI] [PubMed] [Google Scholar]
- 2.Digilio MC, Conti E, Sarkozy A, Mingarelli R, Dottorini T, Marino B, Pizzuti A, Dallapiccola B. Grouping of multiple-lentigines/LEOPARD and Noonan syndromes on the PTPN11 gene. Am J Hum Genet. 2002;71:389–394. doi: 10.1086/341528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pandit B, Sarkozy A, Pennacchio LA, Carta C, Oishi K, Martinelli S, Pogna EA, Schackwitz W, Ustaszewska A, Landstrom A, Bos JM, Ommen SR, Esposito G, Lepri F, Faul C, Mundel P, Lopez Siguero JP, Tenconi R, Selicorni A, Rossi C, Mazzanti L, Torrente I, Marino B, Digilio MC, Zampino G, Ackerman MJ, Dallapiccola B, Tartaglia M, Gelb BD. Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nat Genet. 2007;39(8):1007–12. doi: 10.1038/ng2073. [DOI] [PubMed] [Google Scholar]
- 4.Limongelli G, Pacileo G, Calabro R. Is sudden cardiac death predictable in LEOPARD syndrome? Cardiol Young. 2006;16:599–601. doi: 10.1017/S1047951106001247. [DOI] [PubMed] [Google Scholar]
- 5.Woywodt A, Welzel J, Haase H, Duerholz A, Wiegand U, Potratz J, Sheikhzadeh A. Cardiomyopathic Lentiginosis/LEOPARD syndrome presenting as sudden cardiac arrest. Chest. 1998;113:1415–1417. doi: 10.1378/chest.113.5.1415. [DOI] [PubMed] [Google Scholar]
- 6.Limongelli G, Pacileo G, Marino B, Digilio MC, Sarkozy A, Elliott P, Versacci P, Calabro P, De Zorzi A, Di Salvo G, Syrris P, Patton M, McKenna WJ, Dallapiccola B, Calabro R. Prevalence and clinical significance of cardiovascular abnormalities in patients with the LEOPARD syndrome. Am J Cardiol. 2007;15(100):736–41. doi: 10.1016/j.amjcard.2007.03.093. [DOI] [PubMed] [Google Scholar]
- 7.Limongelli G, Sarkozy A, Pacileo G, Calabro’ P, Digilio MC, Maddaloni V, Gagliardi G, Di Salvo G, Iacomino M, Marino B, Dallapiccola B, Calabro’ R. Genotype-phenotype analysis and natural history of left ventricular hypertrophy in LEOPARD syndrome. Am J Med Gen A. 2008 doi: 10.1002/ajmg.a.32206. [DOI] [PubMed] [Google Scholar]
- 8.Maron BJ, McKenna WJ, Danielson GK, Kappenberger LJ, Kuhn HJ, Seidman CE, Shah PM, Spencer WH 3rd, Spirito P, Ten Cate FJ, Wigle ED. Task Force on Clinical Expert Consensus Documents. American College of Cardiology; Committee for Practice Guidelines. European Society of Cardiology. American College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol. 2003;42:1687–713. doi: 10.1016/s0735-1097(03)00941-0. [DOI] [PubMed] [Google Scholar]
- 9.Nugent AW, Daubeney PE, Chondros P, Carlin JB, Colan SD, Cheung M, Davis AM, Chow CW, Weintraub RG. National Australian Childhood Cardiomyopathy Study. Clinical features and outcomes of childhood hypertrophic cardiomyopathy: results from a national population-based study. Circulation. 2005;112:1332–8. doi: 10.1161/CIRCULATIONAHA.104.530303. [DOI] [PubMed] [Google Scholar]
- 10.Maron BJ, Tajik AJ, Ruttenberg HD, Graham TP, Atwood GF, Victorica BE, Lie JT, Roberts WC. Hypertrophic cardiomyopathy in infants: clinical features and natural history. Circulation. 1982;65:7–17. doi: 10.1161/01.cir.65.1.7. [DOI] [PubMed] [Google Scholar]
- 11.Pedra SR, Smallhorn JF, Ryan G, Chitayat D, Taylor GP, Khan R, Abdolell M, Hornberger LK. Fetal cardiomyopathies: pathogenic mechanisms, hemodynamic findings, and clinical outcome. Circulation. 2002;106:585–91. doi: 10.1161/01.cir.0000023900.58293.fe. [DOI] [PubMed] [Google Scholar]