Abstract
Character displacement – trait evolution stemming from selection to lessen resource competition or reproductive interactions between species – has long been viewed as an important mechanism for enabling closely related species to coexist. Yet, the causes and consequences of character displacement have not been fully explored. Moreover, character displacement in traits associated with resource use (ecological character displacement) has been studied largely independently of that in traits associated with reproduction (reproductive character displacement). Here, we underscore the commonalities of these two forms of character displacement and discuss how they interact. We focus on the causes of character displacement and explore how character displacement can have downstream effects ranging from speciation to extinction. In short, understanding how organisms respond to competitive and reproductive interactions with heterospecifics offers key insights into the evolutionary consequences of species coexistence and diversification.
Keywords: adaptive radiation, coevolution, coexistence, competition, Darwinian extinction, mate choice, phenotypic plasticity, reinforcement, resource polymorphism, sexual selection, speciation
Introduction
How can closely related species coexist in the same habitat? Why are even closely related species often phenotypically different from one another? What role do interactions between species play in the process of diversification? In this article, we describe how the answers to such questions can emerge from knowledge of how organisms respond to a common evolutionary problem. Namely, organisms often face reduced fitness stemming from interactions with other species that reduce access to resources or successful reproduction. Here, we show how selection minimizes competitive or reproductive interactions between species by favoring the evolution of divergent resource-use or reproductive phenotypes. This process, termed “character displacement” (Brown and Wilson, 1956), is potentially a leading cause of adaptive diversification (reviewed in Schluter, 2000). In particular, character displacement: favors the evolution of novel resource-use or reproductive traits; drives divergence between sympatric and allopatric conspecific populations; and both initiates and finalizes the process of speciation.
Despite the significance of character displacement, previous research has focused largely on whether or not it occurs (reviewed in Servedio and Noor, 2003; Coyne and Orr, 2004; Dayan and Simberloff, 2005). The need exists, however, to move beyond establishing the existence of character displacement in order to discover its full implications. Moreover, research has tended to focus separately on ecological character displacement (character displacement in traits associated with resource use) and reproductive character displacement (character displacement in traits associated with reproduction) [for notable exceptions, see research on stickleback fish (reviewed in Rundle and Schluter, 2004) and Darwin's finches (reviewed in Grant and Grant, 2008)]. Consequently, there has been relatively little cross-fertilization of ideas between researchers who study these two forms of character displacement.
Rather than comprehensively review the evidence for character displacement, as has been done elsewhere (Howard, 1993; Schluter, 2000; Servedio and Noor, 2003; Coyne and Orr, 2004; Dayan and Simberloff, 2005; Groning and Hochkirch, 2008), we highlight future directions for research in character displacement. Our specific goals are threefold. First, we seek to unite ecological and reproductive character displacement under the same conceptual framework. Second, we underscore the value of exploring more fully the ecological and evolutionary causes and consequences of character displacement. In particular, we describe why some species may be especially prone to undergo character displacement and discuss some of character displacement's downstream effects. Third, we evaluate how reproductive and ecological character displacement interact and thereby affect the likelihood that either process will unfold.
Unifying Ecological and Reproductive Character Displacement
We begin by presenting a unified framework for making the fields of ecological and reproductive character displacement parallel in focus. To do so, we first discuss what constitutes character displacement and review, albeit briefly, the problems of definitions that have plagued both ecological and reproductive character displacement. We suggest that applying the conceptual framework developed for ecological character displacement to reproductive character displacement will alleviate confusion and place both fields on equal footing.
What is Character Displacement?
Brown and Wilson (1956) coined the term “character displacement,” but the catalyst for the idea can be traced to Gause (1934). Gause (1934) showed experimentally that two species cannot stably coexist if they overlap completely in resource requirements [Darwin (1859) had actually made a similar argument but did not provide empirical support]. In such situations, one species ultimately edges out the other. This hypothesis, termed the competitive exclusion principle (Hardin, 1960), forms a cornerstone of ecology. The competitive exclusion principle has an important corollary: that species can stably coexist if they differ in resource use (Hardin, 1960; Pianka, 2000). Therefore, initially identical, interacting species will experience strong selection to evolve differences in resource use (Lack, 1947; Grant, 1972; Arthur, 1982; Schluter, 1994; Pfennig et al., 2007). Similarly, such species will experience strong selection to evolve differences in reproductive traits (reviewed in Butlin and Ritchie, 1994; Servedio and Noor, 2003; Coyne and Orr, 2004); otherwise, one species may drive the other locally extinct through “reproductive” exclusion (also referred to as “sexual exclusion”; Hochkirch et al., 2007; Groning and Hochkirch, 2008).
Character displacement is likely a general phenomenon in that most species will, at some point in their evolutionary history, confront heterospecifics with which they competitively or reproductively interact. In such situations, individuals most dissimilar from the average resource-use or reproductive traits of another species are expected to procure more resources or successful reproduction than other members of their population (Slatkin, 1980; Taper and Case, 1985, 1992; Abrams, 1986; Butlin and Ritchie, 1994; Doebeli, 1996; Servedio and Noor, 2003; Coyne and Orr, 2004). Consequently, these most divergent individuals should experience highest fitness. If heritable variation exists in these traits, each species will evolve to be less like the other (although, as we note briefly later on, asymmetric character displacement can arise if the species differ in whether and how much they diverge; for fuller discussion see Schluter, 2000; Cooley, 2007). Such selection, acting to lessen competitive or reproductive interactions between species, can promote evolutionary divergence in traits associated with resource use or reproduction; i.e., character displacement (Figure 1; for a review of the theory, see Schluter, 2000; Coyne and Orr, 2004). In the absence of character displacement, competitive or reproductive exclusion may ensue (Gause, 1934; Liou and Price, 1994; Groning and Hochkirch, 2008).
Figure 1. The Process of Character Displacement.
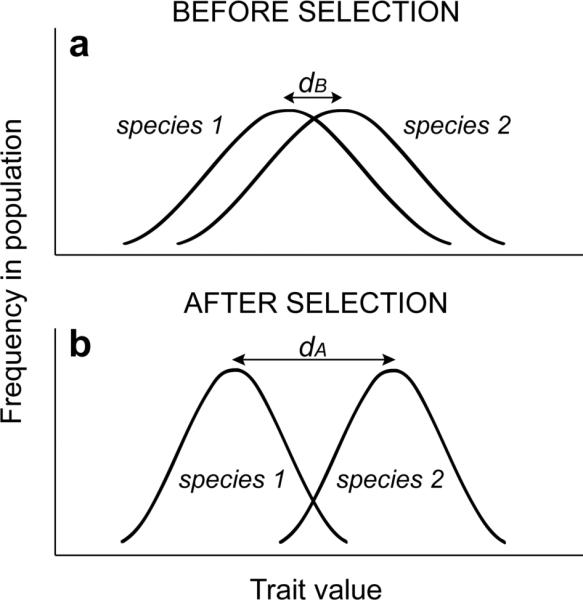
(a) Initially, two species encounter each other and overlap in phenotypes associated with resource use or reproduction (indicated here by the two overlapping bell-shaped curves). Character displacement arises when individuals most dissimilar from the average resource-use or reproductive phenotypes of another species are more successful at acquiring resources or reproduction than other members of their population. Consequently, (b) the most divergent individuals should experience highest fitness and the two species should tend to evolve to be less like the other. Character displacement is indicated when the difference between species in mean trait value is greater after selection (dA) than before selection (dB).
In this article, “character displacement” refers to the evolutionary accentuation of phenotypic differences between species stemming from selection to lessen resource competition or reproductive interactions between them (later, we broaden this definition to include selection acting within species). Character displacement can assume two distinct forms that differ in the agent and target of selection (Brown and Wilson, 1956). “Ecological character displacement” refers to trait evolution stemming from selection to lessen resource competition between species and therefore acts on traits associated with resource use (e.g., morphological structures such as beaks and jaws; Slatkin, 1980; Schluter, 2001). By contrast, “reproductive character displacement” refers to trait evolution stemming from selection to lessen sexual interactions between species and therefore acts on traits associated with reproduction (e.g., sexual signals or female mate preferences; Brown and Wilson, 1956; Crozier, 1974).
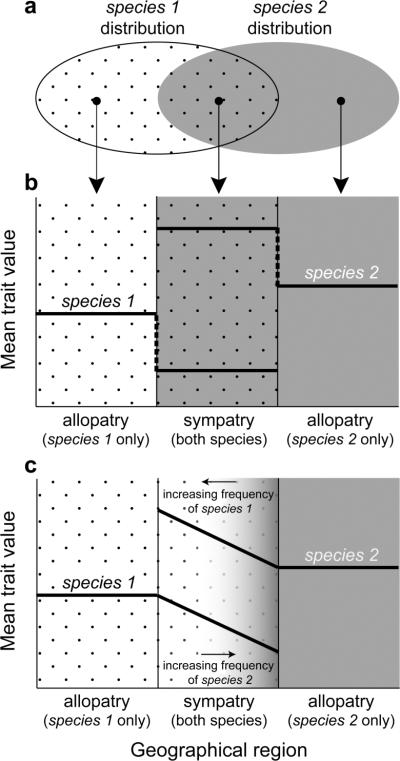
An important prediction of character displacement is that species should differ in traits associated with resource use or reproduction if they occur together (Brown and Wilson, 1956; Grant, 1972; Schluter, 2000). Moreover, selection to lessen resource competition or reproductive interactions should act only where species actually co-occur (Brown and Wilson, 1956; Lack, 1947). Consequently, character displacement should also produce a distinctive pattern in which species are more dissimilar where they occur together than where each occurs alone (Figure 2a, b; Brown and Wilson, 1956; Lack, 1947). Thus, within each species, populations in sympatry with the heterospecific should differ from those in allopatry (Figure 2b; Lack, 1947). Character displacement therefore consists of two hallmark features: (1) the process of phenotypic evolution stemming from selection to lessen resource competition or reproductive interactions between species (Figure 1); and (2) the resulting pattern of geographical variation in which sympatric species show exaggerated divergence, and in which conspecific populations in sympatry with aheterospecific differ from those in allopatry (Figure 2b).
Figure 2. Patterns of Character Displacement.
(a) For two species that occur in both sympatry with each other and in allopatry, character displacement should produce a distinctive pattern of divergence (b) in which the two species are more dissimilar to each other in sympatry (where there is selection for divergence) than in allopatry (where there is no such selection). Moreover, within such species, populations in sympatry with the heterospecific are expected to diverge from conspecific populations in allopatry. (c) Because the likelihood of encountering heterospecifics may increase along a spatial gradient (e.g., as one moves from the edge to the center of a species' geographical range), character displacement may produce a pattern in which, within each species, the magnitude of divergence increases along a gradient with increasing likelihood of encountering heterospecifics.
Conflation of Process and Pattern
Character displacement has often been conflated with the pattern that is predicted to arise from it (reviewed in Grant, 1972; Endler, 1986; Schluter, 2000; Goldberg and Lande, 2006). Defining character displacement as a pattern, however, is problematic, because patterns of divergence between species and populations can be generated via processes other than selection to avoid interactions with heterospecifics (Grant, 1972; Strong et al., 1979; Simberloff and Boecklen, 1981; Arthur, 1982; Endler, 1986; Diamond et al., 1989). In the case of ecological character displacement, the conflation of process and pattern provoked a lengthy and spirited debate over what constituted “true” character displacement (for a review of this debate, see Schluter, 2001). In response, researchers generally agreed to define ecological character displacement as the process described above (i.e., the definition that we presented in the previous section is widely accepted; e.g., see Schluter, 2001, 2002). By defining character displacement in terms of process rather than pattern, researchers could thereby focus strictly on the ecological and evolutionary implications of resource competition between species (Grant, 1972; Schluter, 2000).
Unifying the Conceptual Framework of Ecological and Reproductive Character Displacement
Although the field of ecological character displacement is largely reconciled as to what constitutes character displacement, the field of reproductive character displacement has achieved no such resolution. Consequently, conflation of pattern and process is widespread when dealing with reproductive character displacement (for additional discussion see also Butlin and Ritchie, 1994; Servedio and Noor, 2003; Coyne and Orr, 2004). Yet, as the literature on ecological character displacement clearly illustrates, patterns of trait divergence can be generated via processes other than selection to avoid interactions with heterospecifics (e.g., founder effects; Marko, 2005; reviewed in Grant, 1972; Schluter, 2000). Defining reproductive character displacement as a process, rather than as a pattern, has the same benefit as defining ecological character displacement as a process: researchers can focus specifically on the ecological and evolutionary implications of interactions between species driving reproductive trait divergence. Moreover, taking a parallel process-oriented approach to both ecological and reproductive character displacement allows for a more complete understanding of how they interact. If the two fields continue to define character displacement differently, then the ability to adequately delineate, let alone address, questions regarding how and why ecological and reproductive character displacement interact will be hampered.
Defining reproductive character displacement as a process is not new. Indeed, Butlin and Ritchie (1994) define reproductive character displacement as “the process of divergence in mating signal systems between reproductively isolated species” (p. 62, italics are ours; Butlin and Ritchie, 1994). The definition of reproductive character displacement that we use here is a more general form than that of Butlin and Ritchie (1994). Yet, this more general definition clarifies the relationship of reproductive character displacement to reinforcement – the evolution of traits that minimize hybridization between species (Dobzhansky, 1940; Servedio and Noor, 2003; Coyne and Orr, 2004). By the general definition we use here, reinforcement constitutes a special case of reproductive character displacement. This approach is also not new. Indeed, Blair (1974) – who is credited with coining the term “reinforcement” (Coyne and Orr, 2004) – refers to reinforcement as “a rather restricted form of character displacement” (p. 1119, Blair, 1974).
Defining reproductive character displacement broadly as the selective process by which reproductive traits diverge in order to minimize costly reproductive interactions with heterospecifics, and including reinforcement as a form of character displacement, emphasizes the general importance of selection as the driving force behind reproductive trait divergence. The definition we use here thereby minimizes confusion about what does, and does not, constitute reproductive character displacement versus reinforcement, and overcomes the issues associated with confounding pattern and process when describing reproductive character displacement (e.g., those who study reinforcement typically consider reproductive character displacement to be a signature pattern resulting from reinforcement; see Howard, 1993; Servedio and Noor, 2003 and references therein).
Butlin and Ritchie (1994) argued that reproductive character displacement and reinforcement should be considered separate processes based, in part, on the nature of interactions between species (see also Butlin, 1987). Whereas reinforcement was defined as arising from interactions where species could actually exchange genes during mating, reproductive character displacement was deemed to arise from all other mating interactions (Butlin, 1987; Butlin and Ritchie, 1994). Yet, in the same way that competition for resources can take different forms [i.e., exploitative (indirect) competition and interference (direct) competition] but still generate ecological character displacement (Schluter, 2000), different types of sexual interactions between species can promote reproductive character displacement. In particular, reproductive interactions between species can take two general forms: direct interactions, in which the two species actually risk hybridizing with one another, and indirect interactions, in which the two species utilize similar aspects of their habitat (e.g., signaling space, pollinators) to seek and attract mates.
Direct interactions can produce wasted mating effort (e.g., in terms of lost gametes or investment in searching for a mate) if no viable offspring are produced (for discussion of hybrid fitness see Barton and Hewitt, 1989; Arnold, 1997; Coyne and Orr, 2004). Even if hybrid offspring are viable, hybridization may still result in low fitness if hybrids have lower survivorship or reduced fertility and fecundity (reviewed in Barton and Hewitt, 1989; Arnold, 1997; Coyne and Orr, 2004). Consequently, selection should generally minimize the risks of hybridization by favoring divergence between species in reproductive traits; i.e., reproductive character displacement. In other words, direct interactions that contribute to gene flow between species can lead to reinforcement, which we consider to be a special case of reproductive character displacement (see also Blair, 1974).
By contrast, indirect interactions can generate interference between species that make mate localization difficult and costly in terms of increasing signaling effort or increasing search times and their associated costs (Butlin and Ritchie, 1994; Gerhardt and Huber, 2002). For example, species that use acoustic signals can mask, jam, or attenuate aspects of one another's signal properties, making it difficult to discern either signal (reviewed in Gerhardt and Huber, 2002). Similarly, plants that compete for pollinators may cope with pollen limitation and pollen interference (Levin, 1985; Caruso, 2000; Smith and Rausher, 2008). As with the direct reproductive interactions described above, selection should generally minimize indirect reproductive interference by favoring divergence between species in reproductive traits; i.e., reproductive character displacement.
This dichotomy of direct and indirect interactions should not be taken as mutually exclusive –species can interact in both ways, and how they interact may change spatially and temporally. The key point is that, for direct and indirect reproductive interactions, both the agent and target of selection are the same. Consequently, both types of interactions can promote divergence in reproductive traits as a means of minimizing costly reproductive interactions between species. In other words, both can promote reproductive character displacement (according to the definition we use here).
Given this framework for defining ecological and reproductive character displacement in a similar way, we turn to evaluating the causes of character displacement. In particular, we seek to examine what factors facilitate character displacement and thereby make it more likely to occur than the alternative outcomes of competitive or reproductive exclusion.
Causes of Character Displacement
The consensus that has emerged from decades of work is that character displacement is taxonomically widespread (Schluter, 2000; Servedio and Noor, 2003; Coyne and Orr, 2004; Dayan and Simberloff, 2005). Yet, why character displacement appears to be more likely to transpire in some circumstances and taxa than in others remains relatively unexplored (reviewed in Schluter 2000; Rice and Pfennig 2007). Understanding when and why character displacement is more likely to proceed is important, because differences in the occurrence of character displacement could explain ecological and evolutionary patterns of diversity. For example, communities or taxa that are more prone to undergo character displacement will likely be more diverse that those communities or taxa where character displacement does not occur, for at least two reasons. First, species that undergo character displacement are less likely to go extinct through competitive or reproductive exclusion (see above). Second, as we describe later, character displacement may promote speciation. Hence, as part of a more general theory for why some communities or taxa are more diverse than others (Schluter, 2000), it is important to determine what factors facilitate character displacement.
Factors that Facilitate Character Displacement
Four, nonexclusive factors appear to facilitate character displacement and therefore make it more likely to unfold. Two are evolutionary factors: strong selection disfavoring interactions with heterospecifics, and ecological opportunity. The remaining two are proximate factors: initial trait differences between species, and abundant standing variation. Although these factors facilitate adaptive evolution in general, and are therefore not unique to character displacement, studies are needed to examine how they affect character displacement. Below, we describe each factor and its effect on character displacement in turn.
First, character displacement is more likely to occur when selection against interactions with heterospecifics is strong. For example, reproductive character displacement is increasingly likely to occur as the costs of hybridization increase (Liou and Price, 1994). Moreover, differences between species in the strength of selection to avoid interactions with the other species may explain asymmetric character displacement, where one species diverges less than another species (Cooley, 2007). When one species suffers higher costs in the interaction, it may experience greater divergence than the other species (although asymmetric character displacement can occur for other reasons not described here; see Schluter, 2000; Cooley, 2007). Character displacement should also be more likely to occur when the encounter rate between species is high, and, hence, when selection disfavoring interactions with heterospecifics is strong (see Figure 2c; for examples, see Pfennig and Murphy, 2002; Tynkkynen et al., 2004; Pfennig and Pfennig, 2005).
Second, character displacement is facilitated by “ecological opportunity,” the availability of different resource types underutilized by other species (Simpson, 1953; Schluter, 2000; although the concept of ecological opportunity has traditionally been applied to resources, a similar principle applies to having available signal space in the case of reproductive character displacement). Character displacement often generates new resource-use or reproductive traits in sympatry that differ from the pre-displacement traits in allopatry (Howard, 1993; Schluter, 2000; Servedio and Noor, 2003; Coyne and Orr, 2004; Dayan and Simberloff, 2005; Groning and Hochkirch, 2008). Therefore, for character displacement to occur, exploitable resources or signal space that are not already utilized by another species must be available (i.e., there must be resources or signal space onto which a species can actually be displaced; Pfennig et al., 2006; Groning and Hochkirch, 2008). In the absence of exploitable resources or signal space, competitive or reproductive exclusion may result (Pfennig et al., 2006; Hochkirch et al., 2007; Groning and Hochkirch, 2008).
Third, character displacement occurs most readily if interacting species already differ in phenotypic traits under selection when they come into contact with one another (Slatkin, 1980; Liou and Price, 1994). Although character displacement can occur without such initial differences, character displacement is facilitated if other factors “jump-start” the divergence, prior to interactions with heterospecifics (Slatkin, 1980). Such factors may act in allopatry before the two species come into contact with one another, and they may include drift or spatially divergent natural or sexual selection (Schluter, 2000). Such differences may then be amplified in sympatry by selection acting to lessen interspecific interactions (Schluter, 2000; Rice and Pfennig, 2007). In the absence of initial differences between species, one species will be more likely to drive the other locally extinct; e.g., through competitive or reproductive exclusion (see above). Thus, species that differ initially from heterospecifics should be more prone to undergo character displacement (Slatkin, 1980; Liou and Price, 1994; Schluter, 2000; Rice and Pfennig, 2007).
Finally, character displacement may be more likely to occur when interacting species are phenotypically variable (Milligan, 1985). Phenotypic variation is important, because it increases the chances that character displacement can evolve through the selective filtering of divergent phenotypes in sympatry that were already present in allopatry (for reviews see Rice and Pfennig, 2007; Barrett and Schluter, 2008). Indeed, because this process should unfold relatively rapidly, abundant standing variation should facilitate character displacement as opposed to competitive or reproductive exclusion (Rice and Pfennig, 2007). Thus, species with abundant standing variation should therefore be especially likely to undergo character displacement (Rice and Pfennig, 2007).
Given that abundant standing variation might facilitate character displacement, what evolutionary and developmental mechanisms generate such variation? Answering this question could explain why some populations are predisposed to undergo character displacement. In the next section, we discuss two such mechanisms: intraspecific competition and phenotypic plasticity.
Intraspecific Character Displacement
As noted above, species with abundant standing variation should be especially prone to undergo character displacement. Therefore, identifying the mechanisms that generate and maintain variation within natural populations is crucial for understanding the factors that facilitate character displacement. One such mechanism is disruptive selection, which arises when extreme phenotypes have a fitness advantage over more intermediate phenotypes (Mather, 1953). By favoring extreme phenotypes, disruptive selection maintains, and may even increase, variation in natural populations (Rueffler et al., 2006). Indeed, such selection could ultimately result in the evolution of resource or mating polymorphism – alternative phenotypes within the same population that differ in resource use or mate acquisition tactics (Andersson, 1994; Smith and Skúkason, 1996).
Although numerous agents can generate disruptive selection, intraspecific competition for resources or mates has long been viewed as a leading cause (Rosenzweig, 1978; Wilson and Turelli, 1986; Day and Young, 2004; Rueffler et al., 2006). Such disruptive selection on traits associated with resource or reproduction thereby favors divergence in these characters within populations (for examples, see Smith, 1993; Medel et al., 2003; Bolnick, 2004; Pfennig et al., 2007; Bolnick and Lau, 2008; Calsbeek and Smith, 2008; Hendry et al., 2009; Martin and Pfennig, in press). The resulting trait evolution, arising from interactions within species, is analogous to that stemming from interactions between species (Dayan and Simberloff, 2005) and can be considered as “intraspecific character displacement” (sensu West-Eberhard, 2003).
Once a population has undergone intraspecific character displacement, it may, in turn, be more prone to undergo interspecific character displacement, for at least two reasons. First, intraspecific character displacement may favor the evolution of alternative resource-use or mate-acquisition phenotypes (Martin and Pfennig, in press). The evolution of such alternative phenotypes, prior to interactions between species, may fuel rapid character displacement via differential success of the alternative phenotypic variants (Figure 3; Rice and Pfennig, 2007; Barrett and Schluter, 2008). Second, even when disruptive selection does not favor distinct morphs, it does tend to maintain, and even increase, both phenotypic and genetic variation in natural populations (Mather, 1953; Rueffler et al., 2006). As noted in the previous section, such abundant standing variation increases the chances that interspecific character displacement will occur (Rice and Pfennig, 2007).
Figure 3. How abundant standing variation promotes character displacement.
Initially (a, d), a focal species (species 1, whose trait distribution is indicated by the bell-shaped curve) occurs alone in allopatry, either as a monomorphic species (a) or as a polymorphic species (d) consisting of alternative resource use or reproductive morphs (morphs 1, 2), one of which is initially rarer than the other. Later (b, e), a superior competitor, species 2 (whose trait distribution is indicated by the heavy bell-shaped curve), becomes sympatric with species 1 (either because species 2 invades the habitat of species 1 or vice versa). Finally (c, f), because of selection imposed by species 2, species 1 undergoes an evolutionary shift in resource use and associated phenotypic features (ecological character displacement) or in reproductive traits (reproductive character displacement; in both cases, the trait distributions of species 1 before selection are shown by the dashed bell-shaped curves). When there is little standing variation prior to encountering the heterospecific (as in panel c), character displacement unfolds only if for novel phenotypes that are more dissimilar to the competitor arise and spread in sympatry following the invasion of species 2. Because such novel phenotypes, if they do not already exist in the population, can only be generated through mutation, recombination, or introgression – all of which are relatively slow processes – competitive or reproductive exclusion, as opposed to character displacement, are more likely. By contrast, when there is abundant standing variation (as in panel f), character displacement unfolds when the phenotypic variant that is more dissimilar to the competitor (here, morph 1) is selectively favored and thereby increases in frequency at the expense of the alternative morph. Because such a process can unfold rapidly (e.g., potentially, within a single generation), character displacement, as opposed to exclusion, is more likely to transpire. Although we have illustrated this process as involving discrete morphs (which may have arisen through intraspecific character displacement), it could also occur in populations expressing a wide-range of continuously distributed phenotypes. Modified from Rice and Pfennig (2007).
Phenotypic Plasticity
In the previous section, we focused on a selective agent – intraspecific competition – that favors variation within populations. Such variation might, in turn, predispose populations to subsequently undergo interspecific character displacement. However, it is important to also consider the proximate mechanisms that generate such variation. Elucidating these proximate mechanisms is vital, because different proximate mechanisms can influence the speed at which new phenotypic variants arise. Therefore, different proximate mechanisms may ultimately influence the speed of character displacement, and, hence, whether character displacement even occurs in the first place. In particular, any proximate mechanism that facilitates divergence in resource-use or reproductive phenotypes may render character displacement, as opposed to competitive or reproductive exclusion, more likely to transpire.
An important proximate mechanism for rapidly generating new phenotypic variants is phenotypic plasticity. Phenotypic plasticity is the ability of an individual organism to react to an environmental stimulus with a change in phenotype (reviewed in West-Eberhard, 2003). Phenotypic plasticity enables organisms to respond rapidly to the presence of heterospecifics by altering their phenotype adaptively (see reviews by, and references in, Robinson and Wilson, 1994; Agrawal, 2001; Pfennig and Murphy, 2002; Fordyce, 2006; Pfennig et al., 2006). For example, when faced with resource competition or reproductive interactions from a heterospecific, individuals of many species facultatively express alternative resource-acquisition or reproductive phenotypes that lessen competition or reproductive interactions (e.g., Werner and Hall, 1976; Pfennig and Murphy, 2002; Pfennig, 2007). Such rapid shifts in resource-acquisition or reproductive traits have not traditionally been considered character displacement, because phenotypic plasticity is often regarded as a nongenetic response that is incapable of mediating adaptive evolution (Grant, 1972; Arthur, 1982; Schluter, 2000). Yet, the magnitude and direction of a plastic response is often genetically variable, and, consequently, subject to natural selection and evolutionary change (reviewed in Schlichting and Pigliucci, 1998; West-Eberhard, 2003; DeWitt and Scheiner, 2004).
Moreover, intergenerational plasticity – specifically, maternal effects – might actually promote a form of “canalization,” in which trait differences between species and populations persist, even when individuals are reared under common conditions (Pfennig and Martin, in press). Maternal effects occur when the phenotype of a female influences the phenotype of her offspring, independent of the direct effects of her genes on her offspring's phenotype (Mousseau and Fox, 1998). Because these effects can be acted upon by selection (McAdam and Boutin, 2004) and then cause information to be conveyed reliably between generations (Rossiter, 1996; Agrawal et al., 1999; Plaistow et al., 2006; Allen et al., 2008), they may play an important role in mediating adaptive evolution (Jablonka and Lamb, 1995; Maynard Smith, 1998).
Maternal effects can facilitate either form of character displacement, but they may be especially important in mediating ecological character displacement. Ecological character displacement causes interacting species to utilize different resources (reviewed in Schluter, 2000; Day and Young, 2004). When resource quality is asymmetric, one species will gain the more profitable resource, whereas the other will be forced onto a less profitable resource (e.g., Pfennig and Pfennig, 2005; Grant and Grant, 2006). Consequently, females of the latter species may mature at a smaller body size or in poorer condition (e.g., Gorbushin, 1996; Pfennig and Pfennig, 2005; Grant and Grant, 2006). Because of their smaller size and poorer condition, these females may subsequently produce offspring that are also smaller and in poorer condition, purely because of a maternal effect (e.g., Pfennig and Martin, in press). As a result of this maternal effect, the offspring may ultimately express a resource-use phenotype less like that expressed by the other species (resource use is often correlated with body size). Moreover, because maternal effects can be transmitted reliably between generations (see above), these differences in trait expression between populations in sympatry with a heterospecific competitor and those in allopatry may persist even when individuals are experimentally reared under common conditions. Such a pattern would give the misleading appearance that genetic differences underlie these trait differences. Thus, trait differences between populations undergoing character displacement may be underlain entirely by a maternal effect (for a possible example, see Pfennig and Martin, in press).
The discussion above suggests that phenotypic plasticity can mediate rapid phenotypic divergence between species. Phenotypic plasticity might also promote the evolution of genetic differences that stabilize such phenotypic differences (West-Eberhard, 2003). If individuals in a population begin to facultatively express a novel phenotype that lessens costly interactions with a heterospecific, and if there is underlying genetic variation in the degree to which individuals respond to heterospecifics, then selection should favor those alleles or gene combinations that best stabilize, refine, and extend the new trait's expression (a process know as “genetic accommodation”, West-Eberhard, 2003). Thus, under persistent selection to minimize competition or reproductive interactions with heterospecifics, divergent traits that are initially plastic may eventually become genetically canalized (i.e., “fixed”) in the population (e.g., Pfennig and Murphy, 2000, 2002). Furthermore, phenotypic shifts mediated by phenotypic plasticity may shield populations from extinction (e.g., via competitive or reproductive exclusion) as genetic evolution proceeds.
Phenotypic plasticity therefore plays a potentially important role in facilitating character displacement (Wilson, 1992; Pfennig and Murphy, 2002). Plastic traits themselves may be the targets of selection that initially diverge rapidly between species (i.e., they may undergo character displacement) (Pfennig and Murphy, 2000, 2002). Additionally, plasticity can promote canalized character displacement (sensu Pfennig and Murphy, 2002) or buffer populations from extinction while the evolution of such canalization proceeds (sensu West-Eberhard, 2003).
In the above section, we explored the causes of character displacement by highlighting some diverse factors that might promote character displacement. By fostering character displacement as opposed to extinction through competitive or reproductive exclusion, these factors could ultimately explain why some communities or taxonomic groups are more diverse. In other words, any factors that contribute to character displacement may have far reaching implications beyond simply mediating trait divergence between species. Below, we explore such evolutionary and ecological implications in more detail.
Consequences of Character Displacement
As described above, character displacement results in divergent traits between the interacting species as well as divergent traits within each species between allopatric and sympatric populations (Figure 2b). This hallmark pattern is not the only significant outcome of character displacement, however. For example, as the above discussion indicates, whether character displacement occurs depends on a number of evolutionary and proximate factors. Consequently, some groups may be more likely to undergo character displacement (and therefore be more diverse) than others. Yet, character displacement's role in generating such macroevolutionary patterns of differential taxonomic diversity is largely unknown (but see Schluter, 2000). Similarly, the ecological and evolutionary implications that stem from character displacement remain relatively unexplored.
Here, we discuss how character displacement can influence four key evolutionary processes: correlated evolution, sexual selection, speciation, and extinction. By influencing how these processes unfold, character displacement has potentially far reaching impacts beyond mere trait divergence between species.
Correlated Evolution
During character displacement, sympatric and allopatric populations diverge in traits involved in resource use or reproduction (Figure 2b). However, populations may often also diverge in traits that are not directly involved in resource acquisition or reproduction owing to correlated evolution with those traits actually targeted by selection (sensu Conner and Hartl, 2004). Such divergence in correlated traits can accentuate differences between interacting species and, within each species, between populations in sympatry and allopatry. As we describe below, these differences could, in turn, enhance reproductive isolation among these groups.
When correlated evolution in response to heterospecifics arises from pleiotropy, fitness trade-offs can arise between the benefits of avoiding deleterious interactions with heterospecifics and the costs accrued in other fitness components (Pfennig and Pfennig, 2005). For example, both reproductive and ecological character displacement have caused spadefoot toads to evolve smaller body size in the presence of a heterospecific competitor (Pfennig and Pfennig, 2005). This shift in size appears to have arisen as a by-product, rather than as a direct target, of character displacement (Pfennig and Pfennig, 2005). Yet, the shift to reduced body size in sympatry is associated with reduced offspring survival, female fecundity, and sexual selection on males (Pfennig and Pfennig, 2005). Thus, character displacement may sometimes represent the “best of a bad situation” in that it lessens competition but at a cost: individuals in sympatry with the displaced phenotype may have higher fitness than those without the displaced trait because they experience reduced competition, but they may have reduced fitness relative to individuals in allopatry (Pfennig and Pfennig, 2005).
Fitness trade-offs associated with the benefits of avoiding deleterious interactions with heterospecifics on the one hand, and the costs accrued in other fitness components on the other hand, may have at least three important consequences. First, depending on the nature of the trade-off and the strength of selection to avoid heterospecific interactions, trade-offs may constrain the evolution of adaptive traits that reduce heterospecific interactions (sensu Conner and Hartl, 2004). In other words, pleiotropic interactions may limit evolutionary divergence in response to heterospecifics. Variation within and between species in fitness trade-offs may explain why character displacement varies among populations or why it is sometimes expressed asymmetrically between the interacting species (Schluter, 2000; Cooley, 2007). Second, such fitness trade-offs may explain why traits that evolve in sympatry often do not spread back into allopatry, even in the face of high gene flow (for discussion, see Servedio and Noor, 2003; Higgie and Blows, 2007). Finally, because fitness trade-offs may cause individuals in sympatry to have reduced fitness relative to those in allopatry (as in the spadefoot toad example described in the previous paragraph), sympatric populations may be at higher extinction risk relative to allopatric populations (Pfennig and Pfennig, 2005; see also Webb, 2003; Groning and Hochkirch, 2008). We will return to this point below.
Sexual Selection
Sexual selection explains much of the diversity in sexual signals and mating behaviors in sexually reproducing organisms (Andersson, 1994), and character displacement can have a profound influence on sexual selection. When character displacement alters the expression of mate choice or traits used in sexual signaling or male competition, it necessarily impacts the expression of sexual selection among populations that differ in interactions with heterospecifics (Boughman, 2001, 2007; Pfennig and Ryan, 2007). Indeed, character displacement may impact sexual selection in at least two ways. First, character displacement can preclude mate choice formale traits that are indicative of mate quality and thereby alter the underlying fitness consequences of mate choice and sexual signaling (Higgie and Blows, 2008; Pfennig, 1998, 2000). Second, character displacement can alter the targets of sexual selection in populations that differ in their interactions with heterospecifics without necessarily affecting the fitness accrued through mating decisions or mate attraction. We discuss each of these impacts separately below.
First, character displacement potentially alters sexual selection by precluding the expression of mate choice for fitness-enhancing conspecific mates (Higgie and Blows, 2008; Pfennig, 1998, 2000). Generally, females should choose mates that provide them with fitness benefits, such as enhanced numbers of offspring or better-quality offspring (reviewed in Andersson, 1994). If reproductive character displacement favors the evolution of preferences that ensure mating with the correct species, the resulting preferences that evolve via character displacement may not be those that also enable females to select high-quality conspecific mates (Pfennig, 1998, 2000; Higgie and Blows, 2007, 2008). For example, sexual selection theory generally predicts that females prefer males with more elaborate or costly signals that are indicative of a male's ability to confer benefits to a female (Andersson, 1994; Bradbury and Vehrencamp, 1998). If, however, heterospecifics possess elaborate traits, character displacement may promote the evolution of preferences for less exaggerated signals (Ryan and Rand, 1993; Pfennig, 1998; e.g., Pfennig, 2000; Rosenthal et al., 2002; Higgie and Blows, 2008). By adopting such preferences, females may avoid costly heterospecific interactions, but they may concomitantly forego information about a prospective conspecific mate's ability to convey additional fitness benefits (for examples, see Pfennig, 2000, 2008; Higgie and Blows, 2007, 2008).
Such trade-offs will not always arise via character displacement (Pfennig, 1998). For example, if males with the most elaborate characters are also the most dissimilar from heterospecifics, sexual selection and character displacement reinforce each other (Pfennig, 2000). Yet, when trade-offs do arise, their effects may be far-reaching. As with pleiotropic effects described above, trade-offs in mate choice can explain why divergent mating traits that evolve in sympatry do not spread back into allopatry via gene flow (Pfennig and Pfennig, 2005; Higgie and Blows, 2007). Indeed, when trade-offs in mate choice arise, sympatric and allopatric populations can experience nearly opposing patterns of mate-choice mediated sexual selection. Consequently, not only will mate preferences diverge between sympatry and allopatry, but sexual signals (and any correlated traits) will also diverge (Hoskin et al., 2005; Pfennig and Pfennig, 2005; Pfennig and Ryan, 2006; Higgie and Blows, 2007, 2008). As we discuss below, such divergence in mating behavior can lead to reproductive isolation and, ultimately, speciation of allopatric and sympatric populations (Hoskin et al., 2005; Pfennig and Ryan, 2006). Furthermore, over time, selection may favor the resolution of trade-offs by promoting the evolution of preferences for multiple traits that enable females to avoid heterospecific interactions while simultaneously assessing conspecific quality (Pfennig, 1998). Thus, character displacement can contribute not only to divergence in a given aspect of a signal, but it can also indirectly promote the evolution of multiple or complex signals for discriminating mates (reviewed in Pfennig, 1998; Gerhardt and Huber, 2002; Hebets and Papaj, 2005).
A second major way that character displacement may impact sexual selection is by altering the targets of sexual selection in sympatry versus allopatry (e.g., Gerhardt, 1994; Pfennig, 2000; Higgie and Blows, 2007). As we describe below, character displacement can lead to morphological changes in resource-use traits that concomitantly alter the production of sexual signals (e.g., Podos, 2001; Huber and Podos, 2006). These novel sexual signals might, in turn, become targets for further elaboration by direct sexual selection (e.g., because of their attractiveness to the opposite sex or effectiveness in competition among conspecifics for mates; Andersson 1994). Moreover, because ecological and reproductive character displacement often promote habitat shifts, occupancy of these novel habitats will tend to promote new patterns of sexual selection (sensu Endler and Basolo, 1998; Boughman, 2002). The nature of mate preferences and sexually selected traits often co-vary with habitat, because the transmission and the perception of sexual signals are typically habitat-dependent (reviewed in Wiley, 1994; Bradbury and Vehrencamp, 1998; Endler and Basolo, 1998; Boughman, 2002). Thus, any shifts in habitat use that are mediated by character displacement will likely be accompanied by shifts in patterns of sexual selection (e.g., Boughman, 2007).
As a result of the above effects of character displacement on sexual selection, sympatric and allopatric populations will potentially diverge in mating behaviors that were not necessarily the direct targets of selection to reduce heterospecific interactions. Because mate choice plays a critical role in reproductive isolation (reviewed in Coyne and Orr, 2004), divergent patterns of sexual selection in sympatry versus allopatry could ultimately contribute to speciation (Hoskin et al., 2005; Pfennig and Ryan, 2006). Thus, character displacement may initiate speciation between populations that differ in their interactions with heterospecifics (Hoskin et al., 2005; Pfennig and Ryan, 2006), which is the topic we turn to next.
Speciation
Character displacement potentially plays a critical role in speciation in two ways. First, character displacement can finalize speciation between already divergent groups (reviewed in Servedio and Noor, 2003; Coyne and Orr, 2004; Grant and Grant, 2008). Second, character displacement can initiate divergence and reproductive isolation between populations that differ in their interactions with heterospecifics (Hoskin et al., 2005; Pfennig and Ryan, 2006; Pfennig and Rice, 2007). We discuss each of these avenues to speciation in turn.
Character displacement has long been regarded as important in completing the process of speciation (reviewed in Coyne and Orr, 2004; Grant and Grant, 2008). Ecological character displacement, for example, should cause differentiated, but potentially interbreeding populations (i.e., incipient species) to diverge in resource acquisition traits (reviewed in Grant and Grant, 2008). Specialization on alternate resources may reduce contact between the two incipient species and thereby allow for the accumulation of genetic differences between them that, in turn, contributes to enhanced isolation (reviewed in Coyne and Orr, 2004; Grant and Grant, 2008; Price, 2008). Moreover, if the two species interbreed and produce hybrids of low fitness, reproductive character displacement will cause divergence in reproductive traits and thereby preclude hybridization (Dobzhansky, 1940; reviewed in Howard, 1993; Servedio and Noor, 2003; Coyne and Orr, 2004). This process of reinforcement will therefore finalize speciation by promoting the evolution of complete reproductive isolation (Dobzhansky, 1940; reviewed in Howard, 1993; Servedio and Noor, 2003; Coyne and Orr, 2004).
That character displacement can also initiate speciation has received relatively little attention (but see Hoskin et al., 2005; Pfennig and Ryan, 2006; Pfennig and Rice, 2007). Character displacement may instigate speciation by driving the evolution of divergent traits between populations that differ in their interactions with heterospecifics (Hoskin et al., 2005; Pfennig and Ryan, 2006; Pfennig and Rice, 2007). In particular, because individuals in sympatry will experience a different selective environment than conspecifics in allopatry, conspecific populations in these two types of environments are expected to diverge in resource-use or reproductive traits (Hoskin et al., 2005; Pfennig and Ryan, 2006; Pfennig and Rice, 2007). Such divergence may indirectly promote speciation via two non-mutually exclusive routes.
First, character displacement may promote the evolution of post-mating barriers to gene flow between sympatric and allopatric populations (Pfennig and Rice, 2007). In particular, as an indirect consequence of character displacement between species, offspring produced by matings between conspecific individuals from different selective environments (i.e., sympatric male/female × allopatric male/female) may express an intermediate phenotype that is less well adapted to either selective environment than that expressed by offspring produced by matings between individuals from the same selective environment (i.e., sympatric male/female × sympatric male/female or allopatric male/female × allopatric male/female) (sensu Rice, 1987; Hatfield and Schluter, 1999; Rundle, 2002). For example, individuals produced by matings across sympatry and allopatry may express intermediate resource acquisition phenotypes that make them competitively inferior in either sympatry or allopatry (e.g., Pfennig and Rice, 2007). Similarly, individuals produced from matings across sympatry and allopatry may engage in mating behaviors that are inappropriate for either selective environment (sensu Hatfield and Schluter, 1996; Vamosi and Schluter, 1999; Svedin et al., 2008; van der Sluijs et al., 2008). Such maladaptation essentially serves as post-mating barriers to gene flow between populations in different selective environments.
Second, character displacement may promote the evolution of pre-mating barriers between sympatric and allopatric populations. During reproductive character displacement, female preferences or male traits may become so divergent that females in sympatry fail to recognize allopatric males as acceptable mates (or vice versa). Consequently, populations in sympatry and allopatry will become reproductively isolated from each other (Hoskin et al., 2005; Pfennig and Ryan, 2006). Likewise, ecological character displacement can contribute to pre-mating barriers between conspecific populations in sympatry versus allopatry if shifts in habitat or resource use preclude mating between them (reviewed in Rundle and Schluter, 2004).
Differentiation between conspecific populations in sympatry versus allopatry is especially likely to occur if character displacement generates the kinds fitness trade-offs described above. By precluding the spread of traits from sympatry into allopatry (and vice versa; see correlated evolution above), such trade-offs essentially generate a selective barrier between sympatry and allopatry that fosters local adaptation (Pfennig and Pfennig, 2005). Moreover, because of reduced gene flow between sympatry and allopatry, populations in these divergent selective environments may accumulate further differences that exaggerate both pre- and post-mating isolation between them. Thus, speciation between sympatric and allopatric populations may arise as an indirect consequence of selection for divergence between species during interspecific character displacement (Hoskin et al., 2005; Pfennig and Ryan, 2006; Pfennig and Rice, 2007).
Although we have focused above on interactions between pairs of species, character displacement may also drive numerous, rapid speciation events. If, for example, a given species interacts with different heterospecifics across different populations, local evolution of mating behaviors in response to these interactions may isolate these conspecific populations and generate speciation among them (i.e., “speciation cascades”; Pfennig and Ryan, 2006). Thus, multiple speciation events, and possibly even adaptive radiations (Schluter, 2000), may arise as a by-product of interactions between species.
Coexistence Versus Extinction
Generally, character displacement is expected to promote species coexistence by reducing fitness-decrementing interactions that would otherwise lead to competitive or reproductive exclusion (see above and also Losos, 2000). Yet, even when character displacement promotes coexistence, populations in sympatry may have reduced survival and reproductive rates as a result of character displacement (Pfennig and Pfennig, 2005). Consequently, sympatric populations may experience higher extinction risk than conspecific populations in allopatry (for review and discussion of how adaptive evolution can lead to extinction risk, see Kokko and Brooks, 2002; Webb, 2003). Character displacement can contribute to enhanced extinction risk when it involves trade-offs between the benefits of avoiding heterospecific interactions and the costs of expressing the displaced phenotype (Pfennig and Pfennig, 2005). The costs that accrue to individuals in sympatry may reduce population fitness and thereby render sympatric populations more likely to go extinct (Pfennig and Pfennig, 2005). For example, as we described above (see phenotypic plasticity), ecological character displacement may result in one species being displaced onto a novel resource that is of lower quality or more ephemeral than the pre-displacement resource. Lower quality resources may support smaller populations that are more susceptible to stochastic extinction events, thereby rendering sympatric populations at higher extinction risk relative to allopatric populations. Likewise, displacement onto a more ephemeral resource may make sympatric populations more susceptible to stochastic extinction events than allopatric populations.
Reproductive character displacement also could engender costs if the displaced phenotypes (such as male signals or female preferences) are more costly to express (for discussion of mate choice for costly signals, see Andersson, 1994). More costly signals could reduce reproductive rates and limit population size (Kokko and Brooks, 2002). Additionally, extinction risk may depend on how males trade-off sexual and viability selection (Kokko and Brooks, 2002). Novel sexual signaling in sympatry may be more susceptible to trade-offs that enhance the risk of extinction relative to populations in allopatry. Moreover, as described above (see sexual selection), avoidance of heterospecifics may preclude females from selecting high quality mates and reduce sympatric female fitness relative to allopatric female fitness (Pfennig, 2000; Higgie and Blows, 2008; Pfennig, 2008). Such trade-offs can reduce female fecundity, rates of reproduction, and even offspring growth or survival (Pfennig, 2000, 2008). Indeed, if character displacement suppresses condition-dependent sexual selection in sympatry, sympatric populations may be less able to adapt to changing environments (sensu Lorch et al., 2003). Thus, relative to conspecifics in allopatry, those in sympatry may be smaller, slower growing, and less able to respond to changes in the environment (Pfennig and Pfennig, 2005). As a result, populations that have undergone character displacement may be more likely to experience extinction.
In sum, character displacement generally promotes species coexistence (Losos, 2000). Depending on the way that character displacement unfolds, however, it may, counter intuitively, also enhance the risk of extinction in populations that are sympatric with heterospecifics relative to those that are not (Pfennig and Pfennig, 2005; see also Kokko and Brooks, 2002; Webb, 2003). Thus, the distributions of many species may be patchier in areas where they are sympatric with a heterospecific than where they are allopatric, and this patchy distribution may be associated with stochastic factors, rather than because of the deterministic processes of competitive or reproductive exclusion. Moreover, persistence of sympatric populations may be more variable in both space and time. Indeed, coexistence between species may be more dynamic than originally thought, with sympatric populations experiencing chance extinction, followed by recolonization and coexistence. In other words, the outcome of heterospecific interactions may not be merely one or the other of two alternatives: coexistence or exclusion. Instead, character displacement may promote coexistence while increasing the likelihood of chance extinction.
Relationship Between Ecological and Reproductive Character Displacement
Throughout this article, we have referred to character displacement – rather than to ecological or reproductive character displacement – when the concepts being discussed apply to either process. Although the two processes are similar in many ways, relatively few studies have examined how they interact [for notable exceptions, see research on stickleback fish (reviewed in Rundle and Schluter, 2004) and Darwin's finches (reviewed in Grant and Grant, 2008)]. Yet, because species that compete for resources likely interact during mating and vice versa (Schluter, 2000; Rundle and Schluter, 2004; Grant and Grant, 2008; Price, 2008), reproductive and ecological character displacement may often become intertwined. Below, we discuss how ecological character displacement may affect reproductive character displacement and vice versa.
Ecological Character Displacement in Phenotypic Traits as a Promoter of Reproductive Character Displacement
Ecological character displacement can promote reproductive character displacement when shifts in resource-use traits also alter the production of signals used for reproduction (Huber and Podos, 2006; Grant and Grant, 2008). If these shifts in signal production reduce deleterious reproductive interactions between species, then ecological selection essentially jump-starts reproductive character displacement. For example, shifts in resource use that lead to changes in bird beak and larynx morphology can cause concomitant shifts in a sexual signal – bird song – that is directly involved in species recognition (reviewed in Podos and Nowicki, 2004; Grant and Grant, 2008; Price, 2008). Indeed, in the medium ground finch, Geospiza fortis, populations that consist of a large-beaked morph and a small-beaked morph – which feed on large and small seeds, respectively – produce distinct song types (Huber and Podos, 2006). Females apparently use these different song types during mate choice and mate assortatively with males of their own beak type (Huber et al., 2007). Thus, ecological selection can also alter sexual signals in a way that affects mate choice, and potentially, reproductive isolation (Podos, 2001; Podos and Nowicki, 2004; Podos et al., 2004; Huber et al., 2007; Grant and Grant, 2008).
Although the above example focuses on acoustic signals, shifts in resource use could foster similar changes in other sensory modalities used in sexual signaling. In particular, shifts in resource use could affect visual or olfactory sexual signals depending on how dietary components (e.g., carotenoids) are incorporated into sexual displays. In many fish species, for example, male coloration is diet-dependent (see discussion and references in Andersson, 1994; Olson and Owens, 1998), and coloration can also play an important role in species recognition (e.g., Seehausen and van Alphen, 1998; Boughman, 2001). If ecological character displacement causes divergence in resource use, male signaling can be affected if the dietary components used to generate a given signal are no longer available (or are too costly to acquire) with the new diet (Boughman, 2007). Consequently, resource shifts may be accompanied by shifts in sexual signals, which can then be maintained and further elaborated via reproductive character displacement if they minimize deleterious reproductive interactions between species.
Although recent work has focused on how resource shifts can alter male signals, morphological and physiological changes that accompany ecological character displacement can also directly affect female perception, and, consequently, female mate choice (sensu Endler and Basolo, 1998; Ryan, 1998; Boughman, 2002). Changes in jaw morphology to capture prey, divergence in olfactory or visual sensitivity to localize prey, and even shifts in overall body size for specializing on different resources that result from ecological character displacement could simultaneously alter female perception and discrimination of male signals (Endler and Basolo, 1998; Ryan, 1998; Boughman, 2002). For example, changes in ear morphology caused by changes in jaw structure or body size could affect female perception of and preferences for male calls (Ryan, 1990, 1998; Boughman, 2002). Thus, ecological character displacement could directly alter female mate preferences, and thereby initiate or perpetuate reproductive character displacement, if such preferences also minimize reproductive interactions.
Ecological Character Displacement in Habitat Use as a Promoter of Reproductive Character Displacement
The above discussion illustrates how resource shifts can directly alter male sexual signals or female mate preference, and thereby promote reproductive character displacement. Yet, ecological character displacement may also mediate indirect divergence in reproductive characters. In particular, because habitat critically affects the attenuation and perception of signals (reviewed in Wiley, 1994; Bradbury and Vehrencamp, 1998; Boughman, 2002; Gerhardt and Huber, 2002), shifts in habitat use associated with ecological character displacement may promote selection for the evolution of novel sexual signals that are more suited to the new foraging habitat. The sympatric anoles, Anolis cooki and A. cristatellus, for example, display divergent UV light sensitivity that appears to enable them to co-occur in partitioned light microhabitat (Leal and Fleishman, 2002). Such divergent microhabitat use may facilitate co-occurrence by enabling them to partition resources (Leal and Fleishman, 2002). UV reflectance of male dewlaps has also diverged so that male dewlaps contrast most against the light microhabitat in which each species resides, thereby facilitating species recognition (Leal and Fleishman, 2002). Presumably, divergent habitat use simultaneously selects for signals that optimize communication in the novel habitat while also minimizing reproductive interactions between species (Leal and Fleishman, 2002). In this way, ecological character displacement may indirectly foster the evolution of divergent reproductive characters that minimize reproductive interactions.
Changes in habitat or resource use via ecological character displacement may also generate changes in female mate preferences that promote reproductive character displacement. Such changes could occur in two ways. First, novel habitats may exert direct selection on females to evolve preferences for male traits that are most efficiently detected in those new habitats (Endler and Basolo, 1998; Boughman, 2002). Second, novel habitats or resources may exert natural selection on female sensory systems to better identify prey (Ryan, 1998; Boughman, 2002). These shifts in sensory sensitivity could indirectly alter patterns of female mate choice (Ryan, 1990, 1998; Endler and Basolo, 1998; Boughman, 2002, 2007). If such preferences reduce sexual interactions between species, they may be further enhanced by reproductive character displacement.
In sticklebacks, for example, a benthic ecomorph forages and mates in the littoral zone where red coloration is more difficult to detect. A limnetic ecomorph, by contrast, occurs in open water where red coloration is more discernable (Boughman, 2001). Benthic females are less sensitive to variation in red than are limnetic females, and, unlike limnetic females, benthic females do not tend to prefer redder males (Boughman, 2001, 2007; reviewed in Boughman, 2002). Male red coloration, in turn, is “tuned” to female perception of red color: males are redder in populations where females are actually sensitive to, and thus prefer, redder males (Boughman, 2001, 2007). Perhaps more critically, the extent to which a given limnetic/benthic species pair is reproductively isolated is negatively correlated with female red sensitivity and preference in a given population (Boughman, 2001). Thus, shifts in mate preference tied to different habitats dictates the degree to which reproductive divergence has occurred (Boughman, 2001). Generally, shifts in resource or habitat use via ecological character displacement may play a critical role in initiating and promoting reproductive character displacement by fostering changes in mate preferences and sexual signals that minimize reproductive interactions between species.
Reproductive Character Displacement as a Promoter of Ecological Character Displacement
Although most empirical work has focused on how shifts in resource or habitat use may dictate shifts in reproductive characters, the reverse scenario could unfold (e.g., Boughman, 2001; Podos, 2001; Huber and Podos, 2006; Boughman, 2007). The evolution of reproductive characters stemming from selection to minimize reproductive interference could also cause divergence in traits associated with resource acquisition (Konuma and Chiba, 2007). If, for example, species segregate in space or time to avoid reproductive interactions, they may be concomitantly exposed to novel, underutilized resources, thereby possibly leading to a shift in traits associated with resource use.
Moreover, mate preferences to avoid interactions with heterospecifics may promote the evolution of traits involved in the production of those signals (e.g., body size, beak morphology), which could, in turn, cause a shift in resource use and associated traits (Konuma and Chiba, 2007). In the anole example above, for instance, the evolutionary chain of events is unclear. Although habitat partitioning in different light environments may have fostered the evolution of sexual signals that result in reproductive trait divergence, the converse also could have occurred. That is, reproductive interactions may have generated divergence in perception and signaling that in turn fostered habitat and resource partitioning (Leal and Fleishman, 2002).
Teasing Apart Reproductive and Ecological Character Displacement: Caveats
As discussed above, reproductive and ecological character displacement can promote each other. Yet, correlated evolution in either sexual signals or resource acquisition traits in response to direct selection on the alternative type of trait does not constitute character displacement (Coyne and Orr, 2004). For example, the correlated evolution of sexual signals in response to selection to minimize resource competition would not represent reproductive character displacement per se. Only if those reproductive traits also become the targets of selection to minimize reproductive interactions would divergence in these traits constitute reproductive character displacement. Caution must therefore be exercised when studying character displacement in systems that display divergence in both reproductive and resource use traits (Rundle and Schluter, 1998; Coyne and Orr, 2004).
Nevertheless, two species that are similar enough to compete for resources will also likely utilize similar habitat for mate acquisition and vice versa (Schluter, 2000; Rundle and Schluter, 2004; Grant and Grant, 2008; Price, 2008). Thus, initial changes to resource-use traits or sexual signals brought about by one form of character displacement will fuel the alternate form of character displacement, if these differences are subsequently maintained or enhanced by selection to minimize interspecific interactions. As ecological and reproductive character displacement become intertwined, the degree to which one leads the other may become obscure (Schluter, 2000). Studies specifically aimed at teasing apart the relative contribution of each process are needed to determine if both types of character displacement are occurring in a given system (e.g., Rundle and Schluter, 1998).
Finally, although we have focused on how reproductive and ecological character displacement might reinforce each other, each process can potentially preclude the other from occurring. The inhibition of one process by the other may be especially likely to occur when either process generates habitat partitioning (either spatially or temporally). When habitat partitioning arises via one process, selection to minimize interactions via the alternate process is effectively shut down. Essentially, the operation of one process negates the selective pressure for the other process to occur.
Conclusions
The consensus that has emerged from previous work is that character displacement is taxonomically widespread and that it can act to lessen both competitive and reproductive interactions between species (Howard, 1993; Schluter, 2000; Servedio and Noor, 2003; Coyne and Orr, 2004; Dayan and Simberloff, 2005; Groning and Hochkirch, 2008). Having established that character displacement occurs, researchers can now move on to teasing apart what factors that facilitate character displacement. Because character displacement can drive speciation and adaptive radiations (Schluter, 2000; Grant and Grant, 2008), understanding what species are especially prone to undergoing character displacement may help explain why some taxonomic groups are more diverse than others. Understanding when character displacement proceeds – and when it does not – may therefore reveal how microevolutionary processes generate macroevolutionary patterns of diversity.
Similarly, by understanding how and when character displacement is likely to occur, we may gain insights into patterns of species coexistence, community diversity, and potentially large-scale patterns of species distribution. Character displacement necessarily mediates species coexistence (Losos, 2000), and it has the further potential to alter population dynamics, extinction risk, and concomitantly, species ranges (see discussion above as well as Brown, 1995;Thompson, 2005; Groning and Hochkirch, 2008). Studying character displacement can therefore potentially reveal how the fitness consequences of interactions between species ultimately translate into macroecological patterns of species richness, distributions, and diversity. Thus, the process by which individuals optimize fitness in response to heterospecifics – character displacement – provides a unifying framework for understanding the origins, abundance, and distribution of biodiversity.
Acknowledgements
For comments and discussion we thank T. Price, A. Rice, E. Wojtowicz, R. Martin, S. Dhole, C. Ledón-Rettig, A. Chunco, L. Exline, and M. Servedio. We also thank J. Wiens and two anonymous reviewers, whose comments greatly improved the manuscript, and M. Noor for moral support and a chocolate shake. The National Science Foundation and the National Institutes of Health support our research on character displacement.
Literature Cited
- Abrams PA. Character displacement and niche shift analyzed using consumer-resource models of competition. Theoretical Population Biology. 1986;29:107–160. doi: 10.1016/0040-5809(86)90007-9. [DOI] [PubMed] [Google Scholar]
- Agrawal AA. Phenotypic plasticity in the interactions and evolution of species. Science. 2001;294:321–326. doi: 10.1126/science.1060701. [DOI] [PubMed] [Google Scholar]
- Agrawal AA, Laforsch C, Tollrian R. Transgenerational induction of defences in animals and plants. Nature. 1999;401:60–63. [Google Scholar]
- Allen RM, Buckley YM, Marshall DJ. Offspring size plasticity in response to intraspecific competition: An adaptive maternal effect across life-history stages. American Naturalist. 2008;171:225–237. doi: 10.1086/524952. [DOI] [PubMed] [Google Scholar]
- Andersson M. Sexual selection. Princeton University Press; Princeton, NJ: 1994. [Google Scholar]
- Arnold ML. Natural hybridization and evolution. Oxford University Press; Oxford, UK: 1997. [Google Scholar]
- Arthur W. The evolutionary consequences of interspecific competition. Advances in Ecological Research. 1982;12:127–187. [Google Scholar]
- Barrett RDH, Schluter D. Adaptation from standing genetic variation. Trends in Ecology and Evolution. 2008;23:38–44. doi: 10.1016/j.tree.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Barton NH, Hewitt GM. Adaptation, speciation and hybrid zones. Nature. 1989;341:497–503. doi: 10.1038/341497a0. [DOI] [PubMed] [Google Scholar]
- Blair WF. Character displacement in frogs. American Zoologist. 1974;14:1119–1125. [Google Scholar]
- Bolnick DI. Can intraspecific competition drive disruptive selection? An experimental test in natural populations of sticklebacks. Evolution. 2004;58:608–618. [PubMed] [Google Scholar]
- Bolnick DI, Lau OL. Predictable patterns of disruptive selection in stickleback in postglacial lakes. American Naturalist. 2008;172:1–11. doi: 10.1086/587805. [DOI] [PubMed] [Google Scholar]
- Boughman JW. Divergent sexual selection enhances reproductive isolation in sticklebacks. Nature. 2001;411:944–948. doi: 10.1038/35082064. [DOI] [PubMed] [Google Scholar]
- Boughman JW. How sensory drive can promote speciation. Trends in Ecology and Evolution. 2002;17:571–577. [Google Scholar]
- Boughman JW. Condition-dependent expression of red colour differs between stickleback species. Journal of Evolutionary Biology. 2007;20:1577–1590. doi: 10.1111/j.1420-9101.2007.01324.x. [DOI] [PubMed] [Google Scholar]
- Bradbury JW, Vehrencamp SL. Principles of animal communication. Sinauer Associates, Inc; Sunderland, MA: 1998. [Google Scholar]
- Brown JH. Macroecology. University of Chicago Press; Chicago, IL: 1995. [Google Scholar]
- Brown WL, Wilson EO. Character displacement. Systematic Zoology. 1956;5:49–64. [Google Scholar]
- Butlin R. Speciation by reinforcement. Trends in Ecology and Evolution. 1987;2:8–13. doi: 10.1016/0169-5347(87)90193-5. [DOI] [PubMed] [Google Scholar]
- Butlin RK, Ritchie MG. Behaviour and speciation. In: Slater PJB, Halliday TR, editors. Behaviour and speciation. Cambridge University Press; Cambridge, UK: 1994. [Google Scholar]
- Calsbeek R, Smith TB. Experimentally replicated disruptive selection on performance traits in a Caribbean lizard. Evolution. 2008;62:478–484. doi: 10.1111/j.1558-5646.2007.00282.x. [DOI] [PubMed] [Google Scholar]
- Caruso CM. Competition for pollination influences selection on floral traits of Ipomopsis aggregata. Evolution. 2000;54:1546–1557. doi: 10.1111/j.0014-3820.2000.tb00700.x. [DOI] [PubMed] [Google Scholar]
- Conner JK, Hartl DL. A primer of ecological genetics. Sinauer Associates, Inc.; Sunderland, MA: 2004. [Google Scholar]
- Cooley JR. Decoding asymmetries in reproductive character displacement. Proceedings of the Academy of Natural Sciences of Philadelphia. 2007;156:89–96. [Google Scholar]
- Coyne JA, Orr HA. Speciation. Sinauer Associates, Inc.; Sunderland, MA: 2004. [Google Scholar]
- Crozier RH. Niche shape and genetical aspects of character displacement. American Zoologist. 1974;14:1151–1157. [Google Scholar]
- Darwin C. On the origin of species by means of natural selection. 1st ed. John Murray; London, UK: 1859. [Google Scholar]
- Day T, Young KA. Competitive and facilitative evolutionary diversification. BioScience. 2004;54:101–109. [Google Scholar]
- Dayan T, Simberloff D. Ecological and community-wide character displacement: the next generation. Ecology Letters. 2005;8:875–894. [Google Scholar]
- DeWitt TJ, Scheiner SM. Phenotypic plasticity: functional and conceptual approaches. Oxford University Press; New York, NY: 2004. [Google Scholar]
- Diamond J, Pimm SL, Gilpin ME, LeCroy M. Rapid evolution of character displacement in Myzomelid honeyeaters. American Naturalist. 1989;134:675–708. [Google Scholar]
- Dobzhansky T. Speciation as a stage in evolutionary divergence. American Naturalist. 1940;74:312–321. [Google Scholar]
- Doebeli M. An explicit genetic model for ecological character displacement. Ecology. 1996;77:510–520. [Google Scholar]
- Endler JA. Natural selection in the wild. Princeton University Press; Princeton, NJ: 1986. [Google Scholar]
- Endler JA, Basolo AL. Sensory ecology, receiver biases and sexual selection. Trends in Ecology and Evolution. 1998;13:415–420. doi: 10.1016/s0169-5347(98)01471-2. [DOI] [PubMed] [Google Scholar]
- Fordyce JA. The evolutionary consequences of ecological interactions mediated through phenotypic plasticity. Journal of Experimental Biology. 2006;209:2377–2383. doi: 10.1242/jeb.02271. [DOI] [PubMed] [Google Scholar]
- Gause GF. The struggle for existence. Williams and Wilkins; Baltimore, MD: 1934. [Google Scholar]
- Gerhardt HC. Reproductive character displacement of female mate choice in the gray treefrog, Hyla chrysoscelis. Animal Behaviour. 1994;47:959–969. [Google Scholar]
- Gerhardt HC, Huber F. Acoustic communication in insects and anurans: common problems and diverse solutions. University of Chicago Press; Chicago, IL: 2002. [Google Scholar]
- Goldberg EE, Lande R. Ecological and reproductive character displacement on an environmental gradient. Evolution. 2006;60:1344–1357. [PubMed] [Google Scholar]
- Gorbushin AM. The enigma of mud snail shell growth: asymmetrical competition or character displacement? Oikos. 1996;77:85–92. [Google Scholar]
- Grant PR. Convergent and divergent character displacement. Biological Journal of the Linnean Society. 1972;4:39–68. [Google Scholar]
- Grant PR, Grant BR. Evolution of character displacement in Darwin's finches. Science. 2006;313:224–226. doi: 10.1126/science.1128374. [DOI] [PubMed] [Google Scholar]
- Grant PR, Grant BR. How and why species multiply: the radiation of Darwin's finches. Princeton University Press; Princeton, NJ: 2008. [Google Scholar]
- Groning J, Hochkirch A. Reproductive interference between animal species. Quarterly Review of Biology. 2008;83:257–282. doi: 10.1086/590510. [DOI] [PubMed] [Google Scholar]
- Hardin G. The competitive exclusion principle. Science. 1960;131:1292–1297. doi: 10.1126/science.131.3409.1292. [DOI] [PubMed] [Google Scholar]
- Hatfield T, Schluter D. A test for sexual selection on hybrids of two sympatric sticklebacks. Evolution. 1996;50:2429–2434. doi: 10.1111/j.1558-5646.1996.tb03629.x. [DOI] [PubMed] [Google Scholar]
- Hatfield T, Schluter D. Ecological speciation in sticklebacks: environment-dependent hybrid fitness. Evolution. 1999;53:866–873. doi: 10.1111/j.1558-5646.1999.tb05380.x. [DOI] [PubMed] [Google Scholar]
- Hebets EA, Papaj DR. Complex signal function: developing a framework of testable hypotheses. Behavioral Ecology And Sociobiology. 2005;57:197–214. [Google Scholar]
- Hendry AP, Huber SK, De Leon LF, Herrel A, Podos J. Disruptive selection in a bimodal population of Darwin's finches. Proceedings of the Royal Society B-Biological Sciences. 2009;276:753–759. doi: 10.1098/rspb.2008.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgie M, Blows MW. Are traits that experience reinforcement also under sexual selection? American Naturalist. 2007;170:409–420. doi: 10.1086/519401. [DOI] [PubMed] [Google Scholar]
- Higgie M, Blows MW. The evolution of reproductive character displacement conflicts with how sexual selection operates within a species. Evolution. 2008;62:1192–1203. doi: 10.1111/j.1558-5646.2008.00357.x. [DOI] [PubMed] [Google Scholar]
- Hochkirch A, Groning J, Bucker A. Sympatry with the devil: reproductive interference could hamper species coexistence. Journal of Animal Ecology. 2007;76:633–642. doi: 10.1111/j.1365-2656.2007.01241.x. [DOI] [PubMed] [Google Scholar]
- Hoskin CJ, Higgie M, McDonald KR, Moritz C. Reinforcement drives rapid allopatric speciation. Nature. 2005;437:1353–1356. doi: 10.1038/nature04004. [DOI] [PubMed] [Google Scholar]
- Howard DJ. Reinforcement: origin, dynamics, and fate of an evolutionary hypothesis. In: Harrison RG, editor. Hybrid zones and the evolutionary process. Oxford University Press; New York, NY: 1993. pp. 46–69. [Google Scholar]
- Huber SK, De Leon LF, Hendry AP, Bermingham E, Podos J. Reproductive isolation of sympatric morphs in a population of Darwin's finches. Proceedings of the Royal Society B-Biological Sciences. 2007;274:1709–1714. doi: 10.1098/rspb.2007.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber SK, Podos J. Beak morphology and song features covary in a population of Darwin's finches (Geospiza fortis) Biological Journal of the Linnean Society. 2006;88:489–498. [Google Scholar]
- Jablonka E, Lamb MJ. Epigenetic inheritance and evolution: the Lamarkian dimension. Oxford University Press; New York, NY: 1995. [Google Scholar]
- Kokko H, Brooks R. Sexy to die for? Sexual selection and the risk of extinction. Conference on Extinction Thresholds; Helsinki, Finland. 2002. pp. 207–219. [Google Scholar]
- Konuma J, Chiba S. Ecological character displacement caused by reproductive interference. Journal of Theoretical Biology. 2007;247:354–364. doi: 10.1016/j.jtbi.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Lack D. Darwin's finches. Cambridge University Press; Cambridge, UK: 1947. [Google Scholar]
- Leal M, Fleishman LJ. Evidence for habitat partitioning based on adaptation to environmental light in a pair of sympatric lizard species. Proceedings of the Royal Society of London Series B-Biological Sciences. 2002;269:351–359. doi: 10.1098/rspb.2001.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin DA. Reproductive character dispalcement in Phlox. Evolution. 1985;39:1275–1281. doi: 10.1111/j.1558-5646.1985.tb05693.x. [DOI] [PubMed] [Google Scholar]
- Liou LW, Price TD. Speciation by reinforcement of premating isolation. Evolution. 1994;48:1451–1459. doi: 10.1111/j.1558-5646.1994.tb02187.x. [DOI] [PubMed] [Google Scholar]
- Lorch PD, Proulx S, Rowe L, Day T. Condition-dependent sexual selection can accelerate adaptation. Evolutionary Ecology Research. 2003;5:867–881. [Google Scholar]
- Losos JB. Ecological character displacement and the study of adaptation. Proceedings of the National Academy of Sciences, U.S.A. 2000;97:5693–5695. doi: 10.1073/pnas.97.11.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marko PB. An intraspecific comparative analysis of character divergence between sympatric species. Evolution. 2005;59:554–564. [PubMed] [Google Scholar]
- Martin RA, Pfennig DW. Disruptive selection in natural populations: the roles of ecological selection and resource competition. American Naturalist. doi: 10.1086/600090. in press. [DOI] [PubMed] [Google Scholar]
- Mather K. The genetical structure of populations. Symposium of the Society for Experimental Biology. 1953;2:196–216. [Google Scholar]
- Maynard Smith J. Evolutionary genetics. 2nd ed Oxford University Press; New York, NY: 1998. [Google Scholar]
- McAdam AG, Boutin S. Maternal effects and the response to selection in red squirrels. Proceedings of the Royal Society B. 2004;271:75–79. doi: 10.1098/rspb.2003.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medel R, Botto-Mahan C, Kalin-Arroyo M. Pollinator-mediated selection on the nectar guide phenotype in the Andean monkey flower, Mimulus luteus. Ecology. 2003;84:1721–1732. [Google Scholar]
- Milligan BG. Evolutionary divergence and character displacement in two phenotypically-variable competing species. Evolution. 1985;39:1207–1222. doi: 10.1111/j.1558-5646.1985.tb05687.x. [DOI] [PubMed] [Google Scholar]
- Mousseau TA, Fox CW. Maternal effects as adaptations. Oxford University Press; New York, NY: 1998. [Google Scholar]
- Olson VA, Owens IPF. Costly sexual signals: are carotenoids rare, risky or required? Trends in Ecology and Evolution. 1998;13:510–514. doi: 10.1016/s0169-5347(98)01484-0. [DOI] [PubMed] [Google Scholar]
- Pfennig DW, Martin RA. A maternal effect mediates rapid population divergence and character displacement in spadefoot toads. Evolution. doi: 10.1111/j.1558-5646.2008.00544.x. in press. [DOI] [PubMed] [Google Scholar]
- Pfennig DW, Murphy PJ. Character displacement in polyphenic tadpoles. Evolution. 2000;54:1738–1749. doi: 10.1111/j.0014-3820.2000.tb00717.x. [DOI] [PubMed] [Google Scholar]
- Pfennig DW, Murphy PJ. How fluctuating competition and phenotypic plasticity mediate species divergence. Evolution. 2002;56:1217–1228. doi: 10.1111/j.0014-3820.2002.tb01433.x. [DOI] [PubMed] [Google Scholar]
- Pfennig DW, Rice AM. An experimental test of character displacement's role in promoting postmating isolation between conspecific populations in contrasting competitive environments. Evolution. 2007;61:2433–2443. doi: 10.1111/j.1558-5646.2007.00190.x. [DOI] [PubMed] [Google Scholar]
- Pfennig DW, Rice AM, Martin RA. Ecological opportunity and phenotypic plasticity interact to promote character displacement and species coexistence. Ecology. 2006;87:769–779. doi: 10.1890/05-0787. [DOI] [PubMed] [Google Scholar]
- Pfennig DW, Rice AM, Martin RA. Field and experimental evidence for competition's role in phenotypic divergence. Evolution. 2007;61:257–271. doi: 10.1111/j.1558-5646.2007.00034.x. [DOI] [PubMed] [Google Scholar]
- Pfennig KS. The evolution of mate choice and the potential for conflict between species and mate-quality recognition. Proceedings of the Royal Society of London Series B-Biological Sciences. 1998;265:1743–1748. [Google Scholar]
- Pfennig KS. Female spadefoot toads compromise on mate quality to ensure conspecific matings. Behavioral Ecology. 2000;11:220–227. [Google Scholar]
- Pfennig KS. Facultative mate choice drives adaptive hybridization. Science. 2007;318:965–967. doi: 10.1126/science.1146035. [DOI] [PubMed] [Google Scholar]
- Pfennig KS. Population differences in condition-dependent sexual selection may promote divergence in non-sexual traits. Evolutionary Ecology Research. 2008;10:763–773. [Google Scholar]
- Pfennig KS, Pfennig DW. Character displacement as the “best of a bad situation”: fitness trade-offs resulting from selection to minimize resource and mate competition. Evolution. 2005;59:2200–2208. [PubMed] [Google Scholar]
- Pfennig KS, Ryan MJ. Reproductive character displacement generates reproductive isolation among conspecific populations: an artificial neural network study. Proceedings of the Royal Society B-Biological Sciences. 2006;273:1361–1368. doi: 10.1098/rspb.2005.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfennig KS, Ryan MJ. Character displacement and the evolution of mate choice: an artificial neural network approach. Philosophical Transactions of the Royal Society B-Biological Sciences. 2007;362:411–419. doi: 10.1098/rstb.2006.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pianka ER. Evolutionary ecology. 6th ed Benjamin/Cummings; San Francisco, CA: 2000. [Google Scholar]
- Plaistow SJ, Lapsley CT, Benton TG. Context-dependent intergenerational effects: The interaction between past and present environments and its effect on population dynamics. American Naturalist. 2006;167:206–215. doi: 10.1086/499380. [DOI] [PubMed] [Google Scholar]
- Podos J. Correlated evolution of morphology and vocal signal structure in Darwin's finches. Nature. 2001;409:185–188. doi: 10.1038/35051570. [DOI] [PubMed] [Google Scholar]
- Podos J, Nowicki S. Beaks, adaptation, and vocal evolution in Darwin's finches. Bioscience. 2004;54:501–510. [Google Scholar]
- Podos J, Southall JA, Rossi-Santos MR. Vocal mechanics in Darwin's finches: correlation of beak gape and song frequency. Journal of Experimental Biology. 2004;207:607–619. doi: 10.1242/jeb.00770. [DOI] [PubMed] [Google Scholar]
- Price T. Speciation in birds. Roberts and Company Publishers; Greenwood Village, CO: 2008. [Google Scholar]
- Rice AM, Pfennig DW. Character displacement: in situ evolution of novel phenotypes or sorting of pre-existing variation? Journal of Evolutionary Biology. 2007;20:448–459. doi: 10.1111/j.1420-9101.2006.01187.x. [DOI] [PubMed] [Google Scholar]
- Rice WR. Speciation via habitat specialization: the evolution of reproductive isolation as a correlated character. Evolutionary Ecology. 1987;1:301–315. [Google Scholar]
- Robinson BW, Wilson DS. Character release and displacement in fish: a neglected literature. American Naturalist. 1994;144:596–627. [Google Scholar]
- Rosenthal GG, Wagner WE, Ryan MJ. Secondary reduction of preference for the sword ornament in the pygmy swordtail Xiphophorus nigrensis (Pisces: Poeciliidae) Animal Behaviour. 2002;63:37–45. [Google Scholar]
- Rosenzweig ML. Competitive speciation. Biological Journal of the Linnean Society. 1978;10:274–289. [Google Scholar]
- Rossiter MC. Incidence and consequences of inherited environmental effects. Annual Review of Ecology and Systematics. 1996;27:451–476. [Google Scholar]
- Rueffler C, Van Dooren TJM, Leimar O, Abrams PA. Disruptive selection and then what? Trends in Ecology and Evolution. 2006;21:238–245. doi: 10.1016/j.tree.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Rundle HD. A test of ecologically dependent postmating isolation between sympatric sticklebacks. Evolution. 2002;56:322–329. doi: 10.1111/j.0014-3820.2002.tb01342.x. [DOI] [PubMed] [Google Scholar]
- Rundle HD, Schluter D. Reinforcement of stickleback mate preferences: sympatry breeds contempt. Evolution. 1998;52:200–208. doi: 10.1111/j.1558-5646.1998.tb05153.x. [DOI] [PubMed] [Google Scholar]
- Rundle HD, Schluter D. Natural selection and ecological speciation in sticklebacks. In: Dieckmann U, Doebeli M, Metz JAJ, Tautz D, editors. Adaptive speciation. Cambirdge University Press; Cambridge, UK: 2004. pp. 192–209. [Google Scholar]
- Ryan MJ. Sensory systems, sexual selection, and sensory exploitation. Oxford Surveys in Evolutionary Biology. 1990;7:157–195. [Google Scholar]
- Ryan MJ. Sexual selection, receiver biases, and the evolution of sex differences. Science. 1998;281:1999–2003. doi: 10.1126/science.281.5385.1999. [DOI] [PubMed] [Google Scholar]
- Ryan MJ, Rand AS. Species recognition and sexual selection as a unitary problem in animal communication. Evolution. 1993;47:647–657. doi: 10.1111/j.1558-5646.1993.tb02118.x. [DOI] [PubMed] [Google Scholar]
- Schlichting CD, Pigliucci M. Phenotypic evolution: a reaction norm perspective. Sinauer; Sunderland, MA: 1998. [Google Scholar]
- Schluter D. Experimental evidence that competition promotes divergence in adaptive radiation. Science. 1994;266:798–801. doi: 10.1126/science.266.5186.798. [DOI] [PubMed] [Google Scholar]
- Schluter D. The ecology of adaptive radiation. Oxford University Press; Oxford, UK: 2000. [Google Scholar]
- Schluter D. In: Ecological character displacement. Evolutionary ecology: concepts and case studies. Fox CW, Roff DA, Fairbairn DJ, editors. Oxford University Press; New York, NY: 2001. pp. 265–276. [Google Scholar]
- Schluter D. Character displacement. In: Pagel M, editor. Encyclopedia of evolution. Vol. 1. Oxford University Press; Oxford, UK: 2002. pp. 149–150. [Google Scholar]
- Seehausen O, van Alphen JJM. The effect of male coloration on female mate choice in closely related Lake Victoria cichlids (Haplochromis nyererei complex) Behavioral Ecology and Sociobiology. 1998;42:1–8. [Google Scholar]
- Servedio MR, Noor MAF. The role of reinforcement in speciation: theory and data. Annual Review of Ecology Evolution and Systematics. 2003;34:339–364. [Google Scholar]
- Simberloff DS, Boecklen W. Santa Rosalia reconsidered: size ratios and competition. Evolution. 1981;35:1206–1228. doi: 10.1111/j.1558-5646.1981.tb04990.x. [DOI] [PubMed] [Google Scholar]
- Simpson GG. The major features of evolution. Columbia University Press; New York, NY: 1953. [Google Scholar]
- Slatkin M. Ecological character displacement. Ecology. 1980;61:163–177. [Google Scholar]
- Smith RA, Rausher MD. Experimental evidence that selection favors character displacement in the ivyleaf morning glory. American Naturalist. 2008;171:1–9. doi: 10.1086/523948. [DOI] [PubMed] [Google Scholar]
- Smith TB. Disruptive selection and the genetic basis of bill size polymorphism in the African finch Pyrenestes. Nature. 1993;363:618–620. [Google Scholar]
- Smith TB, Skúkason S. Evolutionary significance of resource polymorphisms in fishes, amphibians, and birds. Annual Review of Ecology and Systematics. 1996;27:111–133. [Google Scholar]
- Strong DR, Jr., Szyska LA, Simberloff DS. Tests for community-wide character displacement against null hypotheses. Evolution. 1979;33:897–913. doi: 10.1111/j.1558-5646.1979.tb04743.x. [DOI] [PubMed] [Google Scholar]
- Svedin N, Wiley C, Veen T, Gustafsson L, Qvarnstrom A. Natural and sexual selection against hybrid flycatchers. Proceedings of the Royal Society B-Biological Sciences. 2008;275:735–744. doi: 10.1098/rspb.2007.0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taper ML, Case TJ. Quantitative genetic models for the coevolution of character displacement. Ecology. 1985;66:355–371. [Google Scholar]
- Taper ML, Case TJ. Coevolution among competitors. Oxford Surveys in Evolutionary Biology. 1992;8:63–109. [Google Scholar]
- Thompson JN. The geographic mosaic of coevolution. University of Chicago Press; Chicago, IL: 2005. [Google Scholar]
- Tynkkynen K, Rantala MJ, Suhonen J. Interspecific aggression and character displacement in the damselfly Calopteryx splendens. Journal of Evolutionary Biology. 2004;17:759–767. doi: 10.1111/j.1420-9101.2004.00733.x. [DOI] [PubMed] [Google Scholar]
- Vamosi SM, Schluter D. Sexual selection against hybrids between sympatric stickleback species: evidence from a field experiment. Evolution. 1999;53:874–879. doi: 10.1111/j.1558-5646.1999.tb05381.x. [DOI] [PubMed] [Google Scholar]
- van der Sluijs I, Van Dooren TJM, Hofker KD, van Alphen JJM, Stelkens RB, Seehausen O. Female mating preference functions predict sexual selection against hybrids between sibling species of cichlid fish. Philosophical Transactions of the Royal Society B-Biological Sciences. 2008;363:2871–2877. doi: 10.1098/rstb.2008.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb C. A complete classification of Darwinian extinction in ecological interactions. American Naturalist. 2003;161:181–205. doi: 10.1086/345858. [DOI] [PubMed] [Google Scholar]
- Werner EE, Hall DS. Niche shifts in sunfishes: experimental evidence and significance. Science. 1976;191:404–406. doi: 10.1126/science.1246626. [DOI] [PubMed] [Google Scholar]
- West-Eberhard MJ. Developmental plasticity and evolution. Oxford University Press; Oxford, UK: 2003. [Google Scholar]
- Wiley RH. Errors, exaggeration, and deception in animal communication. In: Real L, editor. Behavioral mechanisms in ecology. University of Chicago Press; Chicago, IL: 1994. pp. 157–189. [Google Scholar]
- Wilson DS, Turelli M. Stable underdominance and the evolutionary invasion of empty niches. American Naturalist. 1986;127:835–850. [Google Scholar]
- Wilson EO. The diversity of life. Harvard University Press; Cambridge, MA: 1992. [Google Scholar]