Abstract
BACKGROUND
Although histological dating of endometrial biopsies provides little help for prediction or diagnosis of infertility, analysis of individual endometrial proteins, proteomic profiling and transcriptome analysis have suggested several biomarkers with altered expression arising from intrinsic abnormalities, inadequate stimulation by or in response to gonadal steroids or altered function due to systemic disorders. The objective of this study was to delineate the developmental dynamics of potentially important proteins in the secretory phase of the menstrual cycle, utilizing a collection of endometrial biopsies from women of fertile (n = 89) and infertile (n = 89) couples.
METHODS AND RESULTS
Progesterone receptor-B (PGR-B), leukemia inhibitory factor, glycodelin/progestagen-associated endometrial protein (PAEP), homeobox A10, heparin-binding EGF-like growth factor, calcitonin and chemokine ligand 14 (CXCL14) were measured using a high-throughput, quantitative immunohistochemical method. Significant cyclic and tissue-specific regulation was documented for each protein, as well as their dysregulation in women of infertile couples. Infertile patients demonstrated a delay early in the secretory phase in the decline of PGR-B (P < 0.05) and premature mid-secretory increases in PAEP (P < 0.05) and CXCL14 (P < 0.05), suggesting that the implantation interval could be closing early. Correlation analysis identified potential interactions among certain proteins that were disrupted by infertility.
CONCLUSIONS
This approach overcomes the limitations of a small sample number. Protein expression and localization provided important insights into the potential roles of these proteins in normal and pathological development of the endometrium that is not attainable from transcriptome analysis, establishing a basis for biomarker, diagnostic and targeted drug development for women with infertility.
Keywords: endometrium, idiopathic infertility, protein expression, developmental regulation, immunohistochemistry
Introduction
The endometrium, composed of luminal epithelium (LE), glandular epithelium (GE) and stroma (STR; mesenchyme, leukocytes, endothelium and vascular smooth muscle), undergoes molecular and morphological changes during the secretory phase of the menstrual cycle to facilitate embryo attachment and invasion. Implantation requires intercellular signaling to bring the endometrium into a ‘receptive’ interval that is restricted to 4 days of the mid-secretory phase (MSP). Disruption of the receptive interval impacts pregnancy success, increasing spontaneous abortion from 25 to 40% if embryo implantation occurs 1 day after the receptive interval or to 80% with a 2-day delay (Wilcox et al., 1999). Only limited information is available about the histological and molecular properties of the receptive endometrium and disruption of the receptive interval by disease that contributes to infertility and recurrent pregnancy loss (Martel and Psychoyos, 1981; Martel et al., 1987; Nikas et al., 1995; Aghajanova et al., 2010; Fazleabas, 2010; Young and Lessey, 2010). For example, immunohistochemistry (IHC) reveals that the integrin subunit β3 increases abruptly around cycle Day 20 as the receptive interval opens and this is delayed in women with discordant menstrual cycles (Lessey et al., 1992). The objective of the present investigation was to establish the normal expression patterns of developmentally important proteins in the receptive endometrium and determine whether any are altered in conjunction with infertility.
Histological dating of the timed endometrial biopsy, while previously employed to assess endometrial developmental adequacy for implantation, provides no clinically useful information for identifying infertile women owing to the dynamic nature of the endometrium and natural variation between patients (Coutifaris et al., 2004). However, there is strong evidence to support the idea that molecular evaluation of the endometrium can reliably characterize the receptive phase and changes associated with abnormal implantation. Gene array analysis of endometrial biopsies has been utilized extensively to assess the transcriptome across the normal endometrial cycle (Punyadeera et al., 2005; Yanaihara et al., 2005; Talbi et al., 2006; Lai et al., 2007), during the implantation window (Dominguez et al., 2003; Riesewijk et al., 2003; Mirkin et al., 2005; Henriquez et al., 2006), in response to gonadotrophin therapy (Mirkin et al., 2004; Horcajadas et al., 2005; Simon et al., 2005) and in endometriosis (Eyster et al., 2002; Arimoto et al., 2003; Matsuzaki et al., 2004, Matsuzaki et al., 2005; Matsuzaki et al., 2006; Wu et al., 2006) and endometrial cancer (Hever et al., 2006). This approach has identified candidate genes that could play an integral role in endometrial development and that may become dysregulated in disease. Gene knockout or knockdown in animal models further supports the important role of these genes in human endometrium (Stewart et al., 1992; Lydon et al., 1995; Benson et al., 1996; Zhu et al., 1998a; Mulac-Jericevic et al., 2000; Song et al., 2000; Mulac-Jericevic et al., 2003; Xie et al., 2007). The functional significance of candidate genes can be established through validation of the corresponding proteins in endometrial tissue sections sampled across the menstrual cycle under a variety of clinical conditions. Additionally, a direct proteomic approach has been taken to identify differentially expressed proteins in endometrial tissue during the menstrual cycle (Dominguez et al., 2009; Scotchie et al., 2009; Hannan et al., 2010). Together with information about protein spatial distribution in the epithelial and stromal cellular compartments, new insights into the function of key genes may be acquired. This approach has been used to characterize candidate proteins in clinical specimens but is generally limited by a low sample number and imprecise quantification of protein levels.
Several proteins regulated by progesterone potentially play an integral role in endometrial function and pathology based on their accumulation during the secretory phase in the LE, GE and STR. The mRNA transcripts of four proteins, progestagen-associated endometrial protein/glycodelin (PAEP), homeobox A10 (HOXA10), leukemia inhibitory factor (LIF) and chemokine ligand 14 (CXCL14) are up-regulated significantly during the MSP (Talbi et al., 2006). Three are strongly implicated in various etiologies of infertility, including endometriosis (PAEP, HOXA10 and LIF; Kao et al., 2003; Dimitriadis et al., 2006), hydrosalpinges (HOXA10; Daftary et al., 2007) or idiopathic infertility (LIF and PAEP; Laird et al., 1997; Skrzypczak et al., 2005; Mikolajczyk et al., 2007). Three additional proteins, calcitonin (CALCA; Diao et al., 2002; Kumar et al., 2003), progesterone receptor-B (PGR-B; Franco et al., 2008) and heparin-binding epidermal growth factor-like growth factor (HBEGF; Das et al., 1994; Yoo et al., 1997; Lessey et al., 2002), are implicated in uterine receptivity for implantation. CALCA is present in human and rat uteri during the implantation window and PGR-B persists during the MSP of the human menstrual cycle (Kumar et al., 1998; Mote et al., 1999). HBEGF can promote trophoblast invasion and survival (Leach et al., 2004; Armant et al., 2006; Leach et al., 2008; Jessmon et al., 2010) and is dysregulated in pre-eclampsia (Leach et al., 2002). We hypothesize that these seven proteins are regulated during the secretory phase of the cycle in human endometrium, in accordance with their previously studied mRNA transcripts, and that their cell-specific, developmental expression patterns are altered in the infertile population of women. It is anticipated that the developmental signatures of these and other critical proteins in the endometrium during the secretory phase will provide clinically useful information which is not attainable through morphological dating or transcriptome analysis.
To address the diagnostic utility of a proteomic evaluation of the endometrium, we have developed a high-throughput IHC procedure to precisely label protein analytes in sectioned endometrial biopsies. We utilized 178 endometrial biopsies, a resource generated by the National Institute of Child Health and Human Development Cooperative Reproductive Medicine Network's Endometrial Biopsy Project, representing timed secretory phase biopsies, equally distributed between women from fertile and infertile couples. This IHC analysis of candidate regulatory proteins in well-characterized endometrial biopsies collected under standard operating procedures was used to identify protein changes that define the 4-day interval of implantation in normal fertile women and to determine whether the protein profiles are altered in women who are infertile.
Materials and Methods
Sample collection
Timed endometrial biopsies from well-characterized fertile controls (n = 89) and women from infertile couples (n = 89) originally collected by the Reproductive Medicine Network (Coutifaris et al., 2004) as part of a study examining the incidence of luteal phase defects in infertile women were histologically dated (Noyes et al., 1950). The Wayne State University Human Investigation Research Board, and those of all universities that contributed endometrial biopsy specimens, have approved this study. The mean ages of the women from fertile and infertile couples were 31.9 and 30.2 years, respectively. The endometrial biopsies were obtained using soft endometrial biopsy catheters (e.g. Pipelle®, Cooper Surgical, Inc., Trumbull, CT, USA). Tissue was immediately placed in fixative and embedded in paraffin for histological evaluation, according to standard procedures (Coutifaris et al., 2004). Specimens were biopsied in a randomized fashion in the MSP (cycle Days 22–23) or late (cycle Days 26–27) secretory phase (LSP), as assessed by mid-cycle LH surges (Coutifaris et al., 2004). Biopsies from both collection times were developmentally heterogeneous owing to variations in cycle length among individual women. Since endometrial morphology is not significantly altered with fertility status (Coutifaris et al., 2004), the developmental stage of each biopsy could be determined without biasing differences based on fertility. Cycle days of the biopsies were assigned using the histological dating criteria of Noyes et al. (1950), and were grouped into early secretory phase (ESP; cycle Days 14–19), MSP (cycle Days 20–24) and LSP (cycle Days 25–28). While the cycle phase may have been estimated as MSP and LSP by timing to the LH surge, the histologic evaluation revealed that 57 women were in the ESP, 80 in the MSP and 41 in the LSP of the cycle.
Western blot analysis
To test the specificity of all antibodies used for IHC (see Supplementary data, Fig. S1), the Ishikawa endometrial epithelial adenocarcinoma cell line was maintained in a 1:1 mixture of Dulbecco's modified Eagle's medium and Ham's F12 medium (Sigma Chemical Co., St. Louis, MO, USA) containing 10% donor calf serum and cultured at 37°C. Western blots of cell lysates were performed as previously described (Kilburn et al., 2000) using precast 4–20% polyacrylamide gradient gels (BioRad).
Immunohistochemistry
Tissue sections (5 μm) from endometrial biopsies were deparaffinized and rehydrated through a series of xylene, 100, 95 and 70% ethanol, followed by water. Antigen retrieval was carried out by heating slides at 15 psi and 121°C for 15 min in modified citrate buffer, pH 6.1 (DAKO, Carpinteria, CA, USA). After cooling for 20 min, slides were washed three times with Tris-buffered saline (TBS).
IHC staining was performed on sections prepared from each specimen using antibodies against PGR-B (B-form specific; Lab Vision, Freemont, CA, USA; 5 μg/ml), LIF (R&D Systems, Minneapolis, MN, USA; 5 μg/ml), PAEP (Abcam, Cambridge, MA, USA; 10 μg/ml), HOXA10 (Santa Cruz Biotechnology, Santa Cruz, CA, USA; 1 μg/ml), HBEGF (R&D Systems; 5 μg/ml), CALCA (Bachem/Peninsula Laboratories, Inc., San Carlos, CA, USA; 10 μg/ml) and CXCL14 (Abcam; 5 μg/ml). Negative controls were performed using 10 μg/ml non-immune goat immunoglobulin (Ig)G (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA). Additional information about antibody staining is provided in the Supplementary data, Table S1. Optimal concentrations were chosen for each antibody based on the linear region of the IHC labeling curve, prepared using paraformaldehyde-fixed Ishikawa cells (see Supplementary data, Fig. S2).
Antibody staining was performed at room temperature using a DAKO Autostainer Universal Staining System to ensure uniform treatment of all samples, as previously described (Leach et al., 2002). Samples were rinsed with TBS after each of the following steps. The slides were incubated for 30 min in 3% H2O2 before application of 300 μl primary antibody for 1 h. Rabbit anti-goat secondary antibody (Jackson ImmunoResearch) was applied for 1 h only for samples labeled with non-immune IgG, HOXA10 or HBEGF primary antibodies. All samples were then incubated for 30 min with a peroxidase-conjugated polymer coupled to anti-rabbit and anti-mouse IgG (EnVision Systems Peroxidase, DAKO). The peroxidase was visualized with 3,3-diaminobenzidine (DAB, DAKO) and hydrogen peroxide for 5 min. If DAB staining was too light to locate the LE, GE and STR regions, the tissue was counterstained with propidium iodide (1 μg/ml for 30 min) and viewed by epifluorescence microscopy for region selection. All slides were then rinsed in TBS and mounted using a TissueTek film coverslipper (Sakura Finetek USA, Torrance, CA, USA).
Quantitative image analysis
Sections of each specimen stained with hematoxylin and eosin were used to identify and photo-document four to six optimal regions that contained all three of the tissue features (LE, GE and STR) to be studied by IHC. Digital images of each selected region were obtained at ×200 using a Leica (Wetzlar, Germany) DM IRB epifluorescence microscope and mapped using a 1.5 × 1.0 inch glass coverslip containing a 6 × 4 grid. The left edge of the grid was aligned flush with the left edge of the tissue section to locate the regions of interest identified by hematoxylin and eosin staining on adjacent immunostained slides. Monochromatic bright field images of the antibody/DAB stained tissues were obtained at ×400 using a Hamamatsu Orca digital camera (Hamamatsu City, Japan). Brightness was adjusted in a region of each slide devoid of tissue by setting the gray level to 255. When tissue features were difficult to discern because of very light DAB staining, fluorescent images were obtained of the same field to visualize propidium iodide labeling. Using Simple PCI imaging software (Hamamatsu Corp., Sewickley, PA, USA), areas determined to be LE, GE and STR were each digitally traced to determine the mean gray level of the circumscribed areas on the DAB-labeled images. The relationship between antigen level estimated by image analysis and the actual concentration in cell lysates was determined to be linear, although image analysis tended to overestimate the expression at very low levels (see Supplementary data, Fig. S3).
Statistics
All statistics were performed with Statistical Package for the Social Sciences (SPSS) version 12.0 (SPSS, Chicago, IL, USA) using a 95% confidence interval. Data were normally distributed, according to the Shapiro–Wilks test of normality. Three-way mixed design analysis of variance (ANOVA) was conducted with cell types as the repeated measures or ‘within’ factor to compare sample trends from women of fertile and infertile couples across the entire secretory phase. One-way ANOVA with Student–Newman–Keuls post hoc comparisons was used to identify the temporal changes within each cell type across the secretory phase, separately for women of either fertile or infertile couples. Pairwise comparisons with Bonferroni adjustments were used to compare expression levels between different cell types during ESP, MSP and LSP. For the pairwise analyses, data from women of fertile and infertile couples were pooled together for each cell type when no significant effect of fertility was observed in the mixed design ANOVA. A Student's t-test was used to determine the significance between fertile and infertile groups for each cell type during the ESP, MSP or LSP. Pearson's correlation coefficients were determined to examine the relationships between the changing protein levels in each cell type during ESP, MSP and LSP separately for samples from women of fertile or infertile couples. Significance was defined as P < 0.05; all data are expressed as mean ± SD.
Results
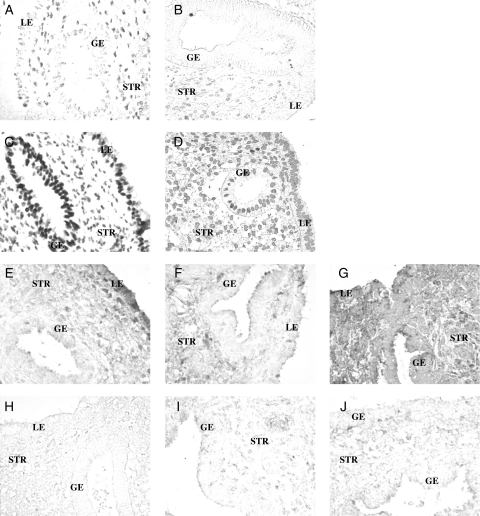
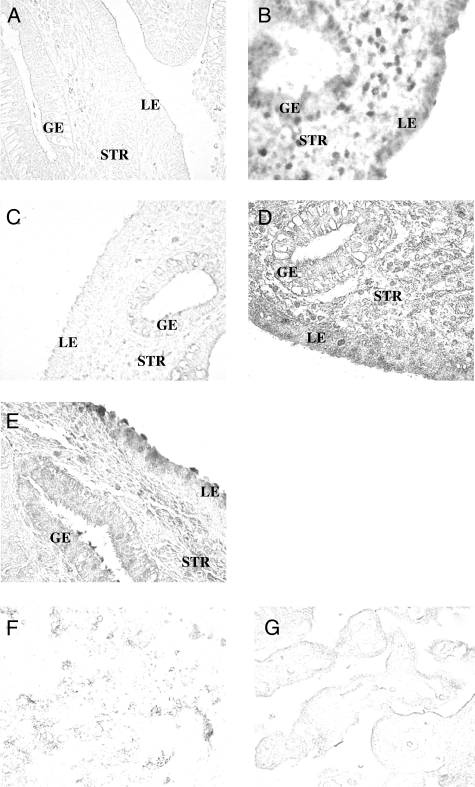
Relative expression of each protein was determined during the ESP, MSP and LSP in each cellular compartment of the endometrium (LE, GE and STR). Images of IHC labeling without a counterstain reveal a variety of compartment-specific patterns that varied from predominantly nuclear staining of PGR-B (Fig. 1A–D) and HOXA10 (Fig. 2B) to broad cytoplasmic staining of the LIF (Fig. 1 E–J), HBEGF (Fig. 2D) and CXCL14 (Fig. 2E). PAEP (Fig. 2A) and CALCA (Fig. 2C) staining was highly localized near the surface of epithelial cells.
Figure 1.
Localization of PGR-B and LIF in endometrial biopsies of women from fertile and infertile couples as assessed by IHC. (A–D) Representative images of PGR-B in fertile controls (A, B) and in women from infertile couples (C, D) during the early (A, C) and mid-secretory (B, D) phases. (E–J) LIF in fertile controls (E–G) and in women from infertile couples (H–J) during the early (E, H), mid (F, I) and late (G, J) secretory phases. Images were not counterstained. Glands (GE), stroma (STR) and luminal epithelium (LE) are indicated in each image. Magnification, ×200.
Figure 2.
IHC localization of PAEP, HOXA10, CALCA, HBEGF and CXCL14 in endometrial biopsies of women from fertile and infertile couples. Representative images of (A) PAEP, (B) HOXA10, (C) CALCA, (D) HBEGF and (E) CXCL14. Pictures F and G represent control staining where a primary antibody for LIF or HBEGF was excluded. Magnification, ×200.
Quantitative IHC analyses
Significant differences were found with respect to tissue specificity, developmental stage and fertility status for most of the proteins examined.
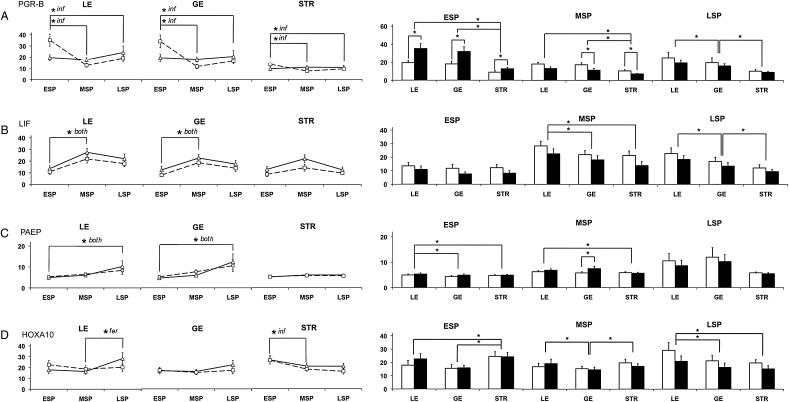
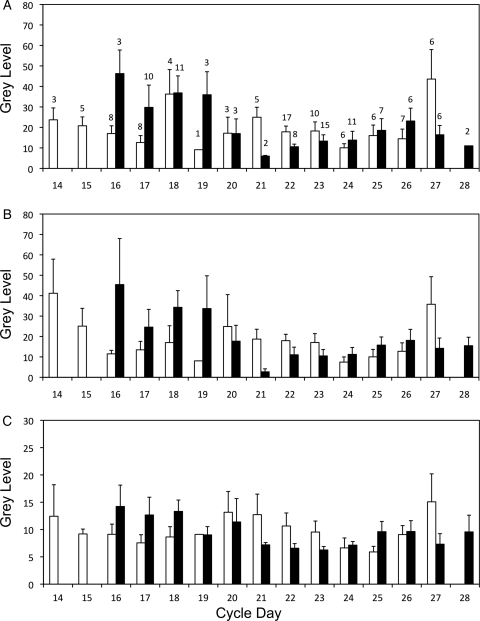
Three-way ANOVA did not demonstrate a significant temporal accumulation of PGR-B in any region of fertile controls (Fig. 3A). However, PGR-B levels fell in all three cell types between the ESP and MSP in biopsies from women of infertile couples (Table I), based on post hoc analysis (P < 0.05 for all). The infertile group had higher levels of PGR-B in LE, GE and STR compared with fertile controls during the ESP (P= 0.01, P = 0.027 and P = 0.032, respectively). This difference reversed during the MSP in which fertile controls had higher levels of PGR-B in the GE and STR than samples from women of infertile couples (P = 0.039 and P = 0.021, respectively). PGR-B levels also differed by cell type. In the ESP, PGR-B levels were similar in the LE and GE (P = 0.534) and were lower in the STR (P < 0.001). In the MSP, regardless of fertility status, PGR-B levels were equivalent in the LE and GE (P = 0.534) and were least prevalent in the STR (P < 0.001). In the LSP, PGR-B levels were highest in the LE and lowest in the STR for both groups (P ≤ 0.001 for all). Overall, while PGR-B did not change temporally in fertile controls, elevated levels appeared during the ESP and diminished during the MSP in women from infertile couples, as seen when the values for each cycle day were examined (Fig. 4).
Figure 3.
Quantitative IHC of PGR-B, LIF, PAEP and HOXA10 in endometrial biopsies of women from fertile and infertile couples. The mean gray levels (arbitrary units; y-axis) were determined for PGR-B (A; fertile, n = 89; infertile, n = 86), LIF (B; fertile, n = 87; infertile, n = 86), PAEP (C; fertile, n = 89; infertile, n = 89) and HOXA10 (D; fertile, n = 86; infertile, n = 87) in each tissue type, as described in the section Materials and Methods, showing temporal changes in each cell type (lines), and cell-type differences (bars) during the ESP (n = 57), MSP (n = 80) and LSP (n = 41). Fertile controls are represented by a solid line (triangles) or open bar, and samples from women of infertile couples by a dashed line (squares) or solid bar. *P < 0.05 for temporal changes (lines) according to Student–Newman–Keuls post hoc comparisons, with indication of changes specific to the fertile controls (fer), infertile couples (inf) or both (both) groups; cell-type-specific differences (bars), or differences with fertility status (bars) according to Student's t-test. Error bars indicate the SD.
Table I.
Changes in protein levels during the opening and closing of the receptive interval for implantation.
| Proteins | ESP to MSP transition |
MSP to LSP transition |
||
|---|---|---|---|---|
| Fertile | Infertile | Fertile | Infertile | |
| PGR-B | −(LE, GE, STR) | |||
| HOXA10 | −(STR) | +(LE), −(STR) | ||
| CXCL14 | +(LE, GE) | +(LE, GE) | ||
| HBEGF | +(LE, GE) | +(GE) | –(STR) | |
| CALCA | −(STR) | +(LE) | ||
| LIF | +(LE, GE) | +(LE, GE) | ||
| PAEP | ||||
Figure 4.
Analysis of PGR-B levels in endometrial biopsies of women from fertile and infertile couples at different stages of the cycle. Mean gray levels were grouped according to histological dating. Tissue type-specific data are shown for LE (A), GE (B) and STR (C), and for fertile controls (open bars) and women of infertile couples (solid bars). The number of patients in each group is indicated above each bar in the first panel. The decrease in expression seen in fertile controls during the early secretory phase appears to be delayed in women of infertile couples. Error bars indicate the SD.
The levels of the LIF fluctuated in cyclical patterns in the LE, GE and STR (three-way ANOVA: P < 0.001, P = 0.002 and P = 0.01, respectively; Fig. 3B). In samples from women of both fertile and infertile couples, LIF expression increased (P < 0.01 for both) according to post hoc testing in the LE and GE between the ESP and MSP (Table I). LIF levels also differed according to cell type. In the ESP, the difference in LIF expression between the LE, GE and STR just escaped significance (P = 0.051). In the MSP, however, LIF expression was higher in the LE than those in GE (P ≤ 0.001), which was not different from levels in the STR (P = 0.249). In the LSP, LIF remained strongest in the LE and was least prevalent in the STR (P ≤ 0.001 for all). The LIF was prominent during the implantation window (c. Day 19) and displayed cell type-specific differences during the MSP and LSP.
According to three-way ANOVA, PAEP exhibited cyclical variations (P < 0.05), with post hoc tests confirming an increase in the LE and GE during the LSP compared with the ESP, independent of fertility status (P < 0.05 for each; Fig. 3C). In addition, a difference was observed during the MSP where the expression in the GE was higher in samples from women of infertile than those of fertile couples (P = 0.049). Differences according to cell type were also observed. In the ESP, the PAEP level was higher in the LE than that in the GE (P = 0.013). In the MSP, PAEP levels were higher in the LE than that in the STR (P = 0.011), but were not different from GE. In the LSP, levels of PAEP in the LE and GE were higher than those in the STR, though these differences did not reach statistical significance (P = 0.059 and P = 0.054, respectively). PAEP expression predominated in the LSP and displayed a minor, but significant, difference in expression associated with fertility during the implantation window.
There was cyclical variation of HOXA10 expression in the LE and STR according to three-way ANOVA. In the LE, post hoc analysis showed that levels in fertile controls were higher in the LSP than ESP or MSP (P < 0.05; Fig. 3D; Table I). In the STR, levels decreased from ESP to MSP in samples from women of infertile couples (P < 0.05). Although HOXA10 was higher in the STR during the ESP in fertile controls, this was not significant (P = 0.051). HOXA10 levels differed according to cell type in each phase. In the ESP, HOXA10 levels were higher in the STR than that in the GE (P < 0.001) and LE (P = 0.035). In the MSP, levels of HOXA10 in the LE did not differ from STR, while it was least abundant in the GE (P < 0.042). In the LSP, HOXA10 protein increased in the LE above the levels observed in both the GE and STR (P = 0.007 and P = 0.013, respectively), which were not significantly different from each other. The cyclical trends for HOXA10 expression were significant and distinguishable between women of infertile couples and fertile controls, with some marked variation in cell type-specific levels that shifted in prominence during the secretory phase from the STR to the LE.
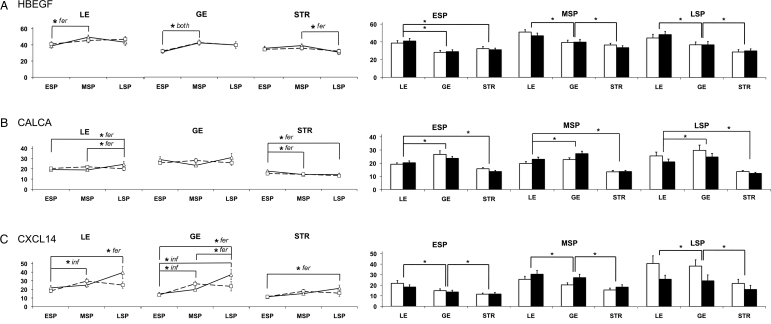
HBEGF displayed significant cyclical variations in all three cell types according to three-way ANOVA (Fig. 5A). As summarized in Table I, HBEGF levels in the LE rose by post hoc analysis between the ESP and MSP for fertile controls (P < 0.05), and in both groups in the GE (P < 0.05). In the STR, the pattern was slightly different; HBEGF levels remained constant during the ESP and MSP, but fell during the LSP in fertile controls (P < 0.05). HBEGF also differed by cell type and appears to be largely epithelial. In the ESP, HBEGF was most abundant in the LE (P< 0.001) while it was not different between GE and STR (P = 0.101). In both the MSP and LSP, HBEGF remained highest in the LE and lowest in the STR (P < 0.002). HBEGF appeared to be expressed primarily in epithelial cells during the secretory phase and did not increase in LE at MSP in women from infertile couples as it did in fertile controls.
Figure 5.
Quantitative IHC of HBEGF, CALCA and CXCL14 in endometrial biopsies of women from fertile and infertile couples. The mean gray levels (arbitrary units; y-axis) were determined for HBEGF (A; fertile, n = 86; infertile, n = 87), CALCA (B; fertile, n= 82; infertile, n = 83) and CXCL14 (C; fertile, n = 82; infertile, n = 83), and are displayed as in Fig. 3. Error bars indicate the SD.
Three-way ANOVA demonstrated that CALCA displayed cyclical variation in the LE and STR, summarized in Table I, but this trend was only significant for fertile controls (Fig. 5B). Post hoc analysis demonstrated that levels in the LE remained low in the ESP and MSP, rising during the LSP (P < 0.05). Levels of CALCA in the STR fell between the ESP and MSP and remained low through the LSP (P < 0.05). Its cell type-specific expression was highest in the GE and lowest in the STR in ESP, MSP and LSP (P < 0.001, P < 0.007 and P < 0.043, respectively). CALCA was predominantly glandular in localization and displayed cyclical trends only in fertile controls.
CXCL14 displayed cyclical changes in the LE, GE and STR (three-way ANOVA: P = 0.006, P < 0.001 and P = 0.015, respectively; Fig. 5C). In samples from women of infertile couples, post hoc tests indicated a rise in the LE and GE in the MSP compared with ESP (P < 0.05 for each), whereas fertile controls showed a rise in the LSP compared with the ESP (P < 0.05). In the STR, only fertile controls displayed a rise in CXCL14 between the ESP and LSP (P < 0.05). This shift in the cyclic expression pattern is summarized in Table I. CXCL14 expression appeared to be predominantly epithelial in all three cycle phases (LE versus STR, P < 0.001, P < 0.001 and P < 0.031, respectively). The cyclical expression of CXCL14 differed between groups, where it was up-regulated earlier in women from infertile couples compared with that in fertile women.
Correlations
Correlation analysis was used to examine the relationships between protein levels in each patient (Table II); that is, for a given patient, a positive correlation will exist where two different proteins are either both abundant or not abundant, whereas a negative correlation will exist where one protein is abundant but the other is not abundant. Correlations were calculated separately for samples from women of fertile and infertile couples, taking into account each cell type during each phase. There were more correlations in samples from women of fertile than infertile couples. In addition, for both groups of women there were correlations that were restricted to specific periods of the secretory phase. Interestingly, CALCA and HOXA10 were correlated with other proteins in all three phases for samples from fertile controls, as was the LIF for the infertile group. Importantly, tissues from fertile and infertile groups did not have any correlations in common.
Table II.
Correlation analysis for protein levels in endometrial biopsies of women from fertile and infertile couples.
| ESP | R2 | MSP | R2 | LSP | R2 | |
|---|---|---|---|---|---|---|
| Fertile | ||||||
| LE | CXCL14, CALCA | 0.40 | LIF, CALCA | 0.48 | CXCL14, CALCA | 0.67 |
| CLCL14, HOXA10 | 0.41 | HBEGF, HOXA10 | −0.33 | PGR-B, HOXA10 | 0.58 | |
| GE | PGR-B, CALCA | 0.40 | CXCL14, CALCA | 0.52 | PGR-B, HOXA10 | 0.62 |
| PAEP, HOXA10 | 0.63 | PAEP, HOXA10 | 0.43 | |||
| PAEP, HBEGF | −0.34 | |||||
| STR | CALCA HOXA10 | 0.61 | ||||
| Infertile | ||||||
| LE | LIF, PGR-B | 0.46 | LIF, HOXA10 | 0.35 | ||
| GE | HBEGF, CALCA | 0.49 | ||||
| STR | CXCL14, CALCA | −0.51 | LIF, PAEP | 0.34 | LIF, PAEP | –0.46 |
| LIF, HOXA10 | 0.38 | CXCL14, CALCA | 0.47 | |||
| LIF, PGR-B | 0.36 | |||||
| HOXA10, PGR-B | 0.38 | |||||
Trend analysis was performed with Pearson's correlation test for each protein separately by tissue feature, cycle phase and fertility status. Proteins that were significantly correlated are shown together with the corresponding Pearson's correlation coefficient. CXCL14; chemokine ligand 14; CALCA, calcitonin; LIF, leukemia inhibitory factor; HBEGF, heparin-binding EGF-like growth factor; HOXA10, homeobox A10; PGR-B, progesterone receptor-B; PAEP, progestagen-associated endometrial protein.
Discussion
High-throughput IHC analysis of a large, well-characterized population of biopsies from women of both fertile and infertile couples provided a comprehensive spatio-temporal view of the levels of proteins that multiple approaches suggest are important for nidation during the secretory phase. As previously reported (Coutifaris et al., 2004), the timed endometrial biopsy does not differentiate the MSP–LSP transition nor fertile from infertile women using morphological criteria previously described by Noyes et al. (1950). However, the ability to use endometrial specimens to determine these end-points by tissue type-specific protein profiling has not been rigorously examined. This study utilized a large sample of endometrial biopsies with individual examination of the LE, GE and STR. The findings confirm that PGR-B, LIF, PAEP, HOXA10, HBEGF, CALCA and CXCL14 are regulated at the protein level in endometrium during the secretory phase of the cycle, and that some proteins displayed an absolute difference, with respect to the fertility status of women, at a given point in time, while others differed in their cyclical levels, suggesting a disruption of the time course of the implantation interval, as depicted by the changes in protein levels at the onset and close of the MSP (Table I). While microarray studies detect the relative abundance of transcripts in endometrial biopsies, the IHC approach provided additional information about cell-specific protein accumulation within secretory phase tissue. Thus, it validates and extends our current understanding based on previous expression studies. For example, Talbi et al. (2006) found that PAEP transcripts are up-regulated during the transition from ESP to MSP, yet we did not find PAEP protein levels to be significantly up-regulated until the LSP, suggesting that mRNA levels are up-regulated well in advance of their translation. In contrast, transcript levels of PGR-B, LIF and HOXA10 (Talbi et al., 2006) correlated well with protein accumulations.
We initially analyzed the data using the two LH surge collection times to examine the developmental differences. Although most of the tissue-specific differences were apparent, the two collection times and fertility status were only distinguishable for a small number of the proteins. Both LH collection groups were heterogeneously dated by morphological criteria, including the reassignment of several in the Days 22–23 collection group to the ESP. No correlations were noted between the degree at which samples were out of phase and their fertility status (data not shown), in agreement with previous findings (Coutifaris et al., 2004). Reassignment of the data to specific cycle dates resulted in clearer distinctions among tissue type, developmental stage and fertility status.
We noted altered protein levels in the tissues from women of infertile versus fertile couples. PGR-B levels were high in women of infertile couples during the ESP, and were relatively low during the MSP. A daily plot of PGR-B levels (Fig. 4) suggests that this shift in the pattern may represent a delay in normal cyclical variation in PGR-B expression in the tissues from the infertile group (daily plots of the other proteins are provided for comparison in the Supplementary data, Figs S4–S9). While PGR-B declined from Day 14 until the implantation window (Day 19), samples from infertile couples expressed high levels of PGR-B that did not decline until Days 20–22, an indication of progesterone resistance observed in the endometria of women with endometriosis (Aghajanova et al., 2010; Fazleabas, 2010; Young and Lessey, 2010). It is important to note that our data do not take into account the expression of PGR-A, an inhibitor of PGR-B. Both PGR-A and PGR-B are expressed in GE during the proliferative phase but PGR-B remains elevated during the MSP as PGR-A, present in distinct subnuclear foci (Arnett-Mansfield et al., 2004), decreases (Mote et al., 1999, 2000). The presence of both isoforms in the GE during late proliferative/ESP has suggested a role in glycogenolysis and vacuolation; however, PGR-B may be most important for glycogen secretion during the MSP (Mote et al., 1999). Studies of endometrial cancer have demonstrated less PGR-A in the nucleus and fewer, but larger, PGR-B subnuclear foci than in non-cancerous cells (Arnett-Mansfield et al., 2004, Arnett-Mansfield et al., 2007), demonstrating altered localization of the receptors. PGR-B subnuclear foci are associated with areas of euchromatin and presumably gene transcription (Arnett-Mansfield et al., 2007). The present results constitute the only other evidence for PGR-B dysregulation associated with female infertility.
LIF expression showed a significant fertility effect in the STR during the secretory phase (3-way ANOVA, P = 0.044) but post hoc t-tests did not reveal significant differences between fertile and infertile groups. The lower levels in infertile patients may, however, point to a biologically significant difference. LIF protein was up-regulated during the MSP, predominantly in the LE, a key location to impact the implanting blastocyst. Others find an increase during the implantation window (Kimber, 2005; Dimitriadis et al., 2006; Dimitriadis et al., 2007) but in the GE (Kimber, 2005; Dimitriadis et al., 2006; Dimitriadis et al., 2007) and decidual STR tissue (Kimber, 2005). Recent experiments demonstrate that an LIF antagonist completely blocks implantation of mouse blastocysts (White et al., 2007) and LIF antibodies reduce the number of implantation sites in hamsters (Ding et al., 2008). Embryos fail to implant in LIF-knockout mice owing to a maternal effect (Kimber, 2005; Quinn et al., 2007); perhaps an endometrial defect, as LIF-null uteri display little decidualization (Kimber, 2005). Indeed, Song et al. (2000) found a second wave of LIF expression in the peri-implantation uterine STR that, when absent, correlated with failed expression of key decidualization factors. LIF also stimulates trophoblast formation, adhesion and migration in mice (Mezhevikina et al., 2006), as well as proliferation and migration of immortalized first trimester human cytotrophoblast cells (Horita et al., 2007). Although required for implantation in mice, the role of the LIF in humans is less certain. LIF concentrations in uterine secretions are higher in normal fertile controls than in women with idiopathic infertility (Laird et al., 1997; Mikolajczyk et al., 2007), but staining in endometrial biopsies reveals no difference in protein levels (Dimitriadis et al., 2007). However, endometriotic biopsies obtained during the implantation window reveal decreased LIF protein in GE of women from infertile couples (Dimitriadis et al., 2006) but similar amounts of LIF mRNA throughout the entire endometrium (Mikolajczyk et al., 2006). In examining a large number of biopsies, significant differences were reported in the present investigation in the STR tissue, where the LIF was reduced in women of infertile couples. Altered LIF expression is associated with recurrent miscarriage (Hambartsoumian, 1998), while others have found no difference between women with implantation failure and fertile women (Inagaki et al., 2003; Kimber, 2005). Interestingly, the decreased LIF secretion in idiopathic infertility is presumably attributed to uterine epithelial cells (Laird et al., 1997) but immunostaining results disagree with this finding (Dimitriadis et al., 2007).
Previous studies detected rising PAEP levels in the secretory phase endometrium, particularly after progesterone levels have peaked and decidualization has occurred (Rutanen et al., 1987; Bischof, 1989). A microarray study by Talbi et al. (2006) revealed that PAEP is the most highly up-regulated gene during the ESP to MSP transition. Recent studies have confirmed that PAEP mRNA increases in the ESP, while the protein accumulates significantly in GE during the LSP (Mylonas et al., 2006; Stavreus-Evers et al., 2006). We also find that PAEP immunoreactivity rose significantly during the LSP in both the LE and GE. Therefore, it appears that the PAEP gene is transcribed well ahead of mRNA translation. PAEP has profound inhibitory effects upon the cytotoxicity of natural killer cells (Okamoto et al., 1991), inhibits monocyte chemotaxis (Vigne et al., 2001) and induces apoptosis in activated T lymphocytes (Mukhopadhyay et al., 2001, 2004; Karande et al., 2005; Poornima and Karande, 2007). These findings support the hypothesis that PAEP suppresses some aspects of the endometrial immune response, contributing to an immune-tolerant environment for invading trophoblast cells. Indeed, there is a decrease in PAEP mRNA and protein during the implantation window in patients with endometriosis, implantation failure, idiopathic infertility, HELLP (hemolysis, elevated liver enzymes and low platelet count) and intrauterine growth restriction (Kao et al., 2003; Jeschke et al., 2005; Skrzypczak et al., 2005; Tapia et al., 2008). A recent study links low levels of PAEP mRNA to patients with documented implantation failure after undergoing IVF with donated oocytes (Tapia et al., 2008). In contrast, the present study demonstrated higher GE levels of PAEP protein in tissues from women of infertile couples during the MSP. Our contradiction of Skrzypczak et al. (2005), where lower levels of PAEP were detected in the uterine fluid of infertile women, suggests that a careful examination of PAEP regulation in various etiologies of infertility is warranted.
HOXA10 varied significantly during the secretory phase in the STR and LE. Its levels fell in the STR during the transition from ESP to MSP and rose in the LE during the MSP. This created a pattern in which HOXA10 became progressively more predominant in the LE than GE or STR. HOXA10 is an important developmental gene, and both mRNA and protein are found in the human endometrium most abundantly during the MSP (Taylor et al., 1998; Gui et al., 1999; Cermik et al., 2001; Li et al., 2002a) when progesterone peaks. It has been suggested that HOXA10 induction by progesterone during the implantation window blocks the STR cell cycle and promotes decidualization in preparation for blastocyst implantation (Qian et al., 2005). Although the cyclical up-regulation of HOXA10 during the MSP is observed in epithelial and stromal cells, the STR displays markedly less mRNA and protein in patients with endometriosis (Gui et al., 1999; Taylor et al., 1999; Li et al., 2002a; Kim et al., 2007). Our studies support these findings, as HOXA10 predominated in LE during the LSP in women from fertile couples.
HBEGF is expressed in the GE, vascular endothelium and on the apical surface of the LE during the implantation window in humans (Days 18–24; (Yoo et al., 1997; Leach et al., 1999)). The LIF is necessary for the expression of HBEGF in the LE of the mouse uterus surrounding a newly implanted blastocyst, as evaluated in LIF−/− mice (Song et al., 2000). In the human endometrium, HBEGF levels greatly decline by the LSP (Leach et al., 1999). Furthermore, its protein regulation mirrors its mRNA (Yoo et al., 1997; Lessey et al., 2002). The LSP decline observed in previous studies was not detected by our present analysis, which was based on a much larger sample size. HBEGF levels rose during the MSP in both LE and GE and remained high in the LSP. As a result of these increases, HBEGF was least prevalent in the STR during the latter half of the secretory phase. This variation of expression between cellular regions suggests that HBEGF functions during initial attachment and invasion of the embryo; however, the rise in prominence in GE during the MSP might also prove to be of functional importance. Other studies indicate that transient HBEGF elevation in the STR occurs during the late proliferative phase (Yoo et al., 1997; Leach et al., 1999). Importantly, HBEGF regulates the development of blastocysts and the motility of trophoblast cells in both mice and humans (Martin et al., 1998; Armant et al., 2000; Wang et al., 2000; Leach et al., 2004). In the mouse, HBEGF is induced in the uterine LE directly at the site of the apposing blastocyst, further suggesting a role in implantation (Das et al., 1994). Beads soaked with HBEGF mimic the blastocyst and induce HBEGF gene expression, elevated local vascular permeability and decidualization in the mouse endometrium (Paria et al., 2001).
CALCA gene expression has been demonstrated in the uteri of rats, humans and non-human primates (Ding et al., 1994; Kumar et al., 1998; Wang et al., 1998; Zhu et al., 1998a; Zhu et al., 1998b; Diao et al., 2002; Li et al., 2002b; Kumar et al., 2003) and its protein expression has been localized to the GE in humans and non-human primates during their respective windows of implantation (Kumar et al., 1998; Diao et al., 2002; Kumar et al., 2003). Notably, the glandular localization and detection of CALCA protein in rat uterine flushings just prior to implantation suggest that it acts in a paracrine fashion to promote blastocyst implantation (Zhu et al., 1998b). Our results corroborate some, but not all, previous studies, as we demonstrate a predominance of CALCA in GE cells throughout the entire secretory phase. However, the previously reported rise in CALCA during the window of implantation (Kumar et al., 1998) was absent in the present survey using a large sample size. Previous investigations found that injection of CALCA antisense oligonucleotides into the uterine horns of rats severely impairs blastocyst implantation without affecting the number of viable embryos, although it is unclear whether CALCA was required for endometrial or blastocyst function (Zhu et al., 1998a). CALCA up-regulation and secretion by the MSP endometrium is perhaps in response to increasing progesterone levels. In surveying a large number of individuals, we now show a significant drop in STR levels of CALCA between the ESP and MSP.
CXCL14 inhibits mouse trophoblast attachment and outgrowth (Kuang et al., 2009a) and suppresses human trophoblast invasion by inhibiting matrix metalloproteinase (MMP)2 and MMP9 (Kuang et al., 2009b). The proposed receptor for this cytokine (CCR1) is found in extravillous trophoblast cells (Sato et al., 2003; Hannan et al., 2006), MSP endometrium and first trimester placental tissue (Hannan et al., 2006). Microarray studies of the human endometrial transcriptome place maximal CXCL14 expression during the MSP (Talbi et al., 2006). IHC analysis demonstrates highest levels of CXCL14 in fertile controls during the LSP, when uterine receptivity for implantation declines (Wilcox et al., 1999). Furthermore, we observe that CXCL14 protein levels rise during the MSP in the LE and GE of women from infertile couples, suggesting a role in premature closure of the receptive phase. CXCL14 transcript levels appear to be up-regulated well ahead of the protein, similarly to the expression patterns for PAEP. Hence, CXCL14 likely plays a key regulatory role late during implantation, as decidualization and trophoblast invasion progress.
In biopsies from fertile women, the transition from the ESP to MSP is characterized by increased HBEGF and LIF in LE and GE, and decreased CALCA in the STR (Table I). Furthermore, the transition from the MSP to the LSP, or closure of the 4-day receptive interval of implantation, is reflected by increased HOXA10, CALCA and CXCL14 in the LE, increased CXCL14 in the GE and decreased HBEGF and HOXA10 in the STR. In contrast, the MSP in infertile women underwent a significant reduction in PGR-B in all regions and increased CXCL14 in LE and GE. The LSP showed no differences in the absolute level or patterns of protein accumulation according to the fertility status. Coordinated regulation of proteins may be indicated by correlation analysis (Table II). Notably, there was coordinated regulation of several proteins during the MSP in the LE and GE but no correlations were observed in the STR. The MSP correlations were absent in the infertile group; however, several correlations were found in the STR that did not occur in the fertile group. These differences suggest extensive dysregulation of protein expression in infertile women, perhaps owing to altered levels of transcription factors, such as PGR-B or HOXA10.
This study demonstrates that, by localizing and quantifying key proteins in endometrial biopsies during the secretory phase of the menstrual cycle, it is possible to begin to delineate the implantation interval and differentiate between fertile and infertile women. These objectives cannot be met using the histological criteria of Noyes et al. (1950). However, our analysis of these clinical specimens has several limitations. The study was designed to include a population representative of individuals seeking infertility treatment; however, the basis of the diagnosis of infertility was unknown (Coutifaris et al., 2004). Our results were also limited to an analysis of the secretory phase of the menstrual cycle. By using a large, well-characterized sample population, we have avoided the limitations posed by smaller IHC studies. In addition, the significantly altered expression of PGR-B in women from infertile couples validates its further study in various etiologies of infertility. More subtle differences were noted between tissues of women from fertile and infertile couples in the cyclical regulation of other proteins, particularly PAEP and CXCL14, that increased prematurely in the GE of infertile patients and HOXA10 that failed to increase in the LE at LSP. Correlation analysis of the seven proteins studied identified relationships in their expression and developmental regulation in the controls, which were not maintained in tissues from women of infertile couples. Further investigation of these relationships could shed new light on the molecular control of normal and pathological development in endometrial tissues. Our study confirms the importance of evaluating endometrial proteins in a cell type-specific manner. Regulation specific to the GE, LE or STR is obscured when whole tissue homogenates are examined. We demonstrated that expression in the GE and LE clearly differs for several proteins, suggesting that they are differentially regulated in those compartments. Finally, this investigation points out the importance of obtaining data for both mRNA and protein, as they are not necessarily regulated in an identical manner and the temporal patterns of protein levels can provide important insights into the function of specific gene products.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors' roles
All named authors have reviewed the International Committee of Medical Journal Editors' guidelines regarding ‘Authorship and Contributorship’ and all of them comply with required elements of the three criteria: (1). (a) substantial contributions to conception and design, (b) acquisition of data or (c) analysis and interpretation of data; (2). (a) drafting the article or (b) revising the article critically for important intellectual content; (3) final approval of the submitted manuscript. Their specific contributions against these criteria are detailed below: R.E.L.: 1a, c, 2a, b, 3; P.J.: 1b, c, 2a, b, 3; C.C.: 1a, b, c, 2b, 3; M.K.: 1b, 2b, 3; E.R.M.: 1b, 2b, 3; R.A.-F.: 1b, 2b, 3; S.A.C.: 1b, 2b, 3; R.S.L.: 1b, 2b, 3; W.D.S: 1b, 2b, 3; B.R.C.: 1a, b, c, 2b, 3; M.P.S.: 1b, 2b, 3; S.S.: 1a,c, 2b, 3; P.C.L.: 1a,c, 2b, 3; L.G.: 1a, c, 2b, 3; M.P.D.: 1a, b, c, 2a, b, 3; D.R.A.: 1a, c, 2a, b, 3.
Funding
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), NIH through Cooperative Agreement (U54HD40093) as part of the Specialized Cooperative Center Program in Reproduction and Infertility Research to R.E.L., grants to R.E.L. (HD040093) and D.R.A. (HD045966), grants from the NICHD National Cooperative Reproductive Medicine Network to C.C. (HD27049), E.R.M. (HD38997), M.P.D. (HD39005), S.A.C. (HD27011), M.P.S. (HD33172), B.R.C. (HD38988), R.S.L. (HD38992) and W.D.S. (HD38998) and the Intramural Research Program of the NICHD, NIH, DHHS (D.R.A.).
Conflict of interest
None declared.
Supplementary Material
Acknowledgements
We would like to thank Brian Kilburn, Po Jen Chiang, Mike Helmreich, S. Papudesi, Dr Laura Detti and Dr Terri Woodard at Wayne State University School of Medicine for their technical assistance.
References
- Aghajanova L, Velarde MC, Giudice LC. Altered gene expression profiling in endometrium: evidence for progesterone resistance. Semin Reprod Med. 2010;28:51–58. doi: 10.1055/s-0029-1242994. [DOI] [PubMed] [Google Scholar]
- Arimoto T, Katagiri T, Oda K, Tsunoda T, Yasugi T, Osuga Y, Yoshikawa H, Nishii O, Yano T, Taketani Y, et al. Genome-wide cDNA microarray analysis of gene-expression profiles involved in ovarian endometriosis. Int J Oncol. 2003;22:551–560. [PubMed] [Google Scholar]
- Armant DR, Wang J, Liu Z. Intracellular signaling in the developing blastocyst as a consequence of the maternal-embryonic dialogue. Semin Reprod Med. 2000;18:273–287. doi: 10.1055/s-2000-12565. [DOI] [PubMed] [Google Scholar]
- Armant DR, Kilburn BA, Petkova A, Edwin SS, Duniec-Dmuchowski ZM, Edwards HJ, Romero R, Leach RE. Human trophoblast survival at low oxygen concentrations requires metalloproteinase-mediated shedding of heparin-binding EGF-like growth factor. Development. 2006;133:751–759. doi: 10.1242/dev.02237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett-Mansfield RL, DeFazio A, Mote PA, Clarke CL. Subnuclear distribution of progesterone receptors A and B in normal and malignant endometrium. J Clin Endocrinol Metab. 2004;89:1429–1442. doi: 10.1210/jc.2003-031111. [DOI] [PubMed] [Google Scholar]
- Arnett-Mansfield RL, Graham JD, Hanson AR, Mote PA, Gompel A, Scurr LL, Gava N, de Fazio A, Clarke CL. Focal subnuclear distribution of progesterone receptor is ligand dependent and associated with transcriptional activity. Mol Endocrinol. 2007;21:14–29. doi: 10.1210/me.2006-0041. [DOI] [PubMed] [Google Scholar]
- Benson GV, Lim H, Paria BC, Satokata I, Dey SK, Maas RL. Mechanisms of reduced fertility in Hoxa-10 mutant mice: uterine homeosis and loss of maternal Hoxa-10 expression. Development. 1996;122:2687–2696. doi: 10.1242/dev.122.9.2687. [DOI] [PubMed] [Google Scholar]
- Bischof P. Three pregnancy proteins (PP12, PP14, and PAPP-A): their biological and clinical relevance. Am J Perinatol. 1989;6:110–116. doi: 10.1055/s-2007-999559. [DOI] [PubMed] [Google Scholar]
- Cermik D, Karaca M, Taylor HS. HOXA10 expression is repressed by progesterone in the myometrium: differential tissue-specific regulation of HOX gene expression in the reproductive tract. J Clin Endocrinol Metab. 2001;86:3387–3392. doi: 10.1210/jcem.86.7.7675. [DOI] [PubMed] [Google Scholar]
- Coutifaris C, Myers ER, Guzick DS, Diamond MP, Carson SA, Legro RS, McGovern PG, Schlaff WD, Carr BR, Steinkampf MP, et al. Histological dating of timed endometrial biopsy tissue is not related to fertility status. Fertil Steril. 2004;82:1264–1272. doi: 10.1016/j.fertnstert.2004.03.069. [DOI] [PubMed] [Google Scholar]
- Daftary GS, Kayisli U, Seli E, Bukulmez O, Arici A, Taylor HS. Salpingectomy increases peri-implantation endometrial HOXA10 expression in women with hydrosalpinx. Fertil Steril. 2007;87:367–372. doi: 10.1016/j.fertnstert.2006.06.041. [DOI] [PubMed] [Google Scholar]
- Das SK, Wang XN, Paria BC, Damm D, Abraham JA, Klagsbrun M, Andrews GK, Dey SK. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development. 1994;120:1071–1083. doi: 10.1242/dev.120.5.1071. [DOI] [PubMed] [Google Scholar]
- Diao HL, Li SJ, Wang HB, Yang ZM. Calcitonin immunostaining in monkey uterus during menstrual cycle and early pregnancy. Endocrine. 2002;18:75–78. doi: 10.1385/ENDO:18:1:75. [DOI] [PubMed] [Google Scholar]
- Dimitriadis E, Stoikos C, Stafford-Bell M, Clark I, Paiva P, Kovacs G, Salamonsen LA. Interleukin-11, IL-11 receptoralpha and leukemia inhibitory factor are dysregulated in endometrium of infertile women with endometriosis during the implantation window. J Reprod Immunol. 2006;69:53–64. doi: 10.1016/j.jri.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Dimitriadis E, Sharkey AM, Tan YL, Salamonsen LA, Sherwin JR. Immunolocalisation of phosphorylated STAT3, interleukin 11 and leukaemia inhibitory factor in endometrium of women with unexplained infertility during the implantation window. Reprod Biol Endocrinol. 2007;5:44. doi: 10.1186/1477-7827-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding YQ, Zhu LJ, Bagchi MK, Bagchi IC. Progesterone stimulates calcitonin gene expression in the uterus during implantation. Endocrinology. 1994;135:2265–2274. doi: 10.1210/endo.135.5.7956949. [DOI] [PubMed] [Google Scholar]
- Ding T, Song H, Wang X, Khatua A, Paria BC. Leukemia inhibitory factor ligand-receptor signaling is important for uterine receptivity and implantation in golden hamsters (Mesocricetus auratus) Reproduction. 2008;135:41–53. doi: 10.1530/REP-07-0013. [DOI] [PubMed] [Google Scholar]
- Dominguez F, Avila S, Cervero A, Martin J, Pellicer A, Castrillo JL, Simon C. A combined approach for gene discovery identifies insulin-like growth factor-binding protein-related protein 1 as a new gene implicated in human endometrial receptivity. J Clin Endocrinol Metab. 2003;88:1849–1857. doi: 10.1210/jc.2002-020724. [DOI] [PubMed] [Google Scholar]
- Dominguez F, Garrido-Gomez T, Lopez JA, Camafeita E, Quinonero A, Pellicer A, Simon C. Proteomic analysis of the human receptive versus non-receptive endometrium using differential in-gel electrophoresis and MALDI-MS unveils stathmin 1 and annexin A2 as differentially regulated. Hum Reprod. 2009;24:2607–2617. doi: 10.1093/humrep/dep230. [DOI] [PubMed] [Google Scholar]
- Eyster KM, Boles AL, Brannian JD, Hansen KA. DNA microarray analysis of gene expression markers of endometriosis. Fertil Steril. 2002;77:38–42. doi: 10.1016/s0015-0282(01)02955-7. [DOI] [PubMed] [Google Scholar]
- Fazleabas AT. Progesterone resistance in a baboon model of endometriosis. Semin Reprod Med. 2010;28:75–80. doi: 10.1055/s-0029-1242997. [DOI] [PubMed] [Google Scholar]
- Franco HL, Jeong JW, Tsai SY, Lydon JP, Demayo FJ. In vivo analysis of progesterone receptor action in the uterus during embryo implantation. Sem Cell Dev Biol. 2008;19:178–186. doi: 10.1016/j.semcdb.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Gui Y, Zhang J, Yuan L, Lessey BA. Regulation of HOXA-10 and its expression in normal and abnormal endometrium. Mol Hum Reprod. 1999;5:866–873. doi: 10.1093/molehr/5.9.866. [DOI] [PubMed] [Google Scholar]
- Hambartsoumian E. Endometrial leukemia inhibitory factor (LIF) as a possible cause of unexplained infertility and multiple failures of implantation. Am J Reprod Immunol. 1998;39:137–143. doi: 10.1111/j.1600-0897.1998.tb00345.x. [DOI] [PubMed] [Google Scholar]
- Hannan NJ, Jones RL, White CA, Salamonsen LA. The chemokines, CX3CL1, CCL14, and CCL4, promote human trophoblast migration at the feto-maternal interface. Biol Reprod. 2006;74:896–904. doi: 10.1095/biolreprod.105.045518. [DOI] [PubMed] [Google Scholar]
- Hannan NJ, Stephens AN, Rainczuk A, Hincks C, Rombauts LJ, Salamonsen LA. 2D-DiGE analysis of the human endometrial secretome reveals differences between receptive and nonreceptive states in fertile and infertile women. J Proteome Res. 2010;9:6256–6264. doi: 10.1021/pr1004828. [DOI] [PubMed] [Google Scholar]
- Henriquez S, Tapia A, Quezada M, Vargas M, Cardenas H, Rios M, Salvatierra AM, Croxatto H, Orihuela P, Zegers-Hochschild F, et al. Deficient expression of monoamine oxidase A in the endometrium is associated with implantation failure in women participating as recipients in oocyte donation. Mol Hum Reprod. 2006;12:749–754. doi: 10.1093/molehr/gal082. [DOI] [PubMed] [Google Scholar]
- Hever A, Roth RB, Hevezi PA, Lee J, Willhite D, White EC, Marin EM, Herrera R, Acosta HM, Acosta AJ, et al. Molecular characterization of human adenomyosis. Mol Hum Reprod. 2006;12:737–748. doi: 10.1093/molehr/gal076. [DOI] [PubMed] [Google Scholar]
- Horcajadas JA, Riesewijk A, Polman J, van Os R, Pellicer A, Mosselman S, Simon C. Effect of controlled ovarian hyperstimulation in IVF on endometrial gene expression profiles. Mol Hum Reprod. 2005;11:195–205. doi: 10.1093/molehr/gah150. [DOI] [PubMed] [Google Scholar]
- Horita H, Kuroda E, Hachisuga T, Kashimura M, Yamashita U. Induction of prostaglandin E2 production by leukemia inhibitory factor promotes migration of first trimester extravillous trophoblast cell line, HTR-8/SVneo. Hum Reprod. 2007;22:1801–1809. doi: 10.1093/humrep/dem125. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Stern C, McBain J, Lopata A, Kornman L, Wilkinson D. Analysis of intra-uterine cytokine concentration and matrix-metalloproteinase activity in women with recurrent failed embryo transfer. Hum Reprod. 2003;18:608–615. doi: 10.1093/humrep/deg139. [DOI] [PubMed] [Google Scholar]
- Jeschke U, Kunert-Keil C, Mylonas I, Hammer A, Schiessl B, Lomba I, Kuhn C, Schulze S, Friese K. Expression of glycodelin A in decidual tissue of preeclamptic, HELLP and intrauterine growth-restricted pregnancies. Virchows Arch. 2005;446:360–368. doi: 10.1007/s00428-004-1201-3. [DOI] [PubMed] [Google Scholar]
- Jessmon P, Kilburn BA, Romero R, Leach RE, Armant DR. Function-specific intracellular signaling pathways downstream of heparin-binding eGF-like growth factor utilized by human trophoblasts. Biol Reprod. 2010;82:921–929. doi: 10.1095/biolreprod.109.082305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, Osteen K, Lessey BA, Giudice LC. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144:2870–2881. doi: 10.1210/en.2003-0043. [DOI] [PubMed] [Google Scholar]
- Karande AA, Mukhopadhyay D, Jayachandran R, Sundarraj S, Alok A. Mechanism of the immunomodulatory activity of glycodelin. Indian J Physiol Pharmacol. 2005;49:271–283. [PubMed] [Google Scholar]
- Kilburn BA, Wang J, Duniec-Dmuchowski ZM, Leach RE, Romero R, Armant DR. Extracellular matrix composition and hypoxia regulate the expression of HLA-G and integrins in a human trophoblast cell line. Biol Reprod. 2000;62:739–747. doi: 10.1095/biolreprod62.3.739. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Taylor HS, Lu Z, Ladhani O, Hastings JM, Jackson KS, Wu Y, Guo SW, Fazleabas AT. Altered expression of HOXA10 in endometriosis: potential role in decidualization. Mol Hum Reprod. 2007;13:323–332. doi: 10.1093/molehr/gam005. [DOI] [PubMed] [Google Scholar]
- Kimber SJ. Leukaemia inhibitory factor in implantation and uterine biology. Reproduction. 2005;130:131–145. doi: 10.1530/rep.1.00304. [DOI] [PubMed] [Google Scholar]
- Kuang H, Chen Q, Fan X, Zhang Y, Zhang L, Peng H, Cao Y, Duan E. CXCL14 inhibits trophoblast outgrowth via a paracrine/autocrine manner during early pregnancy in mice. J Cell Physiol. 2009a;221:448–457. doi: 10.1002/jcp.21877. [DOI] [PubMed] [Google Scholar]
- Kuang H, Chen Q, Zhang Y, Zhang L, Peng H, Ning L, Cao Y, Duan E. The cytokine gene CXCL14 restricts human trophoblast cell invasion by suppressing gelatinase activity. Endocrinology. 2009b;150:5596–5605. doi: 10.1210/en.2009-0570. [DOI] [PubMed] [Google Scholar]
- Kumar S, Zhu LJ, Polihronis M, Cameron ST, Baird DT, Schatz F, Dua A, Ying YK, Bagchi MK, Bagchi IC. Progesterone induces calcitonin gene expression in human endometrium within the putative window of implantation. J Clin Endocrinol Metab. 1998;83:4443–4450. doi: 10.1210/jcem.83.12.5328. [DOI] [PubMed] [Google Scholar]
- Kumar S, Brudney A, Cheon YP, Fazleabas AT, Bagchi IC. Progesterone induces calcitonin expression in the baboon endometrium within the window of uterine receptivity. Biol Reprod. 2003;68:1318–1323. doi: 10.1095/biolreprod.102.007708. [DOI] [PubMed] [Google Scholar]
- Lai TH, King JA, Shih Ie M, Vlahos NF, Zhao Y. Immunological localization of syndecan-1 in human endometrium throughout the menstrual cycle. Fertil Steril. 2007;87:121–126. doi: 10.1016/j.fertnstert.2006.06.042. [DOI] [PubMed] [Google Scholar]
- Laird SM, Tuckerman EM, Dalton CF, Dunphy BC, Li TC, Zhang X. The production of leukaemia inhibitory factor by human endometrium: presence in uterine flushings and production by cells in culture. Hum Reprod. 1997;12:569–574. doi: 10.1093/humrep/12.3.569. [DOI] [PubMed] [Google Scholar]
- Leach RE, Khalifa R, Ramirez ND, Das SK, Wang J, Dey SK, Romero R, Armant DR. Multiple roles for heparin-binding epidermal growth factor-like growth factor are suggested by its cell-specific expression during the human endometrial cycle and early placentation. J Clin Endocrinol Metab. 1999;84:3355–3363. doi: 10.1210/jcem.84.9.5980. [DOI] [PubMed] [Google Scholar]
- Leach RE, Romero R, Kim YM, Chaiworapongsa T, Kilburn B, Das SK, Dey SK, Johnson A, Qureshi F, Jacques S, et al. Pre-eclampsia and expression of heparin-binding EGF-like growth factor. Lancet. 2002;360:1215–1219. doi: 10.1016/S0140-6736(02)11283-9. [DOI] [PubMed] [Google Scholar]
- Leach RE, Kilburn B, Wang J, Liu Z, Romero R, Armant DR. Heparin-binding EGF-like growth factor regulates human extravillous cytotrophoblast development during conversion to the invasive phenotype. Dev Biol. 2004;266:223–237. doi: 10.1016/j.ydbio.2003.09.026. [DOI] [PubMed] [Google Scholar]
- Leach RE, Kilburn BA, Petkova A, Romero R, Armant DR. Diminished survival of human cytotrophoblast cells exposed to hypoxia/reoxygenation injury and associated reduction of heparin-binding epidermal growth factor-like growth factor. Am J Obstet Gynecol. 2008;198 doi: 10.1016/j.ajog.2008.01.009. 471 e471–477 discussion 471 e477–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessey BA, Damjanovich L, Coutifaris C, Castelbaum A, Albelda SM, Buck CA. Integrin adhesion molecules in the human endometrium. Correlation with the normal and abnormal menstrual cycle. J Clin Invest. 1992;90:188–195. doi: 10.1172/JCI115835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessey BA, Gui Y, Apparao KB, Young SL, Mulholland J. Regulated expression of heparin-binding EGF-like growth factor (HB-EGF) in the human endometrium: a potential paracrine role during implantation. Mol Reprod Dev. 2002;62:446–455. doi: 10.1002/mrd.10129. [DOI] [PubMed] [Google Scholar]
- Li H, Chen S, Xing F. Expression of HOXA10 gene in human endometrium and its relationship with unexplained infertility. Zhonghua Fu Chan Ke Za Zhi. 2002a;37:30–32. [PubMed] [Google Scholar]
- Li Q, Wang J, Armant DR, Bagchi MK, Bagchi IC. Calcitonin down-regulates E-cadherin expression in rodent uterine epithelium during implantation. J Biol Chem. 2002b;277:46447–46455. doi: 10.1074/jbc.M203555200. [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O'Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- Martel D, Psychoyos A. Estrogen receptors in the nidatory sites of the rat endometrium. Science. 1981;211:1454–1455. doi: 10.1126/science.7466405. [DOI] [PubMed] [Google Scholar]
- Martel D, Frydman R, Glissant M, Maggioni C, Roche D, Psychoyos A. Scanning electron microscopy of postovulatory human endometrium in spontaneous cycles and cycles stimulated by hormone treatment. J Endocrinol. 1987;114:319–324. doi: 10.1677/joe.0.1140319. [DOI] [PubMed] [Google Scholar]
- Martin KL, Barlow DH, Sargent IL. Heparin-binding epidermal growth factor significantly improves human blastocyst development and hatching in serum-free medium. Hum Reprod. 1998;13:1645–1652. doi: 10.1093/humrep/13.6.1645. [DOI] [PubMed] [Google Scholar]
- Matsuzaki S, Canis M, Vaurs-Barriere C, Pouly JL, Boespflug-Tanguy O, Penault-Llorca F, Dechelotte P, Dastugue B, Okamura K, Mage G. DNA microarray analysis of gene expression profiles in deep endometriosis using laser capture microdissection. Mol Hum Reprod. 2004;10:719–728. doi: 10.1093/molehr/gah097. [DOI] [PubMed] [Google Scholar]
- Matsuzaki S, Canis M, Pouly JL, Dechelotte P, Okamura K, Mage G. The macrophage stimulating protein/RON system: a potential novel target for prevention and treatment of endometriosis. Mol Hum Reprod. 2005;11:345–349. doi: 10.1093/molehr/gah162. [DOI] [PubMed] [Google Scholar]
- Matsuzaki S, Canis M, Pouly JL, Botchorishvili R, Dechelotte PJ, Mage G. Differential expression of genes in eutopic and ectopic endometrium from patients with ovarian endometriosis. Fertil Steril. 2006;86:548–553. doi: 10.1016/j.fertnstert.2006.02.093. [DOI] [PubMed] [Google Scholar]
- Mezhevikina LM, Kapralova IV, Fesenko EE. Stimulating effect of recombinant cytokine LIF on the mouse blastocysts during implantation. Biomed Khim. 2006;52:620–626. [PubMed] [Google Scholar]
- Mikolajczyk M, Wirstlein P, Skrzypczak J. Leukaemia inhibitory factor and interleukin 11 levels in uterine flushings of infertile patients with endometriosis. Hum Reprod. 2006;21:3054–3058. doi: 10.1093/humrep/del225. [DOI] [PubMed] [Google Scholar]
- Mikolajczyk M, Wirstlein P, Skrzypczak J. The impact of leukemia inhibitory factor in uterine flushing on the reproductive potential of infertile women—a prospective study. Am J Reprod Immunol. 2007;58:65–74. doi: 10.1111/j.1600-0897.2007.00492.x. [DOI] [PubMed] [Google Scholar]
- Mirkin S, Nikas G, Hsiu JG, Diaz J, Oehninger S. Gene expression profiles and structural/functional features of the peri-implantation endometrium in natural and gonadotropin-stimulated cycles. J Clin Endocrinol Metab. 2004;89:5742–5752. doi: 10.1210/jc.2004-0605. [DOI] [PubMed] [Google Scholar]
- Mirkin S, Arslan M, Churikov D, Corica A, Diaz JI, Williams S, Bocca S, Oehninger S. In search of candidate genes critically expressed in the human endometrium during the window of implantation. Hum Reprod. 2005;20:2104–2117. doi: 10.1093/humrep/dei051. [DOI] [PubMed] [Google Scholar]
- Mote PA, Balleine RL, McGowan EM, Clarke CL. Colocalization of progesterone receptors A and B by dual immunofluorescent histochemistry in human endometrium during the menstrual cycle. J Clin Endocrinol Metab. 1999;84:2963–2971. doi: 10.1210/jcem.84.8.5928. [DOI] [PubMed] [Google Scholar]
- Mote PA, Balleine RL, McGowan EM, Clarke CL. Heterogeneity of progesterone receptors A and B expression in human endometrial glands and stroma. Hum Reprod. 2000;15(Suppl. 3):48–56. doi: 10.1093/humrep/15.suppl_3.48. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay D, Sundereshan S, Rao C, Karande AA. Placental protein 14 induces apoptosis in T cells but not in monocytes. J Biol Chem. 2001;276:28268–28273. doi: 10.1074/jbc.M010487200. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay D, SundarRaj S, Alok A, Karande AA. Glycodelin A, not glycodelin S, is apoptotically active. Relevance of sialic acid modification. J Biol Chem. 2004;279:8577–8584. doi: 10.1074/jbc.M306673200. [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289:1751–1754. doi: 10.1126/science.289.5485.1751. [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci USA. 2003;100:9744–9749. doi: 10.1073/pnas.1732707100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylonas I, Jeschke U, Kunert-Keil C, Shabani N, Dian D, Bauerfeind I, Kuhn C, Kupka MS, Friese K. Glycodelin A is expressed differentially in normal human endometrial tissue throughout the menstrual cycle as assessed by immunohistochemistry and in situ hybridization. Fertil Steril. 2006;86:1488–1497. doi: 10.1016/j.fertnstert.2006.03.062. [DOI] [PubMed] [Google Scholar]
- Nikas G, Drakakis P, Loutradis D, Mara-Skoufari C, Koumantakis E, Michalas S, Psychoyos A. Uterine pinopodes as markers of the ‘nidation window’ in cycling women receiving exogenous oestradiol and progesterone. Hum Reprod. 1995;10:1208–1213. doi: 10.1093/oxfordjournals.humrep.a136120. [DOI] [PubMed] [Google Scholar]
- Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3–17. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- Okamoto N, Uchida A, Takakura K, Kariya Y, Kanzaki H, Riittinen L, Koistinen R, Seppala M, Mori T. Suppression by human placental protein 14 of natural killer cell activity. Am J Reprod Immunol. 1991;26:137–142. doi: 10.1111/j.1600-0897.1991.tb00713.x. [DOI] [PubMed] [Google Scholar]
- Paria BC, Ma W, Tan J, Raja S, Das SK, Dey SK, Hogan BL. Cellular and molecular responses of the uterus to embryo implantation can be elicited by locally applied growth factors. Proc Natl Acad Sci USA. 2001;98:1047–1052. doi: 10.1073/pnas.98.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poornima BL, Karande AA. Differential sialylation regulates the apoptotic activity of glycodelin A. FEBS Lett. 2007;581:4366–4370. doi: 10.1016/j.febslet.2007.07.078. [DOI] [PubMed] [Google Scholar]
- Punyadeera C, Dassen H, Klomp J, Dunselman G, Kamps R, Dijcks F, Ederveen A, de Goeij A, Groothuis P. Oestrogen-modulated gene expression in the human endometrium. Cell Mol Life Sci. 2005;62:239–250. doi: 10.1007/s00018-004-4435-y. [DOI] [PubMed] [Google Scholar]
- Qian K, Chen H, Wei Y, Hu J, Zhu G. Differentiation of endometrial stromal cells in vitro: down-regulation of suppression of the cell cycle inhibitor p57 by HOXA10? Mol Hum Reprod. 2005;11:245–251. doi: 10.1093/molehr/gah147. [DOI] [PubMed] [Google Scholar]
- Quinn CE, Detmar J, Casper RF. Pinopodes are present in Lif null and Hoxa10 null mice. Fertil Steril. 2007;88:1021–1028. doi: 10.1016/j.fertnstert.2006.11.157. [DOI] [PubMed] [Google Scholar]
- Riesewijk A, Martin J, van Os R, Horcajadas JA, Polman J, Pellicer A, Mosselman S, Simon C. Gene expression profiling of human endometrial receptivity on days LH+2 versus LH+7 by microarray technology. Mol Hum Reprod. 2003;9:253–264. doi: 10.1093/molehr/gag037. [DOI] [PubMed] [Google Scholar]
- Rutanen EM, Koistinen R, Seppala M, Julkunen M, Suikkari AM, Huhtala ML. Progesterone-associated proteins PP12 and PP14 in the human endometrium. J Steroid Biochem. 1987;27:25–31. doi: 10.1016/0022-4731(87)90290-1. [DOI] [PubMed] [Google Scholar]
- Sato Y, Higuchi T, Yoshioka S, Tatsumi K, Fujiwara H, Fujii S. Trophoblasts acquire a chemokine receptor, CCR1, as they differentiate towards invasive phenotype. Development. 2003;130:5519–5532. doi: 10.1242/dev.00729. [DOI] [PubMed] [Google Scholar]
- Scotchie JG, Fritz MA, Mocanu M, Lessey BA, Young SL. Proteomic analysis of the luteal endometrial secretome. Reprod Sci. 2009;16:883–893. doi: 10.1177/1933719109337165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon C, Oberye J, Bellver J, Vidal C, Bosch E, Horcajadas JA, Murphy C, Adams S, Riesewijk A, Mannaerts B, et al. Similar endometrial development in oocyte donors treated with either high- or standard-dose GnRH antagonist compared to treatment with a GnRH agonist or in natural cycles. Hum Reprod. 2005;20:3318–3327. doi: 10.1093/humrep/dei243. [DOI] [PubMed] [Google Scholar]
- Skrzypczak J, Wirstlein P, Mikolajczyk M. Is glycodelin an important marker of endometrial receptivity? Ginekol Pol. 2005;76:770–781. [PubMed] [Google Scholar]
- Song H, Lim H, Das SK, Paria BC, Dey SK. Dysregulation of EGF family of growth factors and COX-2 in the uterus during the preattachment and attachment reactions of the blastocyst with the luminal epithelium correlates with implantation failure in LIF-deficient mice. Mol Endocrinol. 2000;14:1147–1161. doi: 10.1210/mend.14.8.0498. [DOI] [PubMed] [Google Scholar]
- Stavreus-Evers A, Mandelin E, Koistinen R, Aghajanova L, Hovatta O, Seppala M. Glycodelin is present in pinopodes of receptive-phase human endometrium and is associated with down-regulation of progesterone receptor B. Fertil Steril. 2006;85:1803–1811. doi: 10.1016/j.fertnstert.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, Abbondanzo SJ. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA, et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology. 2006;147:1097–1121. doi: 10.1210/en.2005-1076. [DOI] [PubMed] [Google Scholar]
- Tapia A, Gangi LM, Zegers-Hochschild F, Balmaceda J, Pommer R, Trejo L, Pacheco IM, Salvatierra AM, Henriquez S, Quezada M, et al. Differences in the endometrial transcript profile during the receptive period between women who were refractory to implantation and those who achieved pregnancy. Hum Reprod. 2008;23:340–351. doi: 10.1093/humrep/dem319. [DOI] [PubMed] [Google Scholar]
- Taylor HS, Arici A, Olive D, Igarashi P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J Clin Invest. 1998;101:1379–1384. doi: 10.1172/JCI1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HS, Bagot C, Kardana A, Olive D, Arici A. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod. 1999;14:1328–1331. doi: 10.1093/humrep/14.5.1328. [DOI] [PubMed] [Google Scholar]
- Vigne JL, Hornung D, Mueller MD, Taylor RN. Purification and characterization of an immunomodulatory endometrial protein, glycodelin. J Biol Chem. 2001;276:17101–17105. doi: 10.1074/jbc.M010451200. [DOI] [PubMed] [Google Scholar]
- Wang J, Rout UK, Bagchi IC, Armant DR. Expression of calcitonin receptors in mouse preimplantation embryos and their function in the regulation of blastocyst differentiation by calcitonin. Development. 1998;125:4293–4302. doi: 10.1242/dev.125.21.4293. [DOI] [PubMed] [Google Scholar]
- Wang J, Mayernik L, Schultz JF, Armant DR. Acceleration of trophoblast differentiation by heparin-binding EGF-like growth factor is dependent on the stage-specific activation of calcium influx by ErbB receptors in developing mouse blastocysts. Development. 2000;127:33–44. doi: 10.1242/dev.127.1.33. [DOI] [PubMed] [Google Scholar]
- White CA, Zhang JG, Salamonsen LA, Baca M, Fairlie WD, Metcalf D, Nicola NA, Robb L, Dimitriadis E. Blocking LIF action in the uterus by using a PEGylated antagonist prevents implantation: a nonhormonal contraceptive strategy. Proc Natl Acad Sci USA. 2007;104:19357–19362. doi: 10.1073/pnas.0710110104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med. 1999;340:1796–1799. doi: 10.1056/NEJM199906103402304. [DOI] [PubMed] [Google Scholar]
- Wu Y, Kajdacsy-Balla A, Strawn E, Basir Z, Halverson G, Jailwala P, Wang Y, Wang X, Ghosh S, Guo SW. Transcriptional characterizations of differences between eutopic and ectopic endometrium. Endocrinology. 2006;147:232–246. doi: 10.1210/en.2005-0426. [DOI] [PubMed] [Google Scholar]
- Xie H, Wang H, Tranguch S, Iwamoto R, Mekada E, Demayo FJ, Lydon JP, Das SK, Dey SK. Maternal heparin-binding-EGF deficiency limits pregnancy success in mice. Proc Natl Acad Sci USA. 2007;104:18315–18320. doi: 10.1073/pnas.0707909104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanaihara A, Otsuka Y, Iwasaki S, Aida T, Tachikawa T, Irie T, Okai T. Differences in gene expression in the proliferative human endometrium. Fertil Steril. 2005;83((Suppl. 1):1206–1215. doi: 10.1016/j.fertnstert.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Yoo HJ, Barlow DH, Mardon HJ. Temporal and spatial regulation of expression of heparin-binding epidermal growth factor-like growth factor in the human endometrium: a possible role in blastocyst implantation. Dev Genet. 1997;21:102–108. doi: 10.1002/(SICI)1520-6408(1997)21:1<102::AID-DVG12>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Young SL, Lessey BA. Progesterone function in human endometrium: clinical perspectives. Semin Reprod Med. 2010;28:5–16. doi: 10.1055/s-0029-1242988. [DOI] [PubMed] [Google Scholar]
- Zhu LJ, Bagchi MK, Bagchi IC. Attenuation of calcitonin gene expression in pregnant rat uterus leads to a block in embryonic implantation. Endocrinology. 1998a;139:330–339. doi: 10.1210/endo.139.1.5707. [DOI] [PubMed] [Google Scholar]
- Zhu LJ, Cullinan-Bove K, Polihronis M, Bagchi MK, Bagchi IC. Calcitonin is a progesterone-regulated marker that forecasts the receptive state of endometrium during implantation. Endocrinology. 1998b;139:3923–3934. doi: 10.1210/endo.139.9.6178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.