Abstract
BACKGROUND
Developmental diseases, such as birth defects, growth restriction and preterm delivery, account for >25% of infant mortality and morbidity. Several studies have shown that exposure to chemicals during pregnancy is associated with adverse birth outcomes. The aim of this study was to identify whether occupational exposure to various chemicals might adversely influence intrauterine growth patterns and placental weight.
METHODS
Associations between maternal occupational exposure to various chemicals and fetal growth were studied in 4680 pregnant women participating in a population-based prospective cohort study from early pregnancy onwards in the Netherlands (2002–2006), the Generation R Study. Mothers who filled out a questionnaire during mid-pregnancy (response: 77% of enrolment) were included if they conducted paid employment during pregnancy and had a spontaneously conceived singleton live born pregnancy (n = 4680). A job exposure matrix was used, linking job titles to expert judgement on exposure to chemicals in the workplace. Fetal growth characteristics were repeatedly measured by ultrasound and were used in combination with measurements at birth. Placental weight was obtained from medical records and hospital registries. Linear regression models for repeated measurements were used to study the associations between maternal occupational exposure to chemicals and intrauterine growth.
RESULTS
We observed that maternal occupational exposure to polycyclic aromatic hydrocarbons, phthalates, alkylphenolic compounds and pesticides adversely influenced several domains of fetal growth (fetal weight, fetal head circumference and fetal length). We found a significant association between pesticide and phthalate exposure with a decreased placental weight.
CONCLUSIONS
Our results suggest that maternal occupational exposure to several chemicals is associated with impaired fetal growth during pregnancy and a decreased placental weight. Further studies are needed to confirm these findings and to assess post-natal consequences.
Keywords: chemical exposure, occupational exposure, phthalates, pesticides, fetal growth
Introduction
Developmental diseases, such as structural alterations (birth defects), functional alterations, growth restriction and preterm delivery, account for >25% of infant mortality and morbidity (Liu and Roth, 2008; Stillerman et al., 2008). Fetal growth is generally assessed by surrogate measures, including length of gestation and fetal size, and these end-points are important determinants of later health and morbidity (McCormick, 1985; McIntire et al., 1999; Yanney and Marlow, 2004). Common risk factors for adverse fetal development include ethnicity (Thompson et al., 2001), smoking and alcohol use (Jaddoe et al., 2007), previous children with low birthweight or preterm birth, older maternal age and low socioeconomic status (Silva et al., 2010). Recently, it has been suggested that environmental risk factors and parental occupation may also play an important role (Windham and Fenster, 2008; Li et al., 2010a,b).
Women constitute a substantial part of the labour force in the European Union (EU). In 2010, ∼58% of the women aged between 15 and 64 years had paid employment, which was a substantial increase from 54% in 2002 (Eurostat, 2011). With the increasing labour force participation among women in European countries, the likelihood that women will be exposed to a variety of chemical, physical and psychological risk factors at work during pregnancy will also increase (Linos and Kirch, 2008). Although women in paid employment have better pregnancy outcomes than those without paid jobs (Savitz et al., 1996; Jansen et al., 2010; Burdorf et al., 2011), certain work-related factors, such as exposure to chemicals (Mattison, 2010), physically demanding work (Mozurkewich et al., 2000) and psychological job strain (Vrijkotte et al., 2009) may adversely influence the pregnancy outcome.
Exposure to chemicals during fetal development may increase the risk of adverse health consequences, including adverse birth outcomes, childhood morbidity and adult disease and mortality (Gluckman and Hanson, 2004; Stillerman et al., 2008). Chemicals that have been associated with adverse fetal development are lead, other heavy metals (Llanos and Ronco, 2009; Zhu et al., 2010), phthalates (Latini et al., 2006) and pesticides (Perera et al., 2005; Weselak et al., 2007; Gilden et al., 2010). Chemicals can cross the placenta and enter the fetus, and a number of chemicals measured in maternal urine and serum have also been found in amniotic fluid, cord blood and meconium (Barr et al., 2007). A recent study by Woodruff et al. (2011) showed that pregnant women in the USA were exposed to multiple chemicals. The mechanism by which chemicals affect reproductive events are not completely understood; direct toxic effects may occur when normal processes such as differentiation, mitosis, meiosis intracellular communication and DNA repair are altered. In this regard, the fetus is particularly vulnerable due to its fast growth, the process of cellular differentiation, the immaturity of its metabolic pathways and the stage of development of vital organs (Bruckner, 2000).
Since several studies have shown that exposure to chemicals during pregnancy adversely influences fetal development, as demonstrated by an increased occurrence of low birthweight, small-for-gestational age and preterm delivery (Stillerman et al., 2008; Windham and Fenster, 2008; Wigle et al., 2008), we expect that exposure to chemicals might already influence fetal growth in the different trimesters during pregnancy. Although birth outcomes are important from an obstetric perspective, they are rather crude measures of fetal growth during pregnancy.
The aim of this study was to identify, within a population-based prospective birth cohort study, whether occupational exposure to various chemicals might adversely influence intrauterine growth patterns and placental weight.
Materials and Methods
Study design
The Generation R Study is a population-based prospective cohort study on growth, development and health from early fetal life until young adulthood in Rotterdam, the Netherlands. The study design has previously been described in detail (Jaddoe et al., 2006, 2010). Briefly, all pregnant women who had an expected delivery date between April 2002 and January 2006 and lived in the study area of Rotterdam were invited to participate. In total, 9778 pregnant women (response of 61%) were enrolled in the study, of which 8880 women were enrolled during pregnancy and another 898 at birth of their child. Extensive assessments were carried out during the first trimester (gestational age <18 weeks), second trimester (gestational age 18–25 weeks) and third trimester (gestational age >25 weeks), including physical examinations, questionnaires, interviews and biological samples. The study was approved by the Medical Ethics Committee at Erasmus University Medical Centre, Rotterdam, The Netherlands (MEC 198.782/2001/31).
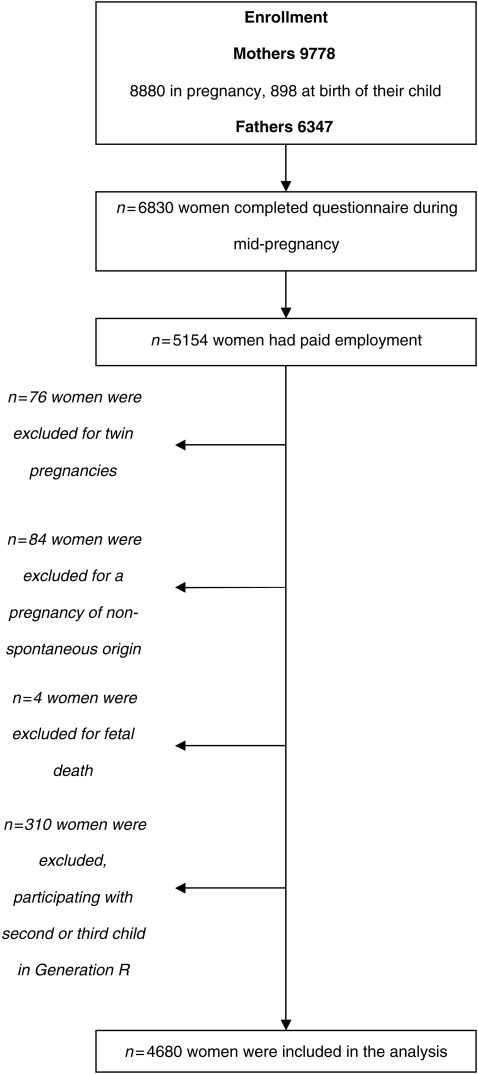
The occupational information required for this study was gathered in the questionnaire completed during mid-pregnancy, which was filled out by 6830 women (77% of enrolment). For this study, we selected women who were prenatally enrolled, with paid employment before or during pregnancy, and with a spontaneously conceived singleton live born pregnancy. For each couple, we included the first pregnancy within the Generation R cohort in our study, since some women participated with more than one child in the study. Finally, the study population consisted of 4680 women; the flowchart of the study population is depicted in Fig. 1. Our results are based on the second and third trimester ultrasonography measures in combination with birth outcomes.
Figure 1.
Flowchart of the study population.
Fetal ultrasounds
For this study, we used the ultrasound measures of fetal head circumference (HC), femur length (FL) and estimated fetal weight (EFW), since these three measures are essential characteristics to describe fetal growth. Fetal ultrasound examinations were carried out in two dedicated research centres in each trimester of pregnancy. We measured fetal HC, abdominal circumference (AC) and FL to the nearest millimetre using standardized ultrasound procedures in the second (median, 20.5; minimum–maximum, 18.0–25.0 weeks) and third (median, 30.4; minimum–maximum, 25.8–37.0 weeks) trimester. Since the use of the last menstrual period for pregnancy dating has several limitations (Verburg et al., 2008a) and a large number of women in our study population did not know the exact date of their last menstrual period (76%), we used crown-rump length for pregnancy dating until a gestational age of 12 weeks (2308 women) and biparietal diameter for pregnancy dating thereafter (2372 women) in all women (Robinson et al., 1979; Altman and Chitty, 1997). First trimester measurements (3459 women) were primarily used to establish gestational age and therefore not included in the growth analysis. EFW was calculated using the formula by Hadlock et al. (1985). Ultrasound examinations were performed using an Aloka model SSD-1700 (Tokyo, Japan) or the ATL-Philips Model HDI 5000 (Seattle, WA, USA). Customized growth curves for the entire study population were constructed, and standard deviation (SD) scores for each individual woman were calculated as a deviation from the ‘overall’ average at that gestational week, and represent the equivalent z-scores (Verburg et al., 2008a). The intraclass correlation coefficient of fetal growth measurements was 0.95, tested on 21 subjects, indicating a high reproducibility of fetal biometry measurements (Verburg et al., 2008b).
Placenta and birth outcomes
Placental weight was obtained from medical records and hospital registries. Information about gender at birth, gestational age, weight, length and HC at birth was obtained from medical records and hospital registries. For the analysis, we used birthweight, HC at birth and length of the infant at birth.
Occupation and working conditions
The mid-pregnancy questionnaire contained questions about work status, occupation and working conditions and focused on the periconception and pregnancy period. The work status, based on a single question on the current economic status with seven categories such as paid labour, self-employed, unemployed, disabled, homemaker, student or other, was used to select women with paid employment. This question was followed by questions whether the mother had worked before conception in this current occupation, and the starting and (optional) closing date of this current occupation. We selected women who started working before conception and women who started working somewhere during the first trimester of pregnancy. Further questions on job title, type of business, name of the employer and activities in the job were used to classify jobs into the Dutch Classification of Occupations (Statistics Netherlands, 1992) and subsequently link these codes to a job exposure matrix (JEM) for chemical exposure (Brouwers et al., 2009). This new JEM was developed according to a general strategy, comprising a literature search to identify chemicals, information gathering on occupations at risk and literature on occupational settings in which the selected chemicals were encountered and exposure measurements were performed. This reference material served as a starting point for the expert assessment. Three experts were asked to estimate exposures based on their knowledge of tasks and working environment in various occupations. Finally, exposure probability scores were added based on the judgement of three experts. For various chemicals, subjects experience a certain level of exposure through diet, environment or widely used consumer products. The JEM exposure score refers to the probability of occupational exposure, which is assumed to exceed the background level in the general population. The exposure probability scores were assigned by means of consensus discussions in which the original scores were taken into account where possible, but no prior individual assessments were performed. The JEM comprises 10 categories of chemicals, namely polycyclic aromatic hydrocarbons (PAHs), polychlorinated organic compounds, pesticides, phthalates, organic solvents, bisphenol A, alkylphenolic compounds, flame retardants, metals and miscellaneous agents (Brouwers et al., 2009). For 353 job titles, probability scores were classified into three levels: ‘unlikely’ (0), ‘possible’ (1) and ‘probable’ (2). Different country-specific JEMs have been used in several studies, and the JEM is a valuable tool for exposure assessment in epidemiological studies on the health risks of chemical exposure (Vrijheid et al., 2003; Pierik et al., 2004; Burdorf et al., 2011; Snijder et al., 2011). For this study, we collated the last two categories into one category indicating the occurrence of exposure to chemicals.
Potential confounders
Information about maternal age, pre-pregnancy weight, educational level, ethnicity, parity and folic acid supplement use was obtained by questionnaire at enrolment in the study. Maternal smoking habits and alcohol use were assessed on the basis of three questionnaires (in early, mid- and late pregnancy) and classified as no, until pregnancy was known or during pregnancy (Jaddoe et al., 2008). Maternal height was measured at intake in the study. The questions on physical work load were obtained from the Dutch Musculoskeletal Questionnaire and concerned questions on long periods of standing, manually handling loads of 5 kg or more, manually handling loads of 25 kg or more and night shifts. The presence of doctor-diagnosed pre-eclampsia, pregnancy-induced hypertension and diabetes gravidarum was retrieved from medical records and was based on the criteria of the International Society for the Study of Hypertension in Pregnancy (Brown et al., 2001; Coolman et al., 2010).
Statistical analysis
We assessed the associations between maternal occupational exposure to various chemicals and longitudinally measured SD scores of HC, length (second and third trimester FL and birth length) and weight (second and third trimester EFW and birthweight) using a mixed model for repeated measurements with an unstructured error term. This is a commonly used method to analyse data from longitudinal studies (Twisk, 2004). First, customized growth curves for the entire study population were constructed, and SD scores for each individual woman were calculated as deviation from the ‘overall’ average at that gestational week (Verburg et al., 2008a). This approach resembles the common measure weight-for-age z-scores, used in international studies on undernutrition and child mortality in order to increase comparability of effects independent of underlying differences in distributions (Fishman et al., 2004). These gestational age-adjusted SD scores were used as parameters of fetal growth, the dependent variables in the statistical analyses. Second, a linear model was used to study the influence of occupational exposure to chemicals on these gestational age-adjusted SD scores. The final model can be written as (for example, for fetal weight): SD score of fetal weight = β0 + β1× gawks + β2× exposure group + β3× gawks × exposure group (gawks = gestational age in weeks). In this model, β0 reflects the intercept and β2 expresses the systematic difference between the exposed and non-exposed groups. The coefficient β3 reflects whether exposed and non-exposed fetus grow at the same rate over time. The later coefficient is the main interest of this analysis, since it represents the average decrease or increase in SD for fetal weight per gestational week for exposed women versus non-exposed women. Different beta coefficients of interaction were estimated for weight, HC and length, representing growth velocity for several domains of fetal growth. The regression models were adjusted for lifestyle and socioeconomic confounders used in previous studies on maternal occupational exposure (Burdorf et al., 2011; Snijder et al. 2011) and known determinants of fetal growth: maternal age, educational level, ethnicity, parity, pre-pregnancy weight, height at intake, smoking during pregnancy, alcohol use during pregnancy, folic acid supplement use, fetal gender, physically demanding work (long periods of standing, handling of loads of >5 kg, handling of loads of >25 kg and night shifts) and pregnancy complications (pre-eclampsia, pregnancy-induced hypertension and diabetes gravidarum). For the important confounders' ethnicity and educational level, potential interaction with exposure was investigated for each multivariate model with a significant effect of exposure on fetal growth.
Missing values in covariates were handled by multiple imputations (Markov chain Monte Carlo method) by generating five independent data sets for all analyses. Imputations were based on the relations between all covariates included in this study and the threshold for imputation was set on a maximum of 30% of missing values. We used the pooled adjusted effect estimates to generate Figs 2–4. No differences were observed between analyses with imputed missing data or complete cases only. We performed a sensitivity analysis in order to evaluate whether women who started working in their current job before conception differed from women who started working during pregnancy. All levels of associations are presented with their 95% confidence intervals (CIs). The repeated-measurement analyses were conducted with the Proc Mixed module of the Statistical Analysis System (version 9.2; SAS Institute, Inc., Cary, NC, USA).
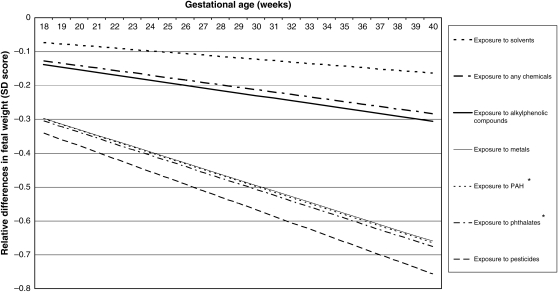
Figure 2.
Adjusted relative differences in fetal weight (SD scores) in various chemical groups compared with the non-exposed group. Values are based on repeated linear regression models and reflect the difference in the SD score of fetal weight measurements (based on 12 748 measurements) in the offspring of mothers occupationally exposed to various groups of chemicals compared with the offspring of non-exposed mothers. The reference value is an SD score of 0. *P < 0.05. Estimates are adjusted for the following confounders: maternal age, educational level, ethnicity, fetal gender, weight before pregnancy, height at intake, smoking during pregnancy, alcohol use during pregnancy, folic acid use, parity, long periods of standing, handling loads of >5 kg, handling loads of >25 kg, night shifts, pre-eclampsia, pregnancy-induced hypertension and diabetes gravidarum.
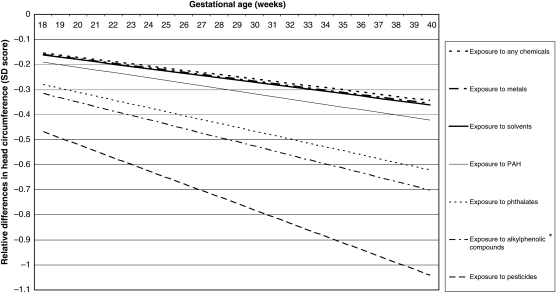
Figure 3.
Adjusted relative differences in head circumference (HC) (SD scores) in various chemical groups compared with the non-exposed group. Values are based on repeated linear regression models and reflect the difference in the SD score of fetal HC measurements (based on 10 789 measurements) in the offspring of mothers occupationally exposed to various groups of chemicals compared with the offspring of non-exposed mothers. The reference value is an SD score of 0. *P< 0.05. Estimates are adjusted for the following confounders: maternal age, educational level, ethnicity, fetal gender, weight before pregnancy, height at intake, smoking during pregnancy, alcohol use during pregnancy, folic acid use, parity, long periods of standing, handling loads of >5 kg, handling loads of >25 kg, night shifts, pre-eclampsia, pregnancy-induced hypertension and diabetes gravidarum.
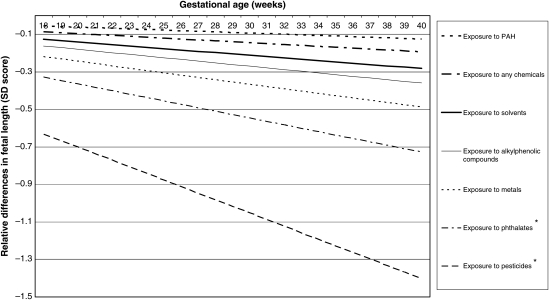
Figure 4.
Adjusted relative differences in fetal length (SD scores) in various chemical groups compared with the non-exposed group. Values are based on repeated linear regression model and reflect the difference in the SD score of fetal length measurements (based on 11 401 measurements) in the offspring of mothers occupationally exposed to various groups of chemicals compared with the offspring of non-exposed mothers. The reference value is an SD score of 0. *P < 0.05. Estimates are adjusted for the following confounders: maternal age, educational level, ethnicity, fetal gender, weight before pregnancy, height at intake, smoking during pregnancy, alcohol use during pregnancy, folic acid use, parity, long periods of standing, handling loads of >5 kg, handling loads of >25 kg, night shifts, pre-eclampsia, pregnancy-induced hypertension and diabetes gravidarum.
Results
Table I shows the baseline characteristics of the study population. The mean age of the women at intake in the study was 31.1 years. Of all women, 30.3% had completed higher education and the largest group was from Dutch origin (64.0%). The majority of women were nulliparous (63.9%). A total of 11.7% of the mothers continued smoking and 39.4% of the mothers continued drinking alcohol after the pregnancy was known. According to the JEM, 1.3% of the women were exposed to PAHs, 0.5% to pesticides, 1.5% to phthalates, 4.7% to organic solvents, 3.3% to alkylphenolic compounds, 1.1% to metals and 6.7% to any chemicals. In total, 4197 (89.7%) women visited our clinic for second trimester ultrasonography and 4294 (91.8%) for third trimester ultrasonography. The median gestational age at birth was 40.1 weeks (minimum, 22.7; maximum, 43.4 weeks), while mean birthweight was 3450g (SD 549g). Slightly more than 50% of the infants were boys. The three characteristics of fetal growth were interrelated with the highest association between fetal weight and length (Pearson correlation coefficient, r = 0.59, at birth) and the smallest association between HC and length (r = 0.43, at birth).
Table I.
Baseline characteristics of pregnant women participating in a birth cohort study, the Generation R Study (n = 4680).
| Maternal characteristics | |
| Age at intake (years) | 31.08 (4.56) |
| Weight before pregnancy (kg) | 64.00 (34–145) |
| Height measured at intake (cm) | 168.80 (7.12) |
| Educational level (%) | |
| Low | 653 (14.0) |
| Mid-low | 1333 (28.5) |
| Mid-high | 1129 (24.1) |
| High | 1419 (30.3) |
| Missing | 146 (3.1) |
| Ethnicity (%) | |
| Netherlands | 2993 (64.0) |
| Surinam and Dutch Antilles | 380 (8.1) |
| Morocco and Turkey | 328 (7.0) |
| Other | 885 (18.9) |
| Missing | 94 (2.0) |
| Parity (%) | |
| Nulliparous | 2992 (63.9) |
| Multiparous | 1565 (33.4) |
| Missing | 123 (2.6) |
| Smoking (%) | |
| Yes, during pregnancy | 546 (11.7) |
| Yes, until pregnancy was known | 355 (7.6) |
| No | 3031 (64.8) |
| Missing | 748 (16.0) |
| Alcohol (%) | |
| Yes, during pregnancy | 1846 (39.4) |
| Yes, until pregnancy was known | 587 (12.5) |
| No | 1524 (32.6) |
| Missing | 723 (15.4) |
| Folic acid use (%) | |
| No | 580 (12.4) |
| Yes, post-conception start | 1163 (24.9) |
| Yes, pre-conception start | 1735 (37.1) |
| Missing | 1202 (25.7) |
| Maternal occupational characteristics (%) | |
| Exposure to | |
| PAH | 63 (1.3) |
| Pesticides | 23 (0.5) |
| Phthalates | 68 (1.5) |
| Organic solvents | 221 (4.7) |
| Alkylphenolic compounds | 156 (3.3) |
| Metals | 52 (1.1) |
| Any chemicals | 313 (6.7) |
| Growth outcomes | |
| Second trimester ultrasonography | 4197 (89.7) |
| Third trimester ultrasonography | 4294 (91.8) |
| Birth outcomes | |
| Gestational age at birth (weeks) | 40.14 (22.71–43.43) |
| Birthweight (g) | 3449.81 (549.28) |
| Male | 2365 (50.5) |
| HC at birth (mm) | 33.89 (1.65) |
| Length at birth (mm) | 50.33 (2.38) |
Values are means (SD) for normal distributed continuous variables or medians (minimum–maximum) for skewed distributed continuous variables, and absolute numbers (percentages) for categorical variables.
Table II shows the results of the linear regression analysis on occupational exposure to chemicals and placental weight. Women occupationally exposed to pesticides and phthalates showed a significantly lower placental weight compared with non-exposed women, respectively, 65.90 g for pesticides (95% CI: −129.86 to −1.94) and 45.88g for phthalates (95% CI: −85.15; −6.60).
Table II.
Associations between occupational exposure to chemicals and placental weight among pregnant women participating in a birth cohort study.
| Occupational chemical exposure | Placental weight (g) |
|
|---|---|---|
| Crudea | Adjustedb | |
| Exposure to | ||
| PAH | −21.21 (−65.17 to 22.75) | −7.64 (−52.03 to 36.76) |
| Pesticides | −74.84 (−138.34 to −11.35)* | −65.90 (−129.86 to −1.94)* |
| Phthalates | −59.55 (−98.11 to −21.00)* | −45.88 (−85.15 to −6.60)* |
| Organic solvents | −17.74 (−39.21 to 3.74) | −10.00 (−32.36 to 12.36) |
| Alkylphenolic compounds | −15.81 (−41.01 to 9.39) | −5.43 (−32.03 to 21.16) |
| Metals | −37.14 (−80.53 to 6.26) | −35.22 (−78.54 to 8.09) |
| Any chemicals | −18.71 (−37.20 to −0.22)* | −11.03 (−30.28 to 8.23) |
Results from simple and multiple linear regression analysis. Values are regression coefficients (95% CIs) and reflect the difference in grams for placental weight between women exposed to chemicals in the workplace compared with non-exposed women. Based on 3185 measurements of placental weight.
aAdjusted for gestational age at birth.
bAdjusted for gestational age at birth, maternal age, educational level, ethnicity, fetal gender, weight before pregnancy, height at intake, smoking during pregnancy, alcohol use during pregnancy, folic acid use, parity, long periods of standing, handling loads of >5 kg, handling loads of >25 kg, night shifts, pre-eclampsia, pregnancy-induced hypertension and diabetes gravidarum.
*P< 0.05.
Table III shows the results of the univariable and multivariable longitudinal models for the associations between occupational exposure to various chemicals and fetal weight, HC and fetal length. The average decline in SD per gestational week is graphically illustrated in Figs 2–4. Maternal occupational exposure to several chemicals showed similar trends with lower growth rates for all three parameters. Women occupationally exposed to PAHs and phthalates showed significant lower fetal weight growth rates (average decline in SD per gestational week: 0.01660 for PAHs and 0.01691 for phthalates) compared with non-exposed mothers, adjusted for potential confounders. In the fully adjusted model, the following covariates statistically significantly influenced fetal growth in order of decreasing importance: weight, height, parity, smoking, ethnicity and diabetes gravidarum, but adjustments did not change the effect estimates of chemical exposure on fetal growth (Supplementary data, SI). No interaction for exposure with ethnicity and educational level was observed in the multivariate models, indicating that ethnicity and education do not moderate or explain the observed associations between occupational exposure and fetal growth parameters.
Table III.
Association between occupational chemical exposure and fetal weight, fetal HC, and fetal length among pregnant women participating in a birth cohort study.
| Occupational exposure | Fetal weight |
Fetal HC |
Fetal length |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted estimate | Adjusted estimate | Standard error | Unadjusted estimate | Adjusted estimate | Standard error | Unadjusted estimate | Adjusted estimate | Standard error | |
| Exposure to | |||||||||
| PAH | −0.01647* | −0.01660* | 0.00798 | −0.01053 | −0.01056 | 0.01114 | −0.00328 | −0.003139 | 0.01020 |
| Pesticides | −0.01892 | −0.01891 | 0.01274 | −0.02619 | −0.02603 | 0.01703 | −0.03610* | −0.035071* | 0.01605 |
| Phthalates | −0.01675* | −0.01691* | 0.00744 | −0.01632 | −0.01553 | 0.00982 | −0.01845* | −0.018183* | 0.00908 |
| Organic solvents | −0.00411 | −0.00410 | 0.00424 | −0.00975 | −0.00902 | 0.00560 | −0.00743 | −0.007048 | 0.00521 |
| Alkylphenolic compounds | −0.00757 | −0.00766 | 0.00500 | −0.01834* | −0.01752* | 0.00661 | −0.00954 | −0.008990 | 0.00621 |
| Metals | −0.01682 | −0.01649 | 0.00872 | −0.00937 | −0.00888 | 0.01163 | −0.01246 | −0.012172 | 0.01087 |
| Any chemicals | −0.00712 | −0.00710 | 0.00363 | −0.00912 | −0.00861 | 0.00482 | −0.00520 | −0.004850 | 0.00449 |
The beta coefficients represent the average decline in SD per gestational week for fetal weight, HC and fetal length. Estimates are adjusted for the following confounders: maternal age, educational level, ethnicity, fetal gender, weight before pregnancy, height at intake, smoking during pregnancy, alcohol use during pregnancy, folic acid use, parity, long periods of standing, handling loads of >5 kg, handling loads of >25 kg, night shifts, pre-eclampsia, pregnancy-induced hypertension and diabetes gravidarum.
*P> 0.05.
For fetal HC, only maternal occupational exposure to alkylphenolic compounds showed a statistically significant lower growth rate (−0.01752 SD per gestational week) compared with non-exposed mothers, adjusted for potential confounders. For fetal length, we observed statistically significant lower growth rates between mothers occupationally exposed to pesticides and phthalates (−0.0361 and −0.0185 SD per gestational week, respectively) compared with non-exposed mothers, with a much steeper decline during the course of pregnancy for pesticides than for other occupational chemicals.
In total, 4177 (89.3%) women filled out the question concerning the starting date of their current occupation, 4068 women (97.4%) started working before conception, whereas 109 (2.6%) women started working somewhere during their first trimester of pregnancy. In the sensitivity analyses, no differences in effect estimates were observed between women who started working before conception compared with women who started working during the first trimester of pregnancy. The differences in SD scores for all fetal growth characteristics for the unadjusted model, the adjusted model, and for the five imputation models are shown in Supplement I. Supplement II and III show the individual data points of exposed and non-exposed women for fetal weight and fetal head circumference in the second trimester, third trimester and at birth.
Discussion
This large population-based prospective cohort study showed that maternal occupational exposure to several chemicals, such as PAHs, phthalates, alkylphenolic compounds and pesticides during pregnancy, adversely influenced their fetal growth rates of weight, HC and length. These differences in fetal growth rates could already be demonstrated during pregnancy, and were partly reflected in a decreased placental weight. These findings suggest that early exposure during the critical window of fetal development is crucial.
In this study, we used ultrasound measurements for pregnancy dating (Robinson et al., 1979; Altman and Chitty, 1997); this method appears to be superior to dating based on the last menstrual period (Verburg et al., 2008a). A disadvantage of pregnancy dating by ultrasound is that growth variations in crown-rump length and biparietal diameter in early pregnancy are assumed to be zero, impairing detailed analysis on fetal growth in the first trimester. In a sensitivity analyses on the subset of women with a certain last menstrual period and regular cycle (n = 1221), the direction of the effect estimates did not change. Reference curves for fetal growth were constructed for our cohort, which enables linear analyses of fetal growth characteristics. These curves are based on a large, urban, non-hospital-based population, which makes these curves generalizable to normal fetal development in industrialized countries (Verburg et al., 2008a). For the repeated measurements concerning fetal length, we used the SD score of birth length in combination with SD scores of FL in second and third trimester in order to assess relative changes in fetal skeletal growth. However, the results should be interpreted with caution, since these measurements reflect different body parts. The repeated measurements based on gestational age-adjusted SD scores were used in previous studies within the same cohort (Jaddoe et al., 2007; Bakker et al., 2010). This method enables us to identify pathological smallness instead of constitutional smallness, which may be normal intrauterine growth. The advantage of SD scores as relative measure of difference is that the SD scores can be used in linear regression models, whereas absolute differences in fetal growth were highly skewed since growth curves during pregnancy have a typical parabolic shape that must be described by fractional polynomials instead of normal distributions. We demonstrated two of these curves with absolute differences in Supplementary data, SII and SIII.
The strength of this study is the population-based approach with recruitment during the prenatal period and the availability of a large number of potential confounders. A limitation of this study is the selective participation with mothers from ethnic minorities and with lower socioeconomic status less represented in the study population (Jaddoe et al., 2006). This selection may have influenced the prevalence of exposure to chemicals at the workplace, but bias is unlikely since exposure status was assessed independently from and prior to the fetal growth characteristics by a recently updated JEM. This approach assured that exposure status was blinded to participants and researchers, both aspects which avoid information bias. The characterization of exposure in the JEM must be interpreted as exposure probabilities, which are only a crude measure of exposure, which have to be interpreted with caution. Background exposure to various chemicals through diet and environment may occur. Previous research within the Generation R Study (Ye et al., 2008), but also within the NHANES national survey, showed that almost all pregnant women are exposed to chemicals, and that levels are comparable between pregnant and non-pregnant women (Woodruff et al., 2011). However, there is a reason to believe that occupational exposure is generally much higher than background exposure through diet and environment (Nieuwenhuijsen, 2003). For example, for phthalates, Hines et al. (2009) showed that for several occupations the urinary phthalate concentrations exceeded the levels of the general population. However, biomonitoring data comparing occupational exposures with exposure from non-occupational sources are scarce. In the current study, we did not assess background exposure and, thus, it is not possible to distinguish the importance of different routes of exposure. Since it is unlikely that the widespread environmental exposure is associated with occupational exposure in specific jobs, background exposure will most likely not confound the observed relation between occupational chemical exposure and fetal growth.
Furthermore, the JEM does not contain specific chemicals, but only contains broad groups of chemicals, and the mechanisms of action can vary between specific chemicals in a group. A major drawback of JEMs is that they do not account for variability in tasks and working environments within job titles. However, from the task description, it may become clear that some subjects within a specific job title, for example, subjects who have odd jobs around a farm (feeding animals) are less likely to be exposed to pesticides. The overlap between the categories phthalates, organic solvents and alkylphenolic compounds was considerable for mothers (κ values of 0.47–0.77), indicating that women exposed to one of these substances were likely to be exposed to other substances as well. We must conclude that due to this interrelationship among exposure groups, it was not possible to disentangle the specific role of phthalates and alkylphenolic compounds in the observed lower fetal growth rates.
Women with lower education and women from ethnic minorities were more often exposed to chemicals in the workplace, but in our study this did not introduce confounding. As can be seen from Table II and Supplementary data, adjustments for education and ethnicity only slightly changed our effect estimates. Even though we were able to control for a large number of potential confounders, residual confounding cannot be ruled out completely. In this study, we used multiple imputations for missing values in covariates. This reduces selection bias due to non-random missing in the covariates.
In this study, we measured fetal growth, comprising three characteristics of fetal growth, namely weight, HC and length. Intrauterine growth restriction has been classified as symmetric and asymmetric, although the clinical relevance of this concept is controversial (Maulik, 2006). Recent studies have shown that asymmetric fetal growth is associated with an increased neonatal morbidity (Dashe et al., 2000). Although it proved to be too difficult to distinguish between symmetric and asymmetric growth restriction in our study, we hypothesized that the comparable effects of occupational exposure to chemicals on all characteristics of fetal growth might be suggestive of symmetric growth restriction.
Several chemicals were associated with impaired fetal weight, resulting in a decrease in SD at birth varying between 0.2 and 0.7. This corresponds to ∼100–400g difference in birthweight. The effect of occupational exposure to chemicals seems of similar magnitude than other well-known lifestyle factors, such as smoking, alcohol use and caffeine intake. Bakker et al. (2010) showed a reduction of 0.3 SD in birthweight for a mother who consumed caffeine >6 units/day. Jaddoe et al. (2007) showed that smoking impaired fetal growth, in particular, HC, FL and AC, with 0.1–0.3 SD. However, the population-attributable fraction is low, due to the low prevalence of exposure to these chemicals compared with other well-known lifestyle factors.
Workplace health is an important topic since women who intend to become pregnant, and pregnant women are at risk for adverse pregnancy outcomes, thus, it is important to identify occupational-related risk factors for prevention. Occupations in which women have a high exposure probability are agricultural and horticultural workers (pesticide exposure), hairdressers, beauticians, furniture makers (phthalate exposure) and cleaners (alkylphenolic compounds). Since the effects of occupational exposures on fetal growth are considerable, one could argue that pregnant women working in agriculture or horticultural trades must be informed about the risks of pesticide exposure in the workplace. However, the underlying mechanism is largely unclear, and results from earlier studies are conflicting, warranting further research into this important topic.
This study supports existing evidence from human studies regarding occupational exposures and adverse pregnancy outcomes (Wigle et al., 2008). Although the chemicals in our study were considered to be potential endocrine disrupters, it remains to be established whether the mode of action is through endocrine disruption. A recent review by Caserta et al. (2011) summarizes the literature regarding exposure to endocrine-disrupting chemicals on the pregnancy outcome. They conclude that epidemiological studies on endocrine disruptors are not always consistent. This is further illustrated by occupational studies, for example, in hairdressers, that show conflicting results (Rylander and Kallen, 2005; Zhu et al., 2006; Axmon and Rylander, 2009). Further studies are urgently needed to identify the molecular basis of the effects, to study the epigenetic effects of these exposures and to develop strategies to prevent exposure to these agents to improve birth outcomes (Robins et al., 2011).
Our results suggest that maternal occupational exposure to several chemicals adversely influence fetal growth patterns. Further studies are needed to confirm these findings and to identify potential targets for prevention.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors' roles
C.S. wrote the first draft of the manuscript and was responsible for the statistical analyses and interpretation of the data, and the revisions of the manuscript. E.S., H.R., A.H. and V.J. initiated and designed the study, were responsible for the infrastructure in which the study is conducted, contributed to the original data collection, critically revised the manuscript and approved the final version of the manuscript. N.R. and E.V. contributed to the interpretation of the data and critically revised the manuscript for important intellectual content. A.B. contributed to all revisions of the manuscript, supervised the data analysis and critically revised the manuscript for important intellectual content. All authors have read the manuscript and agree that the work is ready for submission and accept the responsibility for the manuscript's contents.
Funding
The first phase of the Generation R Study was made possible by financial support from the Erasmus Medical Center Rotterdam, the Erasmus University Rotterdam and the Netherlands Organization for Health Research and Development (ZonMw). The present study was conducted with support from the European Commission Seventh Framework Programme (FP7) for Research and Technology Development for the CONTAMED Project, EU grant agreement no. 212502. The views expressed in this paper are those of the authors alone. The study sponsors had no role in study design, data analysis, interpretation of data or writing this manuscript. The researchers were independent of the funders. Funding to pay the Open Access publication charges for this article was provided by Erasmus Medical Center Rotterdam, the Netherlands.
Conflict of interest
None declared.
Supplementary Material
Acknowledgements
The Generation R Study is conducted by the Erasmus Medical Center in close collaboration with the Erasmus University Rotterdam, School of Law and Faculty of Social Sciences, the Municipal Health Service Rotterdam area, Rotterdam, the Rotterdam Homecare Foundation, Rotterdam and the Stichting Trombosedienst and Artsenlaboratorium Rijnmond (STAR), Rotterdam. We gratefully acknowledge the contribution of general practitioners, hospitals, midwives and pharmacies in Rotterdam.'
References
- Altman DG, Chitty LS. New charts for ultrasound dating of pregnancy. Ultrasound Obstet Gynecol. 1997;10:174–191. doi: 10.1046/j.1469-0705.1997.10030174.x. [DOI] [PubMed] [Google Scholar]
- Axmon A, Rylander L. Birthweight and fetal growth in infants born to female hairdressers and their sisters. Occup Environ Med. 2009;66:198–204. doi: 10.1136/oem.2008.039784. [DOI] [PubMed] [Google Scholar]
- Bakker R, Steegers EA, Obradov A, Raat H, Hofman A, Jaddoe VW. Maternal caffeine intake from coffee and tea, fetal growth, and the risks of adverse birth outcomes: the Generation R Study. Am J Clin Nutr. 2010;91:1691–1698. doi: 10.3945/ajcn.2009.28792. [DOI] [PubMed] [Google Scholar]
- Barr DB, Bishop A, Needham LL. Concentrations of xenobiotic chemicals in the maternal–fetal unit. Reprod Toxicol. 2007;23:260–266. doi: 10.1016/j.reprotox.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Brouwers MM, van Tongeren M, Hirst AA, Bretveld RW, Roeleveld N. Occupational exposure to potential endocrine disruptors: further development of a job exposure matrix. Occup Environ Med. 2009;66:607–614. doi: 10.1136/oem.2008.042184. [DOI] [PubMed] [Google Scholar]
- Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders during pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP) Hypertens Pregnancy. 2001;20:IX–XIV. doi: 10.1081/PRG-100104165. [DOI] [PubMed] [Google Scholar]
- Bruckner JV. Differences in sensitivity of children and adults to chemical toxicity: the NAS panel report. Regul Toxicol Pharmacol. 2000;31:280–285. doi: 10.1006/rtph.2000.1393. [DOI] [PubMed] [Google Scholar]
- Burdorf A, Brand T, Jaddoe VW, Hofman A, Mackenbach JP, Steegers EA. The effects of work-related maternal risk factors on time to pregnancy, preterm birth and birthweight: the Generation R Study. Occup Environ Med. 2011;68:197–204. doi: 10.1136/oem.2009.046516. [DOI] [PubMed] [Google Scholar]
- Caserta D, Mantovani A, Marci R, Fazi A, Ciardo F, La Rocca C, Maranghi F, Moscarini M. Environment and women's reproductive health. Hum Reprod Update. 2011;17:418–433. doi: 10.1093/humupd/dmq061. [DOI] [PubMed] [Google Scholar]
- Coolman M, de Groot CJM, Jaddoe VW, Hofman A, Raat H, Steegers EAP. Medical record validation of maternally reported history of pre-eclampsia: the Generation R Study. J Clin Epidemiol. 2010;63:932–937. doi: 10.1016/j.jclinepi.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Dashe JS, McIntire DD, Lucas MJ, Leveno KJ. Effects of symmetric and asymmetric fetal growth on pregnancy outcomes. Obstet Gynecol. 2000;96:321–327. doi: 10.1016/s0029-7844(00)00943-1. [DOI] [PubMed] [Google Scholar]
- Eurostat. 2011. http://epp.eurostat.ec.europa.eu/portal/page/portal/eurostat/home/ (July 2011, date last accessed)
- Fishman SM, Caulfield LE, de Onis M, Blössner M, Hyder AA, Mullany L, Black RE. Childhood and maternal underweight. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL, editors. Comparative Quantification of Health Risks: The Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. Geneva: World Health Organisation; 2004. pp. 39–131. [Google Scholar]
- Gilden RC, Huffling K, Sattler B. Pesticides and health risks. J Obstet Gynecol Neonatal Nurs. 2010;39:103–110. doi: 10.1111/j.1552-6909.2009.01092.x. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements—a prospective study. Am J Obstet Gynecol. 1985;151:333–337. doi: 10.1016/0002-9378(85)90298-4. [DOI] [PubMed] [Google Scholar]
- Hines CJ, Nilsen Hopf NB, Deddens JA, Calafat AM, Silva MJ, Grote AA, Sammons DL. Urinary phthalate metabolite concentrations among workers in selected industries: a pilot biomonitoring study. Ann Occup Hyg. 2009;53:1–17. doi: 10.1093/annhyg/men066. [DOI] [PubMed] [Google Scholar]
- Jaddoe VW, Mackenbach JP, Moll HA, Steegers EA, Tiemeier H, Verhulst FC, Witteman JC, Hofman A. The Generation R Study: design and cohort profile. Eur J Epidemiol. 2006;21:475–484. doi: 10.1007/s10654-006-9022-0. [DOI] [PubMed] [Google Scholar]
- Jaddoe VW, Verburg BO, de Ridder MA, Hofman A, Mackenbach JP, Moll HA, Steegers EA, Witteman JC. Maternal smoking and fetal growth characteristics in different periods of pregnancy: the Generation R Study. Am J Epidemiol. 2007;165:1207–1215. doi: 10.1093/aje/kwm014. [DOI] [PubMed] [Google Scholar]
- Jaddoe VW, Troe EJ, Hofman A, Mackenbach JP, Moll HA, Steegers EA, Witteman JC. Active and passive maternal smoking during pregnancy and the risks of low birthweight and preterm birth: the Generation R Study. Paediatr Perinat Epidemiol. 2008;22:162–171. doi: 10.1111/j.1365-3016.2007.00916.x. [DOI] [PubMed] [Google Scholar]
- Jaddoe VW, van Duijn CM, van der Heijden AJ, Mackenbach JP, Moll HA, Steegers EA, Tiemeier H, Uitterlinden AG, Verhulst FC, Hofman A. The Generation R Study: design and cohort update 2010. Eur J Epidemiol. 2010;25:823–841. doi: 10.1007/s10654-010-9516-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen PW, Tiemeier H, Verhulst FC, Burdorf A, Jaddoe VW, Hofman A, Moll HA, Verburg BO, Steegers EA, Mackenbach JP, et al. Employment status and the risk of pregnancy complications: the Generation R Study. Occup Environ Med. 2010;67:387–394. doi: 10.1136/oem.2009.046300. [DOI] [PubMed] [Google Scholar]
- Latini G, Del Vecchio A, Massaro M, Verrotti A, DE Felice C. In utero exposure to phthalates and fetal development. Cur Med Chem. 2006;13:2527–2534. doi: 10.2174/092986706778201666. [DOI] [PubMed] [Google Scholar]
- Li X, Sundquist J, Sundquist K. Parental occupation and risk of small-for-gestational-age births: a nationwide epidemiological study in Sweden. Hum Reprod. 2010a;25:1044–1050. doi: 10.1093/humrep/deq004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Sundquist J, Kane K, Jin Q, Sundquist K. Parental occupation and preterm births: a nationwide epidemiological study in Sweden. Paediatr Perinat Epidemiol. 2010b;24:555–563. doi: 10.1111/j.1365-3016.2010.01149.x. [DOI] [PubMed] [Google Scholar]
- Linos A, Kirch W. Promoting Health for Working Women. 1st edn. New York, USA: Springer Science; 2008. [Google Scholar]
- Liu X, Roth J. Development and validation of an infant morbidity index using latent variable models. Stat Med. 2008;27:971–989. doi: 10.1002/sim.2951. [DOI] [PubMed] [Google Scholar]
- Llanos MN, Ronco AM. Fetal growth restriction is related to placental levels of cadmium, lead and arsenic but not with antioxidant activities. Reprod Toxicol. 2009;27:88–92. doi: 10.1016/j.reprotox.2008.11.057. [DOI] [PubMed] [Google Scholar]
- Mattison DR. Environmental exposures and development. Curr Opin Pediatr. 2010;22:208–218. doi: 10.1097/MOP.0b013e32833779bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maulik D. Fetal growth compromise: definitions, standards, and classification. Clin Obstet Gynecol. 2006;49:214–218. doi: 10.1097/00003081-200606000-00004. [DOI] [PubMed] [Google Scholar]
- McCormick MC. The contribution of low birthweight to infant mortality and childhood morbidity. N Engl J Med. 1985;82:378–382. doi: 10.1056/NEJM198501103120204. [DOI] [PubMed] [Google Scholar]
- McIntire DD, Bloom SL, Casey BM, Levano K. Birthweight in relation to morbidity and mortality among newborn infants. N Engl J Med. 1999;340:1234–1238. doi: 10.1056/NEJM199904223401603. [DOI] [PubMed] [Google Scholar]
- Mozurkewich EL, Luke B, Avni M, Wolf FM. Working conditions and adverse pregnancy outcome: a meta-analysis. Obstet Gynecol. 2000;95:623–635. doi: 10.1016/s0029-7844(99)00598-0. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuijsen MJ. Exposure Assessment in Occupational and Environmental Epidemiology. 1st edn. Oxford, UK: Oxford University Press; 2003. [Google Scholar]
- Perera FP, Rauh V, Whyatt RM, Tang D, Tsai WY, Bernert JT, Tu YH, Andrews H, Barr DB, Camann DE, et al. A summary of recent findings on birth outcomes and developmental effects of prenatal ETS, PAH, and pesticide exposures. Neurotoxicology. 2005;26:573–587. doi: 10.1016/j.neuro.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Pierik FH, Burdorf A, Deddens JA, Juttmann RE, Weber RF. Maternal and paternal risk factors for cryptorchidism and hypospadias: a case–control study in newborn boys. Environ Health Perspect. 2004;112:1570–1576. doi: 10.1289/ehp.7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins JC, Marsit CJ, Padbury JF, Sharma SS. Endocrine disruptors, environmental oxygen, epigenetics and pregnancy. Front Biosci. 2011;3:690–700. doi: 10.2741/e279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson HP, Sweet EM, Adam AH. The accuracy of radiological estimates of gestational age using early fetal crown-rump length measurements by ultrasound as a basis for comparison. BJOG. 1979;86:525–528. doi: 10.1111/j.1471-0528.1979.tb10804.x. [DOI] [PubMed] [Google Scholar]
- Rylander L, Kallen B. Reproductive outcomes among hairdressers. Scan J Work Environ Health. 2005;31:212–217. doi: 10.5271/sjweh.871. [DOI] [PubMed] [Google Scholar]
- Savitz DA, Olshan AF, Gallagher K. Maternal occupation and pregnancy outcome. Epidemiology. 1996;7:269–274. doi: 10.1097/00001648-199605000-00009. [DOI] [PubMed] [Google Scholar]
- Silva LM, Jansen PW, Steegers EA, Jaddoe VW, Arends LR, Tiemeier H, Verhulst FC, Moll HA, Hofman A, Mackenbach JP, et al. Mother's educational level and fetal growth: the genesis of health inequalities. Int J Epidemiol. 2010;39:1250–1261. doi: 10.1093/ije/dyq069. [DOI] [PubMed] [Google Scholar]
- Snijder CA, Brouwers MM, Jaddoe VW, Hofman A, Roeleveld N, Burdorf A. Occupational exposure to endocrine disruptors and time to pregnancy among couples in a large birth cohort study: the Generation R Study. Fertil Steril. 2011;95:2067–2072. doi: 10.1016/j.fertnstert.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Statistics Netherlands. Dutch Standard Classification of Occupations (SBC) 1992. The Hague: Statistics Netherlands. [Google Scholar]
- Stillerman KP, Mattison DR, Giudice LC, Woodruff TJ. Environmental exposures and adverse pregnancy outcomes: a review of the science. Reprod Sci. 2008;15:631–650. doi: 10.1177/1933719108322436. [DOI] [PubMed] [Google Scholar]
- Thompson JM, Clark PM, Robinson E, Becroft DM, Pattison NS, Glavish N, Pryor JE, Wild CJ, Rees K, Mitchell EA. Risk factors for small-for-gestational age babies: the Auckland Birthweight collaborative study. J Paediatr Child Health. 2001;37:369–375. doi: 10.1046/j.1440-1754.2001.00684.x. [DOI] [PubMed] [Google Scholar]
- Twisk JW. Longitudinal data analysis. A comparison between generalized estimating equations and random coefficient analysis. Eur J Epidemiol. 2004;19:769–776. doi: 10.1023/b:ejep.0000036572.00663.f2. [DOI] [PubMed] [Google Scholar]
- Verburg BO, Steegers EA, De Ridder M, Snijders RJ, Smith E, Hofman A, Moll HA, Jaddoe VW, Witteman JC. New charts for ultrasound dating of pregnancy and assessment of fetal growth: longitudinal data from a population-based cohort study. Ultrasound Obstet Gynecol. 2008a;31:388–396. doi: 10.1002/uog.5225. [DOI] [PubMed] [Google Scholar]
- Verburg BO, Mulder PG, Hofman A, Jaddoe VW, Witteman JC, Steegers EA. Intra- and interobserver reproducibility study of early fetal growth parameters. Prenat Diagn. 2008b;28:323–331. doi: 10.1002/pd.1972. [DOI] [PubMed] [Google Scholar]
- Vrijheid M, Armstrong B, Dolk H, van Tongeren M, Botting B. Risk of hypospadias in relation to maternal occupational exposure to potential endocrine disrupting chemicals. Occup Environ Med. 2003;60:543–550. doi: 10.1136/oem.60.8.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijkotte TG, van der Wal MF, van Eijsden M, Bonsel GJ. First-trimester working conditions and birthweight: a prospective cohort study. Am J Public Health. 2009;99:1409–1416. doi: 10.2105/AJPH.2008.138412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weselak M, Arbuckle TE, Foster W. Pesticide exposures and developmental outcomes: the epidemiological evidence. J Toxicol Environ Health. 2007;10:41–80. doi: 10.1080/10937400601034571. [DOI] [PubMed] [Google Scholar]
- Wigle DT, Arbuckle TE, Turner MC, Berube A, Yang Q, Liu S, Krewski D. Epidemiologic evidence of relationships between reproductive and child health outcomes and environmental chemical contaminants. J Toxicol Environ Health. 2008;11:373–517. doi: 10.1080/10937400801921320. [DOI] [PubMed] [Google Scholar]
- Windham G, Fenster L. Environmental contaminants and pregnancy outcomes. Fertil Steril. 2008;89:111–116. doi: 10.1016/j.fertnstert.2007.12.041. [DOI] [PubMed] [Google Scholar]
- Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect. 2011;119:878–885. doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanney M, Marlow N. Paediatric consequences of fetal growth restriction. Semin Fetal Neonatal Med. 2004;9:411–418. doi: 10.1016/j.siny.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Ye X, Pierik FH, Hauser R, Duty S, Angerer J, Park MM, Burdorf A, Hofman A, Jaddoe VW, Mackenbach JP, et al. Urinary metabolite concentrations of organophosphorous pesticide, bisphenol-A, and phthalates among pregnant women in Rotterdam, the Netherlands: the Generation R study. Environ Res. 2008;108:260–267. doi: 10.1016/j.envres.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JL, Vestergaard M, Hjollund NH, Olsen J. Pregnancy outcomes among female hairdressers who participated in the Danish National Birth Cohort. Scan J Work Environ Health. 2006;32:61–66. doi: 10.5271/sjweh.977. [DOI] [PubMed] [Google Scholar]
- Zhu M, Fitzgerald EF, Gelberg KH, Lin S, Druschel CM. Maternal low-level lead exposure and fetal growth. Environ Health Perspect. 2010;118:1471–1475. doi: 10.1289/ehp.0901561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.