Abstract
BACKGROUND
Our aim was to study ways to improve IVF success rates in women with suspected endometrial receptivity defects.
METHODS
We conducted a retrospective cohort study examining the effect of letrozole (aromatase inhibitor) on integrin expression as a marker of endometrial receptivity. We compared IVF outcomes in 97 infertile women who had undergone ανβ3 integrin assessment by immunohistochemistry in mid-luteal endometrial biopsies. Of 79 women undergoing standard IVF, 29 (36.7%) lacked normal integrin expression. Eighteen other women with low integrin were studied after receiving letrozole during early IVF stimulation. An independent set of ανβ3 integrin-negative patients (n = 15) who had undergone repeat endometrial biopsy for integrin testing while taking letrozole were re-evaluated.
RESULTS
Clinical pregnancy and delivery rates were higher in women with normal ανβ3 integrin expression compared with those who were integrin negative [20/50 (40%) versus 4/29 (13.8%); P = 0.02 and 19/50 (38%) versus 2/29 (7%); P < 0.01, respectively]. In 18 women who received letrozole early in IVF, 11 conceived (61.1%; P < 0.001) compared with integrin-negative patients who did not receive letrozole. In integrin-negative women who were rebiopsied on letrozole, 66.7% reverted to normal integrin expression. Positive endometrial aromatase immunostaining using a polyclonal antibody was a common finding in infertile patients compared with controls.
CONCLUSIONS
Lack of endometrial ανβ3 integrin expression is associated with a poor prognosis for IVF that might be improved with letrozole co-treatment. Prospective studies are needed to confirm and extend these findings but the data suggest that aromatase expression may contribute to implantation failure in some women.
Keywords: female infertility, endometrial receptivity, endometriosis, IVF/ICSI outcome, implantation
Introduction
Implantation is a major determinant of success or failure for couples undergoing IVF and embryo transfer (IVF-ET) cycles. It is estimated that up to two-thirds of implantation failures are due to defects in endometrial receptivity (Achache and Revel, 2006). Across the USA and Puerto Rico, the overall implantation rate for IVF-ET remains around 29% and has not improved significantly over the past 5 years of reporting (www.sart.org). During this same interval, efforts to reduce multiple gestation rates have become widespread, first in European countries and now in the USA. High-order gestations are viewed as undesirable and put mother and offspring at risk (Grunfeld et al., 2008). Therefore, concerted effort toward reducing the number of embryos transferred while improving implantation rates has become a focus at our center and many others (Miller et al., 2005).
While overall improvement in IVF success over the past decades has been multi-factorial in nature, involving attention to patient selection, culture techniques and technical expertise, testing for uterine receptivity defects in women contemplating IVF has slowly gained momentum (Meyer et al., 1997; Donaghay and Lessey, 2007; Serafini et al., 2009). Correctable causes of implantation failure have been identified that are associated with endometrial integrin deficiency, such as communicating hydrosalpinges (Meyer et al., 1997; Bildirici et al., 2001; Savaris et al., 2006) and endometriosis (Lessey et al., 1994a,b). In cases of unexplained IVF failure, low integrin expression has been described (Tei et al., 2003), while positive integrin expression is predictive of future IVF success (Thomas et al., 2003a, b). In our practice, we routinely test for integrin status in those couples with unexplained infertility or suspected endometriosis, based on meta-analyses suggesting a decrease in IVF success associated with this disease (Barnhart et al., 2002).
GnRH agonist therapy has been shown to improve IVF outcome in women with endometriosis (Surrey et al., 2002) but in a small series Surrey et al., 2009 did not show improved pregnancy rates in integrin-negative patients. The enzyme P450 aromatase is the expression in the eutopic endometrium of women with endometriosis (Noble et al., 1996; Kitawaki et al., 1999) and endometrial aromatase expression has been associated with poor reproductive outcomes in IVF (Brosens et al., 2004). One of the actions of GnRH agonist is the suppression of endometrial aromatase expression (Ishihara et al., 2003). Letrozole is an orally active aromatase antagonist that has gained attention as an adjunct for fertility treatment, especially in women with ovulatory dysfunction and polycystic ovary syndrome (PCOS; Requena et al., 2008). In the present study, we retrospectively examined our own experience in IVF, in women who had undergone integrin testing to examine whether the suppression of the P450 aromatase enzyme improves outcomes in those women with suspected defects in endometrial receptivity.
Materials and Methods
Patients
All subjects included in this study had undergone a timed endometrial biopsy and were patients at the Fertility Center of the Carolinas, Greenville Hospital System (GHS), Greenville, South Carolina or the University of North Carolina at Chapel Hill. The use of endometrial tissue obtained from endometrial biopsy (EMB) for research and for the study of outcomes of pregnancy based on integrin testing was approved by the institutional review committee at GHS. The human subjects committee at the University of North Carolina had previously approved the use of endometrial tissues from fertile controls who were biopsied twice at the same time in the menstrual cycle. All of the studies described conform to that standards of the Declaration of Helsinki for Medical Research involving human subjects.
Letrozole treatment as part of IVF stimulation has been used for the past 4 years to increase follicle recruitment and implantation rates in poor prognosis patients. Each patient signed a consent form prior to the use of letrozole related to its use for ovulation induction. The use of letrozole in this study was not based on integrin testing, and all outcome data were compiled retrospectively.
Tissues
Timed endometrial biopsies were obtained using a Pipelle biopsy device (Cooper Surgical, Trumbull, CT, USA) after informed written consent, 8–10 days after positive urinary LH surge tests performed by subjects during unstimulated, natural menstrual cycles or cycles in which the patients had received letrozole, 5 mg on Days 5–9 of stimulation. Endometrial tissue was divided, with one portion fixed immediately in 10% buffered formalin and later embedded in paraffin for histological dating according to Noyes criteria (Noyes et al., 1950). The remaining was immediately snap frozen in liquid nitrogen, and stored until cryosectioned for immunohistochemical analysis of ανβ3 integrin expression.
Randomly assigned, timed EMB were also obtained from healthy, fertile subjects with regular menstrual cycles, as previously described (Murray et al., 2004). Samples were evaluated for integrin expression. Twenty-six women underwent EMB in two non-consecutive cycles within a 6-month period of time, assigned either to the proliferative phase or to a specific post-ovulatory day in the secretory phase using timed urinary LH-surge testing. Eight additional endometrial biopsies from women without endometriosis (proved by laparoscopsy) and 20 samples from the IVF group (10 with positive integrins and 10 with negative integrins) were used to compare aromatase expression by immunohistochemistry.
Immunohistochemistry
Immunostaining for ανβ3 was performed on endometrial biopsies on 7-μm cryosections using the Vectastain Elite ABC kits (Vector Laboratories, Burlingame, CA, USA) as previously described (Lessey et al., 1994a,b). In addition, in a subset of samples, immunohistochemistry was performed for P450 aromatase as previously reported (Kitaoka et al., 2004). Diaminobenzidine (Sigma, St. Louis, MO, USA) was used as the chromagen. Tissue sections were fixed in 4% paraformaldehyde for 15 min. Following a rinse in phosphate buffered saline (PBS), pH 7.4, endogenous peroxidase activity was quenched upon incubation for 30min with 0.3% H2O2 in absolute methanol, followed by a 6-min rehydration in PBS. Slides were then incubated with 0.4% Triton-X 100 for 10 min. After incubation with blocking serum for 30min at room temperature (4% normal goat serum), sections were incubated with SSA6, a mouse monoclonal antibody against human ανβ3 (1:2000; Lessey et al., 1992). Negative controls were analyzed on adjacent sections incubated without primary antibody. A PBS rinse was followed by treatment with a secondary antibody consisting of biotinylated goat anti-mouse IgG antibody for 30 min (Vector Laboratories). After this incubation, sections were washed and incubated with avidin:biotinylated horseradish peroxidase macromolecular complex for 60 min. Visualization of peroxidase was carried out by adding diaminobenzidine and incubated for 8 min to complete the reaction. As a final step, sections were counterstained with hematoxylin for 5 min, dehydrated in a graded series of ethanols and cleared with xylene. A coverslip was placed over Permount for evaluation by light microscopy. The resulting staining was evaluated using a Nikon microscope (Tokyo, Japan), by a single-blinded observer (B.A.L.) without the knowledge of the subject's identity.
Assessment of staining intensity and distribution was made using the semi-quantitative histologic score (HSCORE) system. HSCORE was calculated using the following equation:
where i is the intensity of staining with a value of 1, 2 or 3 (weak, moderate or strong, respectively) and Pi is the percentage of stained endometrial epithelial cells at each intensity, varying from 0 to 100%. Low intraobserver (r = 0.983; P < 0.0001) and interobserver (r = 0.994; P < 0.0001) differences for HSCORE in uterine tissues have been previously reported using this technique (Budwit-Novotny et al., 1986). The numerical cut-off for a negative result for the ανβ3 integrin was an HSCORE of ≤0.7, based on previous ROC analysis (Lessey et al., 1994a,b).
In vitro fertilization protocols
This retrospective study included 79 women who had undergone traditional IVF and 18 integrin-negative women who had received letrozole during the early stimulation for IVF. During IVF, subjects were stimulated with a long luteal protocol as previously reported (Miller et al., 2005). In cycles where letrozole was used, 5 mg was administered on Days 2–6 (gonadotrophins beginning on Day 3). Ovulation was triggered when lead follicles were 18 mm or greater. Embryo transfer was performed on Day 3 using SART recommendations regarding the number of transfer, unless poor embryo quality or prior IVF failure warranted transferring more embryos. The results of IVF success, defined as clinical pregnancy and delivery rates, were later correlated with the integrin expression status and the use of letrozole. Supervising physicians were not aware of the patient's integrin status during the process of IVF stimulation, egg retrieval and embryo transfer and the integrin status did not influence the number of embryos transferred or the medications used in IVF cycles.
Statistical analysis
Comparisons between groups for continuous and categorical variables were performed with independent t-test and χ2 test, respectively. Comparison of demographics and cycle characteristics during Phase I, between integrin-positive and -negative patients was performed using a Mann–Whitney non-parametric test. ANOVA with the Scheffe's correction was used to compare the differences in aromatase expression among the three groups (controls, IVF success and IVF failure).
Results
We examined IVF outcomes in 97 women who had undergone EMB with an integrin testing. Of these, 37 (38.1%) tested negative for the ανβ3 integrin. Twenty-nine women with absent integrins underwent traditional IVF and 18 had received letrozole during their early IVF stimulation. The demographics for these patients are shown in Table I. Women with normal integrin expression were not different from those who did not express the ανβ3 integrin in terms of age, BMI, total dose of gonadotrophins, peak endometrial thickness, peak estradiol levels, number of oocytes retrieved or fertilization rate. We did find a statistically significant difference in clinical pregnancy rates between integrin-positive and -negative women, with 20/50 (40%) integrin-positive women conceiving with traditional IVF compared with 4/29 (13.7%) for those who were ανβ3 negative (P = 0.02). Similarly, implantation rates (22.4 versus 8%; P = 0.01) and ongoing or delivered pregnancy rates (38 versus 7%; P = 0.003) were improved in the integrin-positive group relative to integrin-negative patients, respectively (Table II).
Table I.
Comparisons in different groups with or without letrozole treatment.
| Parameters, mean (SD) | Standard IVF |
IVF with letrozole (n = 18) | |
|---|---|---|---|
| Positive integrin (n = 50) | Negative integrin (n = 29) | ||
| Age (years) | 32.5 (4.5) | 34.4 (4.5) | 33.7 (3.0) |
| BMI (kg/m2) | 24.3 (5.0) | 23.6 (3.7) | 22.6 (2.4) |
| Gonadotrophins (units) | 2985 (1240) | 2985 (1245) | 2986 (1283) |
| Endometrial thickness (mm) | 10.89 (2.31) | 11.1 (3.28) | 10.16 (2.32) |
| Peak estradiol (pg/ml) | 2332 (1147)a | 2162 (1053) | 1660 (1112)a |
| No. oocytes retrieved | 15.76 (8.2) | 15.96 (8.1) | 15.89 (8.3) |
| Fertilization rate (%) | 62.2 (0.18) | 59.1 (0.17) | 59.6 (0.18) |
| No. embryos transferred | 2.32 (0.55) | 2.6 (0.78) | 2.11 (0.47) |
| Embryo gradeb | 1.66 (0.71)c | 1.92 (0.95) | 1.54 (0.7)c |
| Race (%) | |||
| Caucasian | 44 (86.2) | 22 (75.8) | 16 (88.8) |
| Asian | 2 (3.9) | 3 (10.3) | 2 (11.1) |
| African American | 1 (2) | 3 (10.3) | |
| Other | 4 (7.8) | 1 (3.4) | |
aP = 0.023, Mann–Whitney non-parametric testing.
bEmbryo grade using 1–5 scales.
cP = 0.08, Mann–Whitney non-parametric testing.
Table II.
Endometriosis and pregnancy outcome by defect type.
| Diagnosed with endometriosis (%) | Clinical pregnancy rate (%) | Implantation rate (%) | Ongoing/delivered pregnancy rate (%) | |
|---|---|---|---|---|
| Standard IVF normal (n = 50) | 33/50 (66.6) | 20/50 (40) | 26/116 (22.4) | 19/50 (38) |
| Standard IVF combined | 20/29 (69) | 4/29 (14)a | 6/74 (8) | 2/29 (7)b |
| Type I defect (n = 16) | 12/16 (75) | 1/16 (6.3)c | 3/40 (7.5) | 1/16 (6.25)d |
| Type II defect (n = 13) | 8/13 (62) | 3/13 (23.1) | 3/34 (8.8) | 1/13 (7.7) |
| Letrozole IVF combined | 16/18 (89) | 11/18 (61)e | 11/39 (28)f | 9/18 (50) |
| Type I defect (n = 10) | 8/10 (80) | 7/10 (70)b | 6/23 (26.1) | 5/10 (50) |
| Type II defect (n = 8) | 8/8 (100) | 4/8 (50) | 5/16 (31.3) | 4/8 (50) |
Type I defects refer to EMB that is lacking integrin expression because of delayed histology, while Type II defects represent samples that are histologically in phase between cycle Days 20 and 24 but lacking in integrin expression.
All statistical comparisons were made by χ2 testing.
aP = 0.02, combined (Types I and II) in standard IVF versus letrozole IVF.
bP = 0.001 for Type I defect letrozole IVF versus Type I standard IVF.
cP = 0.01, Type I standard IVF versus normal.
dP = 0.01, combined Type I standard IVF versus normaI.
eP < 0.001 for Types I and II combined in letrozole IVF versus combined Types I and II in standard IVF.
fP = 0.004, combined (Types I and II) letrozole IVF versus combined standard IVF.
Eighteen women with a negative integrin HSCORE (≤0.7) who received letrozole during early gonadotrophin stimulation (Days 2–6) in their IVF cycles were compared with those who did not receive letrozole. There was no difference in age, BMI, amount of gonadotrophin used, endometrial thickness, oocytes retrieved, fertilization rate or number of embryos transferred in this group, compared with those with normal integrin expression. In contrast, peak estradiol levels were significantly lower in the group who received letrozole (P = 0.023; Table I).
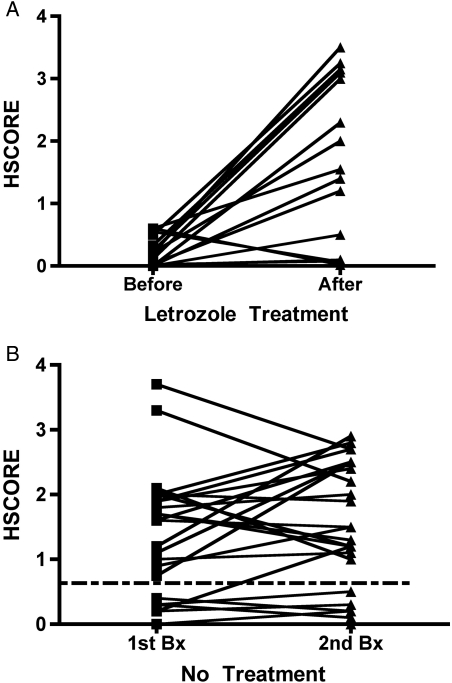
To begin to understand how letrozole given in the proliferative phase of stimulation might impact the secretory phase of ανβ3 integrin expression, we examined the biopsy results of 15 other women with a negative ανβ3 integrin HSCORE who underwent a second, non-consecutive biopsy procedure after taking letrozole in the proliferative phase. As shown in Fig. 1, 10 of 15 women (66.7%) corrected their negative integrin test after receiving letrozole, with a mean HSCORE of 1.66 ± 1.4 (SD) compared with those with a score of 0.07 ± 0.19 before treatment (P < 0.01). The characteristics of these patients are shown in Table III. Of note, BMI was significantly lower in those who corrected (P = 0.04). The absence of integrin expression must be interpreted in the context of histologic dating as previously defined (Lessey et al., 1994b). The proportion of absent integrins that were Type I (histologically out of phase) and Type II defects (histologically in the phase relative to the time of biopsy, but absent integrins) that corrected was similar, as was the prevalence of known endometriosis (80%) in both groups.
Figure 1.
(A) The effect of oral letrozole on endometrial integrin expression was studied in women with a negative HSCORE for the ανβ3 integrin. Integrin assessment was compared in the tissue of women before or after taking 5 mg of letrozole on cycle Days 5–9. A HSCORE cut-off for a negative test is shown by the dotted line (≤0.7) based on earlier analysis (Lessey et al., 1994b). (B) Reproducibility of integrin testing was examined in mid-luteal endometrial biopsies in normal controls in two separate menstrual cycles. HSCORE (0–4) was compared in the first and second biopsies performed at the same time in the proliferative or secretory phase in the same fertile volunteer.
Table III.
Characteristics of the integrin-negative, letrozole-treated subjects in mid-secretory phase endometrial biopsies.
| Characteristic | Correction (n = 10) | No correction (n = 5) | P value |
|---|---|---|---|
| Age (mean ± SD) | 34.7 (5.4) | 32.33 (3.2) | NS |
| BMI | 22.9 (3.2) | 27.5 (3.2) | 0.04 |
| Proportion I/II defects (%) | 5/5 (50) | 3/5 (60) | NS |
| Nulliparity (%) | 80 | 80 | NS |
| Endometriosis (%) | 80 | 80 | NS |
| HSCORE (mean ± SD) | 2.49 (0.89) | 0 | 0.001 |
All statistical comparisons were made using independent t-tests.
To demonstrate the reproducibility of integrin testing, we studied the integrin testing results (HSCORE) in fertile volunteers who had undergone two consecutive endometrial biopsies in either the proliferative or secretory phase as part of an earlier study (Murray et al., 2004). One of 26 (3.8%) fertile controls had a discordant integrin result in repeat biopsy (Fig. 1B), suggesting that repeat biopsy at the same time in the cycle in the same woman provides reproducible results for integrin testing.
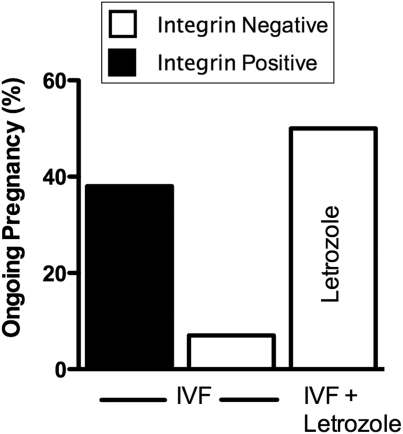
To further clarify the significance of a negative integrin test, we calculated the proportion of ‘in-phase’ (Type II defects) and ‘out-of-phase’ (Type I defects) biopsies and examined the correlation with a diagnosis of endometriosis. As shown in Table II, the proportion of women with endometriosis was similar in each group, reflecting our bias toward integrin testing in women with otherwise unexplained infertility or a history of endometriosis. In the group who received letrozole, 80% of Type I patients and 100% of Type II defect patients had endometriosis. Eleven of these 18 integrin-negative women successfully conceived (61.1%; P < 0.001, comparing integrin-negative patients who did not receive letrozole; Fig. 2). Interestingly, only 1/16 (6.3%) of the Type I defect patients successfully conceived without letrozole, with ongoing or delivered pregnancy rates similar to that of the Type II patients (1/13; 7.6%). With letrozole treatment, 7/10 (70%) and 4/8 (50%) of Type I and II patients successfully conceived, respectively. There was a trend toward improved embryo grade in the letrozole treatment group (P = 0.08).
Figure 2.
Ongoing pregnancy rate in women undergoing IVF with positive (black) or negative integrins (white). In standard IVF protocols women with a negative integrin test had a significantly worse outcome than those who tested positive (P < 0.02). In integrin-negative women who underwent IVF with letrozole (2.5–5 mg/day on Days 2–6), outcomes were similar to integrin-positive women in non-letrozole cycles.
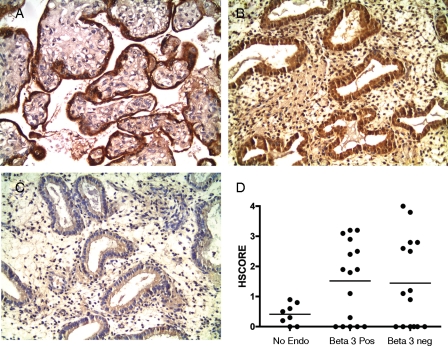
To determine if P450 aromatase was present in this population of women, we performed immunohistochemistry for P450 aromatase in a subset of endometrial samples including 10 women who conceived with normal integrin expression, compared with 10 women who failed IVF with absent integrin immunostaining with 8 controls known to be free of endometriosis. Compared with normal controls (Fig. 3B), aromatase expression was common in the eutopic endometrium of these women (Fig. 3C), present in about half of the patients in both groups, compared with low expression in controls (Fig. 3D).
Figure 3.
The level of p450 aromatase expression was studied in women with and without endometriosis using immunohistochemistry as described in the Materials and methods section. Placenta served as a positive control (A). In women without endometriosis, aromatase expression was low (B), while in those with endometriosis it is generally increased (C), independent of IVF outcome or integrin status. Women without endometriosis (D, controls) did not exhibit detectable aromatase expression, compared with the IVF patients in this study.
Discussion
A proportion of infertility and recurrent pregnancy loss can be attributed to defects in endometrial receptivity (de los Santos et al., 2003; Donaghay and Lessey, 2007; Tapia et al., 2008). Up to half of all unsuccessful IVF cycles may be due to implantation failure (de los Santos et al., 2003) with diminished embryo quality taking on greater importance in older women. With the availability of specific mono- and polyclonal antibodies to potential biomarkers, immunohistochemistry has supplanted histologic dating alone and remains an active area of research on endometrial receptivity (Lessey et al., 1994a,b; Kliman et al., 2006; Wang et al., 2008).
We and others have reported that biomarkers of endometrial receptivity may predict patients who are at increased risk for endometriosis, hydrosalpinges and implantation failure (Lessey et al., 1994a,b; Nip et al., 1995; Miller et al., 1996; Meyer et al., 1997; Van Voorhis and Stovall, 1997; Thomas et al., 2003a,b). Testing for endometrial receptivity defects prior to IVF has received limited attention with only a few studies finding reduced integrin expression in women with IVF failure (Tei et al., 2003; Thomas et al., 2003a,b). In the present study, we retrospectively examined the relationship between ανβ3 integrin and IVF outcomes in women who did or did not receive letrozole treatment during IVF cycle stimulation. Women undergoing IVF with low integrin expression had a significantly reduced pregnancy rate and implantation rate compared with women with normal integrin expression, although the number of eggs retrieved and fertilization rates were similar. This effect was the same for both ‘in-phase’ (Type II defects) as well as ‘out-of-phase’ (Type I defects) histology. All three types (I, II and normal integrin expression) had a high prevalence of endometriosis, reflecting a bias toward integrin testing in unexplained infertility and endometriosis patients. In those women who underwent IVF with low integrin expression who received letrozole, the pregnancy rate was similar to women with normal integrin expression. Finally, endometrial aromatase expression appears to be elevated in women with endometriosis, in general, but did not correlate with IVF outcome or integrin expression by itself.
IVF appears to be a good model to study factors that affect implantation (Margalioth et al., 2006) and according to one prior meta-analysis, endometriosis contributes to IVF failure (Barnhart et al., 2002). While the endometrium of women with infertility and endometriosis can be fundamentally different from normal fertile women (Kao et al., 2003; Talbi et al., 2006; Burney et al., 2007), not all women with endometriosis lack integrin expression (Lessey et al., 1994a,b), nor are they all infertile (American Society of Reproductive Medicine, 2006).
Inadequate progesterone or secondary progesterone resistance has been described in the eutopic endometrium of some women with endometriosis, contributing to persistent mid-luteal estrogen or progesterone receptors (Lessey et al., 1996; Lessey, 2003). In addition, endometriosis is associated with increased aromatase expression (Kitawaki et al., 1999). Brosens et al. (2004) reported an association between unexpected IVF failure and endometrial aromatase expression by RT–PCR. Pretreatment with GnRH agonist have been shown to improve IVF outcomes (Lessey, 2000), perhaps in part by reduction in aromatase expression (Ishihara et al., 2003). Herein, we confirm the expression of aromatase protein by immunohistochemistry in a population of women with a high prevalence of endometriosis undergoing IVF, consistent with prior reports (Kitawaki et al., 1999), but did not find that protein expression itself was related to IVF success or failure. Studies are ongoing to compare integrin expression and aromatase expression in infertility and pregnancy loss.
Letrozole has previously been reported to be a medical treatment for endometriosis (Ailawadi et al., 2004; Razzi et al., 2004; Mousa et al., 2007). This drug is a direct aromatase antagonist and has found a niche for ovulation induction, especially in women with PCOS,and in IVF patients who are low responders (Goswami et al., 2004; Mitwally and Casper, 2004; Garcia-Velasco et al., 2005; Bedaiwy et al., 2006; Verpoest et al., 2006; Garcia-Velasco et al., 2008; Schoolcraft et al., 2008; Yarali et al., 2009). While pregnancy rates appear similar in letrozole cycles, cancellation rates (Ozmen et al., 2009) and miscarriage rates (Lee et al., 2011) are reduced in IVF cycles where letrozole was added. To date no studies have reported the use of letrozole to specifically improve endometrial receptivity during IVF in women who lack ανβ3 integrin expression.
In addition to a lower peak estradiol level, we found little difference in the cycle parameters of patients receiving letrozole compared with those who did not take this medication. Based on the findings that letrozole improved integrin expression along with pregnancy and implantation rates, it might be a useful adjunct therapy during IVF protocols, especially in women with unexplained IVF failure and/or endometriosis. Given that aromatase expression itself did not correlate with IVF outcome, integrin expression may be the better predictor of IVF outcome. Why some women with aromatase expression succeed while others fail, remains unanswered. As letrozole appears to restore integrin expression in those with low expression, integrin-negative patients may represent the subset in which aromatase inhibitors have a greater impact.
We have previously reported that loss of integrin expression is seen in milder cases of endometriosis (Lessey et al., 1994a,b), while aromatase expression has been reported to be diagnostic of endometriosis, in general (Kitawaki et al., 1999). Others have reported an increase in eutopic endometrial aromatase expression in patients with active disease (Aghajanova et al., 2009). A key regulator of the ανβ3 integrin is the transcription factor, HOXA10 (Daftary et al., 2002), which is also reduced in mild endometriosis (Szczepanska et al., 2010). Letrozole treatment has been shown to increase ανβ3 expression, compared with clomiphene citrate in the rat uterus model (Bao et al., 2009). The increase in integrin expression in response to letrozole in natural cycles, reflects the improved implantation and pregnancy rates noted during IVF in patients who took letrozole. Inhibition of aberrant aromatase expression in women with endometriosis likely changes the intracellular balance between estrogen and progesterone action, and thus could restore the ability of progesterone to effectively down-regulate estrogen receptors, a phenomenon that appears to be universal at the time of implantation in most mammals studied (Donaghay and Lessey, 2007). Since the ανβ3 integrin is indirectly regulated by progesterone, this is the most plausible explanation. Further prospective studies will be needed to confirm these results and to better understand how letrozole improves endometrial receptivity in the setting of IVF.
Conclusion
We report an association between low pregnancy rates in IVF and an abnormal integrin expression by EMB obtained in a natural cycle. A lack of integrin expression was highly associated with endometriosis. Unexplained IVF failure in a subset of women with endometriosis may be avoidable using a simple 5-day treatment of the aromatase inhibitor, letrozole. Recognition of the importance of undiagnosed endometriosis in women with IVF failure (Littman et al., 2005), recurrent pregnancy loss or infertility (Donaghay and Lessey, 2007), offers enhanced opportunities to treat patients with suspected implantation failure. Based on our findings and previous studies in IVF (Brosens et al., 2004), the use aromatase inhibitors might improve the IVF success rates in a subset of women with endometriosis. Further, integrin testing may be indicated in women with unexplained infertility or mild endometriosis to better define the risk for unanticipated implantation failure with ART.
Authors' roles
P.B.M. and B.A.L. were involved in the concept and oversight of data collection, interpretation and drafting the manuscript. D.A.F. was involved in the interpretation of data, writing of the manuscript and critical analysis of the data with P.B.M. and B.A.L. B.A.L. also provided laboratory oversight for the immunohistochemical studies. J.K. provided substantial material and technical support, provided critical oversight in drafting the manuscript and consultation regarding aromatase expression. B.A.P., G.B. and N.T. were involved in data collection and analysis and immunohistochemical staining along with our statistician, H.L.H.
Funding
This study was supported from the NICHD/NIH grant that provided support for reagents and technical procedures including immunohistochemistry as part of these studies and salary for one of the authors (B.A.L.).
Conflict of interest
B.A.L. is the inventor of licensed intellectual property owned by University of Pennsylvania.
Acknowledgements
This study was supported by the Eunice Kennedy Shriver NICHD/NIH through cooperative agreement U54-HD35041-12 (B.A.L.) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research. We would like to acknowledge the outstanding technical assistance of Angela Houwing.
References
- Achache H, Revel A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum Reprod Update. 2006;12:731–746. doi: 10.1093/humupd/dml004. doi:10.1093/humupd/dml004. [DOI] [PubMed] [Google Scholar]
- Aghajanova L, Hamilton A, Kwintkiewicz J, Vo KC, Giudice LC. Steroidogenic enzyme and key decidualization marker dysregulation in endometrial stromal cells from women with versus without endometriosis. Biol Reprod. 2009;80:105–114. doi: 10.1095/biolreprod.108.070300. doi:10.1095/biolreprod.108.070300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ailawadi RK, Jobanputra S, Kataria M, Gurates B, Bulun SE. Treatment of endometriosis and chronic pelvic pain with letrozole and norethindrone acetate: a pilot study. Fertil Steril. 2004;81:290–296. doi: 10.1016/j.fertnstert.2003.09.029. doi:10.1016/j.fertnstert.2003.09.029. [DOI] [PubMed] [Google Scholar]
- American Society of Reproductive Medicine. Endometriosis and infertility. Fertil Steril. 2006;86:S156–S160. doi: 10.1016/j.fertnstert.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Bao SH, Sheng SL, Peng YF, Lin QD. Effects of letrozole and clomiphene citrate on the expression of HOXA10 and integrin alpha v beta 3 in uterine epithelium of rats. Fertil Steril. 2009;91:244–248. doi: 10.1016/j.fertnstert.2007.11.024. doi:10.1016/j.fertnstert.2007.11.024. [DOI] [PubMed] [Google Scholar]
- Barnhart KT, Dunsmoor R, Coutifaris C. The effect of endometriosis on in vitro fertilization. Fertil Steril. 2002;77:1148–1155. doi: 10.1016/s0015-0282(02)03112-6. doi:10.1016/S0015-0282(02)03112-6. [DOI] [PubMed] [Google Scholar]
- Bedaiwy MA, Forman R, Mousa NA, Al Inany HG, Casper RF. Cost-effectiveness of aromatase inhibitor co-treatment for controlled ovarian stimulation. Hum Reprod. 2006;21:2838–2844. doi: 10.1093/humrep/del273. doi:10.1093/humrep/del273. [DOI] [PubMed] [Google Scholar]
- Bildirici I, Bukulmez O, Ensari A, Yarali H, Gurgan T. A prospective evaluation of the effect of salpingectomy on endometrial receptivity in cases of women with communicating hydrosalpinges. Hum Reprod. 2001;16:2422–2426. doi: 10.1093/humrep/16.11.2422. [DOI] [PubMed] [Google Scholar]
- Brosens J, Verhoeven H, Campo R, Gianaroli L, Gordts S, Hazekamp J, Hagglund L, Mardesic T, Varila E, Zech J, et al. High endometrial aromatase P450 mRNA expression is associated with poor IVF outcome. Hum Reprod. 2004;19:352–356. doi: 10.1093/humrep/deh075. doi:10.1093/humrep/deh075. [DOI] [PubMed] [Google Scholar]
- Budwit-Novotny DA, McCarty KS, Sr, Cox EB, Soper JR, Mutch DG, Creasman WT, Flowers JL, McCarty KS., Jr Immunohistochemical analyses of estrogen receptor in endometrial adenocarcinoma using a monoclonal antibody. Cancer Res. 1986;46:5419–5425. [PubMed] [Google Scholar]
- Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148:3814–3826. doi: 10.1210/en.2006-1692. doi:10.1210/en.2006-1692. [DOI] [PubMed] [Google Scholar]
- Daftary GS, Troy PJ, Bagot CN, Young SL, Taylor HS. Direct regulation of beta3-integrin subunit gene expression by HOXA10 in endometrial cells. Mol Endocrinol. 2002;16:571–579. doi: 10.1210/mend.16.3.0792. doi:10.1210/me.16.3.571. [DOI] [PubMed] [Google Scholar]
- de los Santos MJ, Mercader A, Galan A, Albert C, Romero JL, Pellicer A. Implantation rates after two, three, or five days of embryo culture. Placenta. 2003;24(Suppl. B):S13–S19. doi: 10.1016/s0143-4004(03)00172-3. doi:10.1016/S0143-4004(03)00172-3. [DOI] [PubMed] [Google Scholar]
- Donaghay M, Lessey BA. Uterine receptivity: alterations associated with benign gynecological disease. Semin Reprod Med. 2007;25:461–475. doi: 10.1055/s-2007-991044. doi:10.1055/s-2007-991044. [DOI] [PubMed] [Google Scholar]
- Garcia-Velasco JA, Moreno L, Pacheco A, Guillen A, Duque L, Requena A, Pellicer A. The aromatase inhibitor letrozole increases the concentration of intraovarian androgens and improves in vitro fertilization outcome in low responder patients: a pilot study. Fertil Steril. 2005;84:82–87. doi: 10.1016/j.fertnstert.2005.01.117. doi:10.1016/j.fertnstert.2005.01.117. [DOI] [PubMed] [Google Scholar]
- Garcia-Velasco JA, Quea G, Piro M, Mayoral M, Ruiz M, Toribio M, Requena A. Letrozole administration during the luteal phase after ovarian stimulation impacts corpus luteum function: a randomized, placebo-controlled trial. Fertil Steril. 2008;92:222–225. doi: 10.1016/j.fertnstert.2008.04.042. [DOI] [PubMed] [Google Scholar]
- Goswami SK, Das T, Chattopadhyay R, Sawhney V, Kumar J, Chaudhury K, Chakravarty BN, Kabir SN. A randomized single-blind controlled trial of letrozole as a low-cost IVF protocol in women with poor ovarian response: a preliminary report. Hum Reprod. 2004;19:2031–2035. doi: 10.1093/humrep/deh359. doi:10.1093/humrep/deh359. [DOI] [PubMed] [Google Scholar]
- Grunfeld L, Luna M, Mukherjee T, Sandler B, Nagashima Y, Copperman AB. Redefining in vitro fertilization success: should triplets be considered failures? Fertil Steril. 2008;90:1064–1068. doi: 10.1016/j.fertnstert.2007.07.1360. doi:10.1016/j.fertnstert.2007.07.1360. [DOI] [PubMed] [Google Scholar]
- Ishihara H, Kitawaki J, Kado N, Koshiba H, Fushiki S, Honjo H. Gonadotropin-releasing hormone agonist and danazol normalize aromatase cytochrome P450 expression in eutopic endometrium from women with endometriosis, adenomyosis, or leiomyomas. Fertil Steril. 2003;79(Suppl. 1):735–742. doi: 10.1016/s0015-0282(02)04813-6. doi:10.1016/S0015-0282(02)04813-6. [DOI] [PubMed] [Google Scholar]
- Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, Osteen K, Lessey BA, Giudice LC. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144:2870–2881. doi: 10.1210/en.2003-0043. doi:10.1210/en.2003-0043. [DOI] [PubMed] [Google Scholar]
- Kitaoka Y, Kitawaki J, Koshiba H, Inoue S, Ishihara H, Teramoto M, Honjo H. Aromatase cytochrome P450 and estrogen and progesterone receptors in uterine sarcomas: correlation with clinical parameters. J Steroid Biochem Mol Biol. 2004;88:183–189. doi: 10.1016/j.jsbmb.2003.11.013. doi:10.1016/j.jsbmb.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Kitawaki J, Kusuki I, Koshiba H, Tsukamoto K, Honjo H. Detection of aromatase cytochrome P-450 in endometrial biopsy specimens as a diagnostic test for endometriosis. Fertil Steril. 1999;72:1100–1106. doi: 10.1016/s0015-0282(99)00424-0. doi:10.1016/S0015-0282(99)00424-0. [DOI] [PubMed] [Google Scholar]
- Kliman HJ, Honig S, Walls D, Luna M, McSweet JC, Copperman AB. Optimization of endometrial preparation results in a normal endometrial function test (EFT) and good reproductive outcome in donor ovum recipients. J Assist Reprod Genet. 2006;23:299–303. doi: 10.1007/s10815-006-9061-1. doi:10.1007/s10815-006-9061-1. [DOI] [PubMed] [Google Scholar]
- Lee VC, Chan CC, Ng EH, Yeung WS, Ho PC. Sequential use of letrozole and gonadotrophin in women with poor ovarian reserve: a randomized controlled trial. Reprod Biomed Online. 2011;23:380–388. doi: 10.1016/j.rbmo.2011.05.012. doi:10.1016/j.rbmo.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Lessey BA. Medical management of endometriosis and infertility. Fertil Steril. 2000;73:1089–1096. doi: 10.1016/s0015-0282(00)00519-7. doi:10.1016/S0015-0282(00)00519-7. [DOI] [PubMed] [Google Scholar]
- Lessey BA. Two pathways of progesterone action in the human endometrium: implications for implantation and contraception. Steroids. 2003;68:809–815. doi: 10.1016/j.steroids.2003.09.004. doi:10.1016/j.steroids.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Lessey BA, Damjanovich L, Coutifaris C, Castelbaum A, Albelda SM, Buck CA. Integrin adhesion molecules in the human endometrium. Correlation with the normal and abnormal menstrual cycle. J Clin Invest. 1992;90:188–195. doi: 10.1172/JCI115835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessey BA, Castelbaum AJ, Buck CA, Lei Y, Yowell CW, Sun J. Further characterization of endometrial integrins during the menstrual cycle and in pregnancy. Fertil Steril. 1994a;62:497–506. [PubMed] [Google Scholar]
- Lessey BA, Castelbaum AJ, Sawin SW, Buck CA, Schinnar R, Bilker W, Strom BL. Aberrant integrin expression in the endometrium of women with endometriosis. J Clin Endocrinol Metab. 1994b;79:643–649. doi: 10.1210/jcem.79.2.7519194. doi:10.1210/jc.79.2.643. [DOI] [PubMed] [Google Scholar]
- Lessey BA, Yeh I, Castelbaum AJ, Fritz MA, Ilesanmi AO, Korzeniowski P, Sun J, Chwalisz K. Endometrial progesterone receptors and markers of uterine receptivity in the window of implantation. Fertil Steril. 1996;65:477–483. [PubMed] [Google Scholar]
- Littman E, Giudice L, Lathi R, Berker B, Milki A, Nezhat C. Role of laparoscopic treatment of endometriosis in patients with failed in vitro fertilization cycles. Fertil Steril. 2005;84:1574–1578. doi: 10.1016/j.fertnstert.2005.02.059. doi:10.1016/j.fertnstert.2005.02.059. [DOI] [PubMed] [Google Scholar]
- Margalioth EJ, Ben-Chetrit A, Gal M, Eldar-Geva T. Investigation and treatment of repeated implantation failure following IVF-ET. Hum Reprod. 2006;21:3036–3043. doi: 10.1093/humrep/del305. doi:10.1093/humrep/del305. [DOI] [PubMed] [Google Scholar]
- Meyer WR, Castelbaum AJ, Somkuti S, Sagoskin AW, Doyle M, Harris JE, Lessey BA. Hydrosalpinges adversely affect markers of endometrial receptivity. Hum Reprod. 1997;12:1393–1398. doi: 10.1093/humrep/12.7.1393. doi:10.1093/humrep/12.7.1393. [DOI] [PubMed] [Google Scholar]
- Miller KA, Deaton JL, Pittaway DE. Evaluation of serum CA 125 concentrations as predictors of pregnancy with human in vitro fertilization. Fertil Steril. 1996;65:1184. doi: 10.1016/s0015-0282(16)58336-8. [DOI] [PubMed] [Google Scholar]
- Miller PB, Forstein DA, Usadi RS, Lessey BA, Higdon HL, III, Boone WR. Assisted reproductive technology (ART) in the upstate: reducing the risks of multiple births. J S C Med Assoc. 2005;101:373–377. [PubMed] [Google Scholar]
- Mitwally MF, Casper RF. Aromatase inhibition reduces the dose of gonadotropin required for controlled ovarian hyperstimulation. J Soc Gynecol Investig. 2004;11:406–415. doi: 10.1016/j.jsgi.2004.03.006. doi:10.1016/j.jsgi.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Mousa NA, Bedaiwy MA, Casper RF. Aromatase inhibitors in the treatment of severe endometriosis. Obstet Gynecol. 2007;109:1421–1423. doi: 10.1097/01.AOG.0000265807.19397.6d. doi:10.1097/01.AOG.0000265807.19397.6d. [DOI] [PubMed] [Google Scholar]
- Murray MJ, Meyer WR, Zaino RJ, Lessey BA, Novotny DB, Ireland K, Zeng D, Fritz MA. A critical analysis of the accuracy, reproducibility, and clinical utility of histologic endometrial dating in fertile women. Fertil Steril. 2004;81:1333–1343. doi: 10.1016/j.fertnstert.2003.11.030. doi:10.1016/j.fertnstert.2003.11.030. [DOI] [PubMed] [Google Scholar]
- Nip MMC, Taylor PV, Rutherford AJ, Hancock KW. Autoantibodies and antisperm antibodies in sera and follicular fluids of infertile patients; relation to reproductive outcome after in-vitro fertilization. Hum Reprod. 1995;10:2564. doi: 10.1093/oxfordjournals.humrep.a135746. [DOI] [PubMed] [Google Scholar]
- Noble LS, Simpson ER, Johns A, Bulun SE. Aromatase expression in endometriosis. J Clin Endocrinol Metab. 1996;81:174–179. doi: 10.1210/jcem.81.1.8550748. doi:10.1210/jc.81.1.174. [DOI] [PubMed] [Google Scholar]
- Noyes RW, Hertig AI, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3–25. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- Ozmen B, Sonmezer M, Atabekoglu CS, Olmus H. Use of aromatase inhibitors in poor-responder patients receiving GnRH antagonist protocols. Reprod Biomed Online. 2009;19:478–485. doi: 10.1016/j.rbmo.2009.05.007. doi:10.1016/j.rbmo.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Razzi S, Fava A, Sartini A, De Simone S, Cobellis L, Petraglia F. Treatment of severe recurrent endometriosis with an aromatase inhibitor in a young ovariectomised woman. BJOG. 2004;111:182–184. doi: 10.1046/j.1471-0528.2003.00038.x. doi:10.1046/j.1471-0528.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- Requena A, Herrero J, Landeras J, Navarro E, Neyro JL, Salvador C, Tur R, Callejo J, Checa MA, Farre M, et al. Use of letrozole in assisted reproduction: a systematic review and meta-analysis. Hum Reprod Update. 2008;14:571–582. doi: 10.1093/humupd/dmn033. doi:10.1093/humupd/dmn033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savaris RF, Pedrini JL, Flores R, Fabris G, Zettler CG. Expression of alpha 1 and beta 3 integrins subunits in the endometrium of patients with tubal phimosis or hydrosalpinx. Fertil Steril. 2006;85:188–192. doi: 10.1016/j.fertnstert.2005.06.039. doi:10.1016/j.fertnstert.2005.06.039. [DOI] [PubMed] [Google Scholar]
- Schoolcraft WB, Surrey ES, Minjarez DA, Stevens JM, Gardner DK. Management of poor responders: can outcomes be improved with a novel gonadotropin-releasing hormone antagonist/letrozole protocol? Fertil Steril. 2008;89:151–156. doi: 10.1016/j.fertnstert.2007.02.013. doi:10.1016/j.fertnstert.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Serafini PC, Silva ID, Smith GD, Motta EL, Rocha AM, Baracat EC. Endometrial claudin-4 and leukemia inhibitory factor are associated with assisted reproduction outcome. Reprod Biol Endocrinol. 2009;7:30. doi: 10.1186/1477-7827-7-30. doi:10.1186/1477-7827-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surrey ES, Silverberg KM, Surrey MW, Schoolcraft WB. Effect of prolonged gonadotropin-releasing hormone agonist therapy on the outcome of in vitro fertilization-embryo transfer in patients with endometriosis. Fertil Steril. 2002;78:699–704. doi: 10.1016/s0015-0282(02)03373-3. doi:10.1016/S0015-0282(02)03373-3. [DOI] [PubMed] [Google Scholar]
- Surrey ES, Lietz AK, Gustofson RL, Minjarez DA, Schoolcraft WB. Does endometrial integrin expression in endometriosis patients predict enhanced in vitro fertilization cycle outcomes after prolonged GnRH agonist therapy? Fertil Steril. 2009;93:649–651. doi: 10.1016/j.fertnstert.2008.12.023. [DOI] [PubMed] [Google Scholar]
- Szczepanska M, Wirstlein P, Luczak M, Jagodzinski PP, Skrzypczak J. Reduced expression of HOXA10 in the midluteal endometrium from infertile women with minimal endometriosis. Biomed Pharmacother. 2010;64:697–705. doi: 10.1016/j.biopha.2010.09.012. doi:10.1016/j.biopha.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA, et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology. 2006;147:1097–1121. doi: 10.1210/en.2005-1076. doi:10.1210/en.2005-1076. [DOI] [PubMed] [Google Scholar]
- Tapia A, Gangi LM, Zegers-Hochschild F, Balmaceda J, Pommer R, Trejo L, Pacheco IM, Salvatierra AM, Henriquez S, Quezada M, et al. Differences in the endometrial transcript profile during the receptive period between women who were refractory to implantation and those who achieved pregnancy. Hum Reprod. 2008;23:340–351. doi: 10.1093/humrep/dem319. doi:10.1093/humrep/dem319. [DOI] [PubMed] [Google Scholar]
- Tei C, Maruyama T, Kuji N, Miyazaki T, Mikami M, Yoshimura Y. Reduced expression of alphavbeta3 integrin in the endometrium of unexplained infertility patients with recurrent IVF-ET failures: improvement by danazol treatment. J Assist Reprod Genet. 2003;20:13–20. doi: 10.1023/A:1021254620888. doi:10.1023/A:1021254620888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K, Thomson A, Wood S, Kingsland C, Vince G, Lewis-Jones I. Endometrial integrin expression in women undergoing in vitro fertilization and the association with subsequent treatment outcome. Fertil Steril. 2003a;80:502–507. doi: 10.1016/s0015-0282(03)00792-1. doi:10.1016/S0015-0282(03)00792-1. [DOI] [PubMed] [Google Scholar]
- Thomas K, Thomson AJ, Wood SJ, Kingsland CR, Vince G, Lewis-Jones DI. Endometrial integrin expression in women undergoing IVF and ICSI: a comparison of the two groups and fertile controls. Hum Reprod. 2003b;18:364–369. doi: 10.1093/humrep/deg104. doi:10.1093/humrep/deg104. [DOI] [PubMed] [Google Scholar]
- Van Voorhis BJ, Stovall DW. Autoantibodies and infertility: a review of the literature. J Reprod Immunol. 1997;33:239. doi: 10.1016/s0165-0378(97)00025-9. [DOI] [PubMed] [Google Scholar]
- Verpoest WM, Kolibianakis E, Papanikolaou E, Smitz J, Van Steirteghem A, Devroey P. Aromatase inhibitors in ovarian stimulation for IVF/ICSI: a pilot study. Reprod Biomed Online. 2006;13:166–172. doi: 10.1016/s1472-6483(10)60611-6. doi:10.1016/S1472-6483(10)60611-6. [DOI] [PubMed] [Google Scholar]
- Wang B, Sheng JZ, He RH, Qian YL, Jin F, Huang HF. High expression of L-selectin ligand in secretory endometrium is associated with better endometrial receptivity and facilitates embryo implantation in human being. Am J Reprod Immunol. 2008;60:127–134. doi: 10.1111/j.1600-0897.2008.00604.x. doi:10.1111/j.1600-0897.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- Yarali H, Esinler I, Polat M, Bozdag G, Tiras B. Antagonist/letrozole protocol in poor ovarian responders for intracytoplasmic sperm injection: a comparative study with the microdose flare-up protocol. Fertil Steril. 2009;92:231–235. doi: 10.1016/j.fertnstert.2008.04.057. doi:10.1016/j.fertnstert.2008.04.057. [DOI] [PubMed] [Google Scholar]