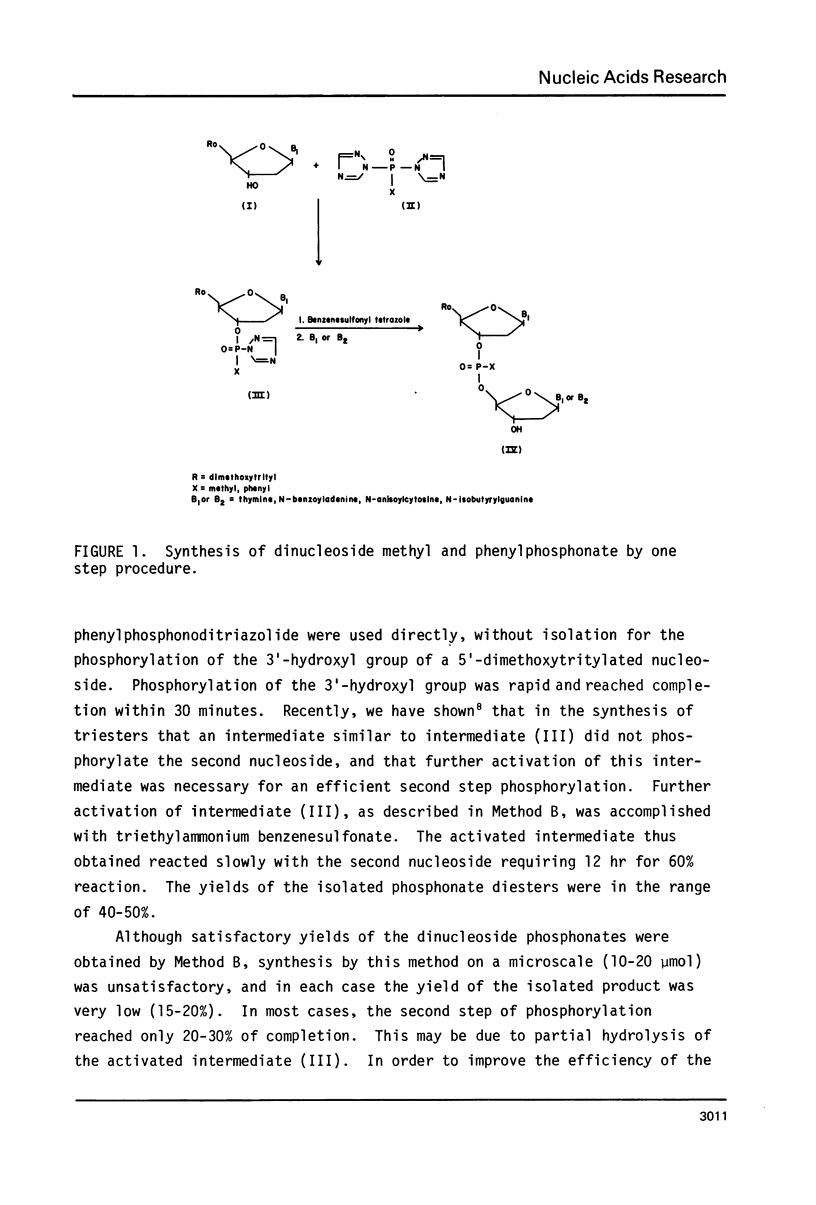
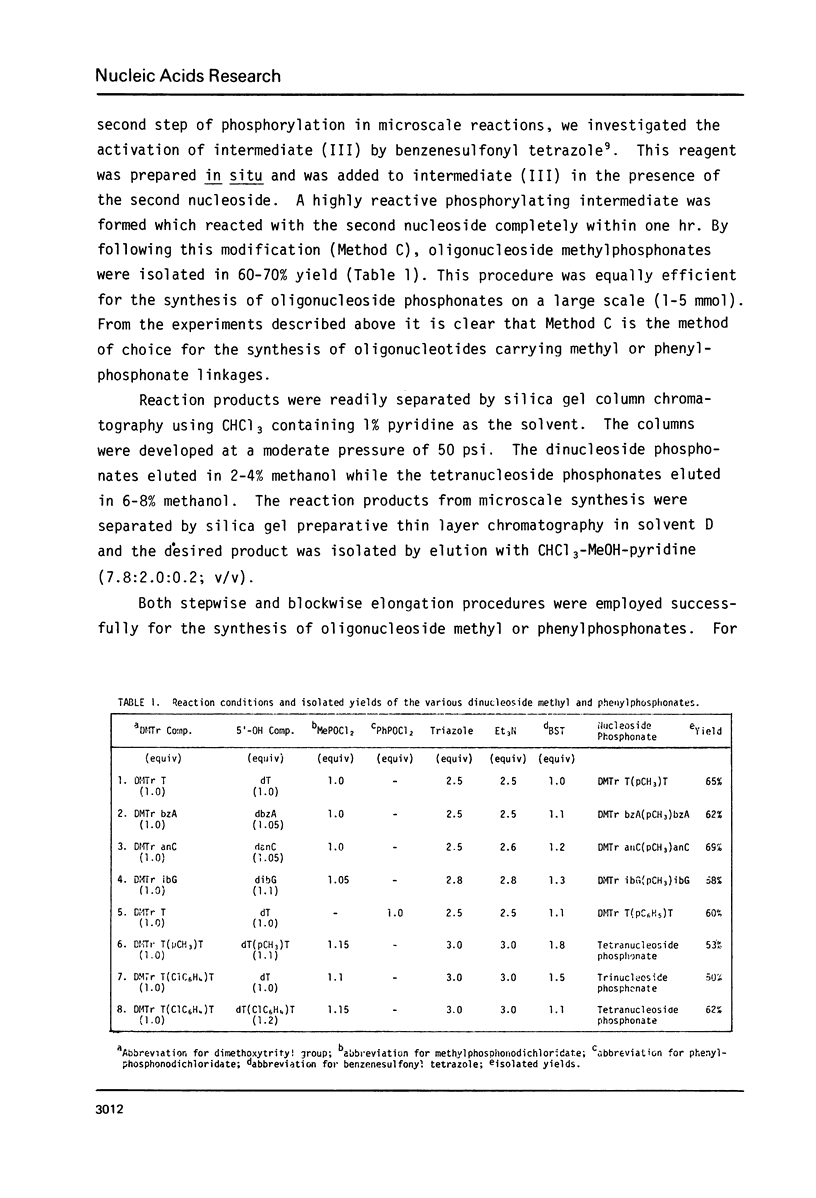
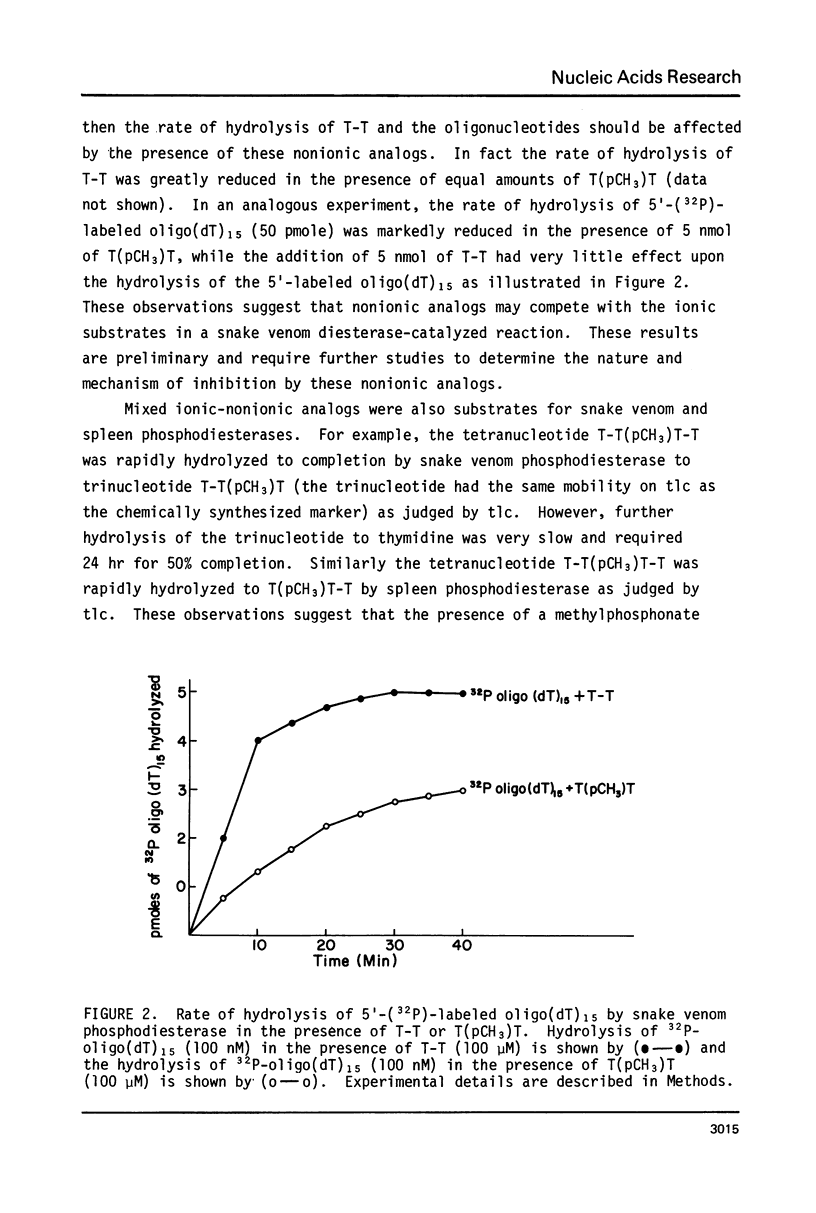
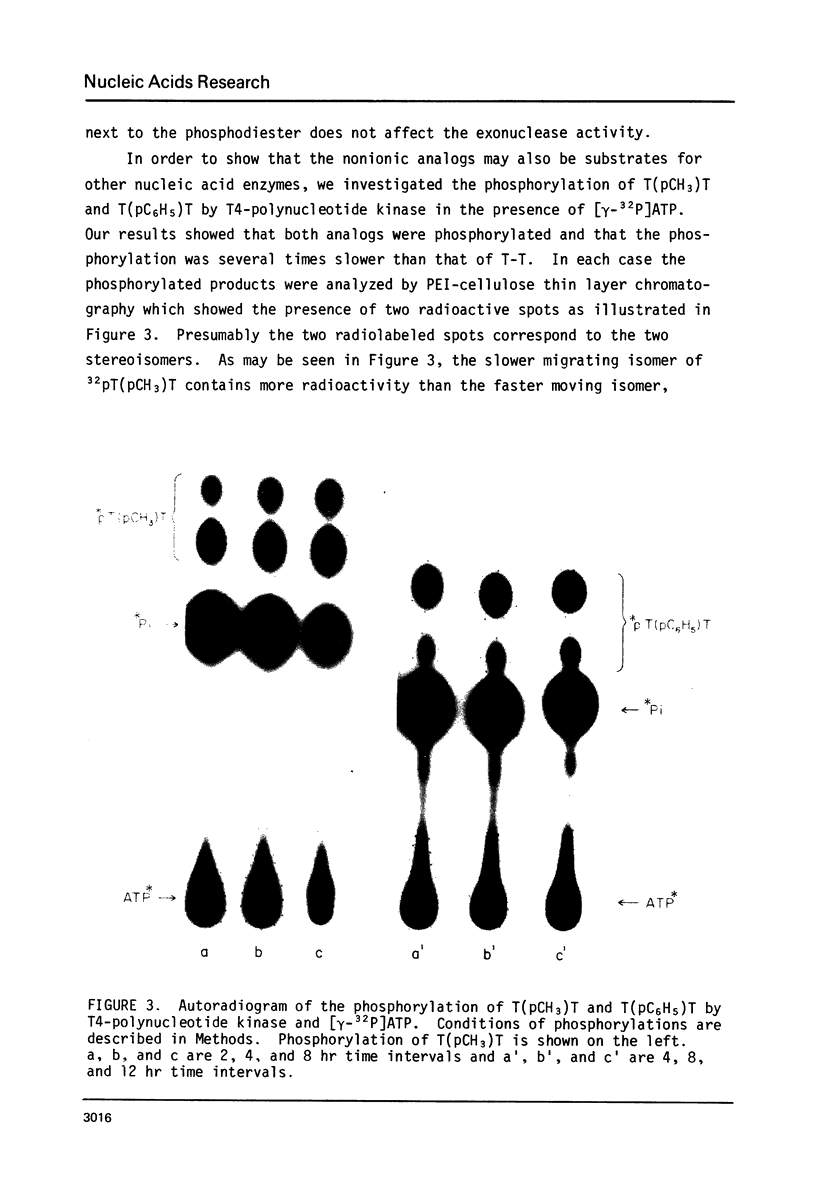
Abstract
Chemical methods for the synthesis of short deoxyribooligonucleotides containing methyl and phenylphosphonodiester linkages have been developed. The interaction of two such nonionic dinucleotide analogs, T(pCH3)T and T(pC6H5)T, with several enzymes has been investigated. Because of the phosphonate linkage each dinucleotide exists as a diastereomeric pair as shown by thin layer chromatography and enzymatic studies. Both isomers of each dinucleotide can be phosphorylated by T4-polynucleotide kinase in the presence of [gamma-32P]ATP. Only one of the diastereoisomers of each dinucleotide is slowly hydrolyzed by snake venom phosphodiesterase and acts as an inhibitor of the enzyme-catalyzed hydrolysis of 5'-labeled oligothymidylic acid. Both isomers of each dinucleotide analog are completely resistant to hydrolysis by spleen phosphodiesterase.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal K. L., Khorana H. G. Studies on polynucleotides. CII. The use of aromatic isocyanates for selective blocking of the terminal 3'-hydroxyl group in protected deoxyribooligonucleotides. J Am Chem Soc. 1972 May 17;94(10):3578–3585. doi: 10.1021/ja00765a054. [DOI] [PubMed] [Google Scholar]
- Agarwal K. L., Riftina F. Chemical synthesis of a self-complementary octanucleotide, dG-G-T-T-A-A-C-C by a modified triester method. Nucleic Acids Res. 1978 Aug;5(8):2809–2823. doi: 10.1093/nar/5.8.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgers P. M., Eckstein F. Stereochemistry of internucleotide bond formation by polynucleotide phosphorylase from Micrococcus luteus. Biochemistry. 1979 Feb 6;18(3):450–454. doi: 10.1021/bi00570a010. [DOI] [PubMed] [Google Scholar]
- Jay E., Bambara R., Padmanabhan R., Wu R. DNA sequence analysis: a general, simple and rapid method for sequencing large oligodeoxyribonucleotide fragments by mapping. Nucleic Acids Res. 1974 Mar;1(3):331–353. doi: 10.1093/nar/1.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. S., Braiterman L. T., Ts'o P. O. Effects of a trinucleotide ethyl phosphotriester, Gmp(Et)Gmp(Et)U, on mammalian cells in culture. Biochemistry. 1977 May 3;16(9):1988–1996. doi: 10.1021/bi00628a036. [DOI] [PubMed] [Google Scholar]
- Panet A., van de Sande J. H., Loewen P. C., Khorana H. G., Raae A. J., Lillehaug J. R., Kleppe K. Physical characterization and simultaneous purification of bacteriophage T4 induced polynucleotide kinase, polynucleotide ligase, and deoxyribonucleic acid polymerase. Biochemistry. 1973 Dec 4;12(25):5045–5050. doi: 10.1021/bi00749a003. [DOI] [PubMed] [Google Scholar]
- Pless R. C., Ts'o P. O. Duplex formation of a nonionic oligo(deoxythymidylate) analogue (heptadeoxythymidylyl-(3'-5')-deoxythymidine heptaethyl ester (d-(Tp(Et))7T)) with poly(deoxyadenylate). Evaluation of the electrostatic interaction. Biochemistry. 1977 Mar 22;16(6):1239–1250. doi: 10.1021/bi00625a033. [DOI] [PubMed] [Google Scholar]
- Rammler D. H., Yengoyan L., Paul A. V., Bax P. C. Nucleoside phosphonic acids. II. The synthesis of 5'-deoxythymidine 5'-phosphonic acid and its pyrophosphate derivatives. Biochemistry. 1967 Jun;6(6):1828–1837. doi: 10.1021/bi00858a034. [DOI] [PubMed] [Google Scholar]
- Stawinski J., Hozumi T., Narang S. A., Bahl C. P., Wu R. Arylsulfonyltetrazoles, new coupling reagents and further improvements in the triester method for the synthesis of deoxyribooligonucleotides. Nucleic Acids Res. 1977 Feb;4(2):353–371. doi: 10.1093/nar/4.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]