Abstract
For more than a century dendritic spines have been a source of fascination and speculation. The long-held belief that these anatomical structures are involved in learning and memory are addressed. Specifically, two lines of evidence that support this claim are reviewed. In the first, we review evidence that experimental manipulations that affect dendritic spine number in the hippocampus also affect learning processes of various sorts. In the second, we review evidence that learning itself affects the presence of dendritic spines in the hippocampus. Based on these observations, we propose that the presence of spines enhances synaptic efficacy and thereby the excitability of the network involved in the learning process. With this scheme, learning is not dependent on changes in spine density but rather changes in the presence of dendritic spines provide anatomical support for the processing of novel information used in memory formation.
Index Entries: Dendritic spines, synapse, experience, plasticity, hippocampus, NMDA
Introduction
The capacity to learn and remember is undeniably critical for our livelihood. Without these abilities, life would be a series of fragmented snapshots lacking unified meaning. The neurobiological mechanisms that underlie learning and memory however have proved quite difficult to understand. In part, this is because they must account for the very rapid induction of memory as well as its persistent expression. Because memories persist long after the molecules involved in their induction have degraded, it is likely that the expression of new memories involves long-term changes in neuronal plasticity, and in particular synaptic plasticity. Indeed, it has long been assumed that changes in the structure and organization of synapses can be brought about by anatomical modifications (1). Dendritic spines, small protrusions found on the shaft of dendrites in the mammalian brain, are one aspect of cellular anatomy that may play a role in the expression of memory (see Fig. 1). Because spines represent potential sites of postsynaptic excitatory input, an increase in their number can translate into an increase in the number of excitatory synapses (2,3). Thus, changes in the density of spines can have a major impact on the amount of excitatory neurotransmission in a particular brain region and presumably on the processing of information by that region.
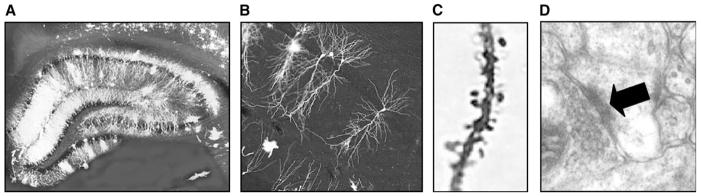
Fig. 1.
Dendritic spines in the hippocampus. Photomicrograph of Golgi-impregnated hippocampus (A). Pyramidal cells in the hippocampus (B) possess dendritic spines protrusions on the shaft of the dendrite that represent sites of postsynaptic excitatory input (C). Electron micrograph of a spine synapse (arrow) in hippocampal area CA1 (D).
There are numerous reports that behavioral training can alter aspects of dendritic anatomy, such as length and branching (4,5) but few have directly tested the effects of new experience on the presence of dendritic spines themselves. However, upon examination of these studies, it does appear that there is a relationship between the presence of dendritic spines and the formation and expression of new memories. The evidence to support such a relationship comes in several forms. There are studies suggesting that experimental manipulations affecting spine density also affect learning processes. In turn, there are a number of studies showing that processes of learning affect the presence of dendritic spines. In this review, the data that account for these two different relationships between spines and memories are summarized. Finally, we discuss what these two types of findings may reveal about a functional relationship between the presence of dendritic spines and the formation of memories.
Dendritic Spines and Memory: The Estrogen Connection
One of the most potent modulators of dendritic spines so far established is the ovarian hormone estrogen. It has been shown that exposure to estrogen either exogenously or endogenously during proestrus greatly enhances the density of dendritic spines on pyramidal cells in area CA1 of the hippocampus (6–8). Over the 5-d estrous cycle of the rat, spine density can fluctuate as much as 30% (9). Moreover, these changes in dendritic spines have been shown to reflect changes in synapse density and to be accompanied by changes in astrocytic volume (10,11). This regulation of pyramidal cell spine density by estrogen is not specific to rats but also occurs in nonhuman primates (12).
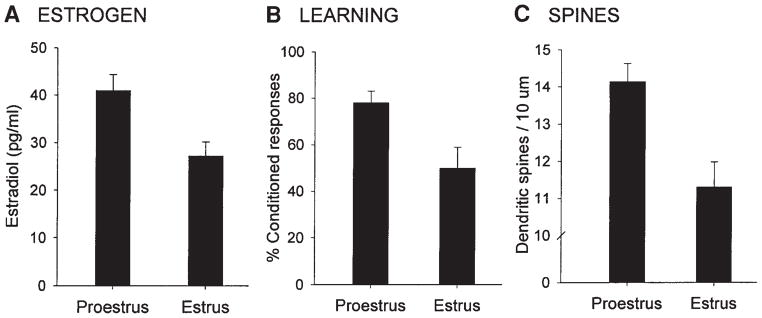
That estrogen exerts such a profound influence on synaptic morphology in the hippocampus, a brain region implicated in various forms of learning and memory, changed the way ovarian hormones were traditionally regarded. Although areas associated with reproduction, such as the hypothalamus, were known to exhibit estrogen-induced changes in synaptic morphology (13,14), changes in hippocampal spine density provided a means whereby estrogen could potentially have an important impact on cognitive processes. Indeed, the stage of proestrus, when both estrogen levels and spine density are highest, is positively related to enhanced performance of both hippocampal-dependent and independent types of classical eyeblink conditioning (see Fig. 2) (15,16). Moreover, estrogen replacement in ovariectomized animals increases CA1 spine density and also improves spatial memory retention in the Morris water maze (17).
Fig. 2.
Ovarian hormones regulate learning and dendritic spine density in the hippocampus (8,15). Females in proestrus, when estrogen levels are highest (A), emit significantly more conditioned responses on the classical eyeblink conditioning task (B) and possess a greater density of dendritic spines (C) relative to females in estrus.
Progesterone has been shown to modulate the increase in spine density and associated memory enhancement following estrogen treatment (7). In ovariectomized females, a single injection of progesterone after estradiol initially augments the estradiol-induced increase in spine density but results in a more rapid decrease than estradiol treatment alone. That is, without progesterone, spine density gradually diminishes over a 7–8 d period whereas progesterone accelerates the decline to baseline levels within 24 h. Thus, there are differing time courses of estrogen-mediated spine density regulation which depend on progesterone. Similarly, the memory enhancing effects of estradiol have been reported to be modulated by progesterone. Animals are better able to remember the location of an escape platform in the water maze within 8 h of progesterone administration (when spine density is augmented), but not 24 h after progesterone administration (when spine density has returned to baseline) (17). This temporal relationship between hormonally regulated changes in hippocampal connectivity and learning ability suggests that the presence of dendritic spines may provide anatomical structures that can be used for learning.
It was recently reported that in the male hippocampus, gonadal hormones are also important for maintaining spine number with gonadectomy producing a 50% decrease in spine density (18). Although the loss of dendritic spines is not affected by estrogen, treatment with testosterone reverses the decline. Thus, the hormonal regulation of hippocampal spine density differs in males vs females. As with estrogen, there are data to suggest that testosterone-mediated changes in spine density may be associated with cognitive ability. In both rats and humans, testosterone depletion reduces cognitive performance, an effect that can be prevented by testosterone replacement (19,20).
Sex Differences and Opposite Effects of Stress on Learning and Dendritic Spines
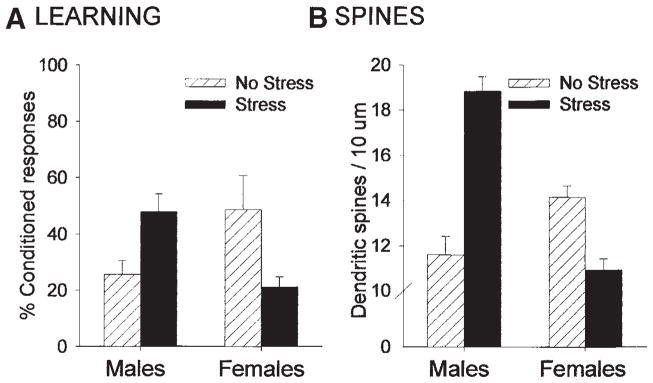
In general, there appears to be a positive relationship between estrogen-induced increases in spines and learning processes of various sorts. However, one might imagine that there are ways in which an increase in spines could affect learning indirectly and not be substrates for learning, per se. For example, changes in spine density in response to estrogen may alter motor activity or motivation and if a task required either, the animals would appear to learn better under conditions when spines were increased. One approach to resolving some of these issues is to examine responses that are directed in opposite directions. Under such circumstances, dissociations would be most evident. In past studies, we found sex differences and opposite effects of stress on associative memory formation (16,21). Under unstressed conditions, females outperform males on the task of classical eyeblink conditioning (see Fig. 3A). Moreover, exposure to an acute stressful experience of intermittent tailshocks greatly increases classical eyeblink conditioning in male rats but decreases conditioning in female rats (see Fig. 3A). Given these opposite effects of sex and stress on memory formation, we examined whether there would be sex differences in spine density (8,8a) and whether exposure to a stressful experience would have opposite effects on spine density. Using Golgi impregnation to examine dendritic spines in the hippocampus, we found that exposure to the stressor was associated with an increase in spine density on CA1 pyramidal neurons in males but a decrease in females (see Fig. 3B). Thus, exposure to an acute experience had a similar effect on spine density as it did on new learning, at least for this particular learned response. In combination with the sex differences in learning, it appears that the presence of spines is positively related to the later acquisition of new associative memories.
Fig. 3.
Opposite effects of stress on learning and dendritic spine density in males vs females (8,16). Under unstressed conditions, females outperform males on the classical eyeblink conditioning task. Following exposure to an acute stressful event, conditioning is enhanced in males but impaired in females (A). Spine density in the hippocampus is positively related to performance. Females in proestrus have a greater density of dendritic spines than males under unstressed conditions. After exposure to the acute stressful event, spine density is increased in males but decreased in females (B).
Environmental Complexity Enhances Dendritic Spines and Learning
In addition to these data on stressful experience, there are studies indicating that a more general experience of environmental complexity can alter the presence of dendritic spines and this experience can affect learning ability on a variety of tasks (22–24). Moser et al. (23,25) exposed separate groups of animals to a complex environment after which one group underwent spatial learning in the water maze and the other group was sacrificed for spine density analysis. Enriched animals possessed a higher density of dendritic spines on pyramidal cells in the CA1 region of the hippocampus. Moreover, animals exposed to the complex environment showed faster acquisition in the water maze task. Similarly, Rampon et al. (24) reported a 20% increase in hippocampal dendritic spine density as well as enhanced performance on both object recognition and fear conditioning tasks following environmental enrichment. Taken together, such results suggest that experience leading to increased spine density can also improve new learning. It should be noted that enrichment also induces changes in spine number in cortical regions (26). Thus, even though there may be a positive relationship between the presence of spines and learning, it is at this point unclear how one region participates, either individually or together with other regions, to improve learning ability.
Trace Conditioning and Dendritic Spines in the Hippocampus
As the discussion above demonstrates, there are numerous studies which report, albeit indirectly, a positive association between dendritic spines and learning. There is also evidence to suggest that learning itself affects dendritic spines in the hippocampus. In one study, it was found that exposure to a passive-avoidance task increased the presence of dendritic spines in the dentate gyrus (27). A similar increase in spine density was found after training on a spatial water maze task, although others did not observe a change (28,29). While these data suggest that training experiences affect the presence of dendritic spines in the hippocampus, again, there are some issues that should be addressed. The primary concern is that these types of changes may be due to the training experience and not to learning, per se. These are difficult issues to resolve using tasks in which control experiences that do not involve learning do not exist. The other problem as noted earlier, is that changes in other behaviors such as activity could alter spines and thus present a positive relationship, but not a direct one, between new memories and new spines. Although there are no irrefutable ways to resolve these issues, there are tasks that are more useful for addressing them, such as classical conditioning procedures. Several studies have used such procedures, most commonly, trace eyeblink conditioning. During this task, an auditory conditioned stimulus (CS) is preceded by and predicts the occurrence of an unconditioned stimulus (US), which is a periorbital shock to the eyelid. The shock to the eyelid elicits a blink, which is the unconditioned response (UR). When the shock to the eyelid is repeatedly paired with and preceded by the auditory stimulus, the auditory stimulus itself comes to elicit an eyeblink conditioned response (CR). Because the CS is separated in time from the US, the animal must presumably form a “memory trace” of the CS in order to make the appropriate association across the temporal gap. Trace conditioning is a form of associative learning dependent on area CA1 of the hippocampal formation (30–32). Moreover, it is a useful task for evaluating changes in structure in response to learning since one can compare the effects of learning a positive relationship between the stimuli (paired) to those that might occur in response to learning a negative relationship between the same stimuli (unpaired).
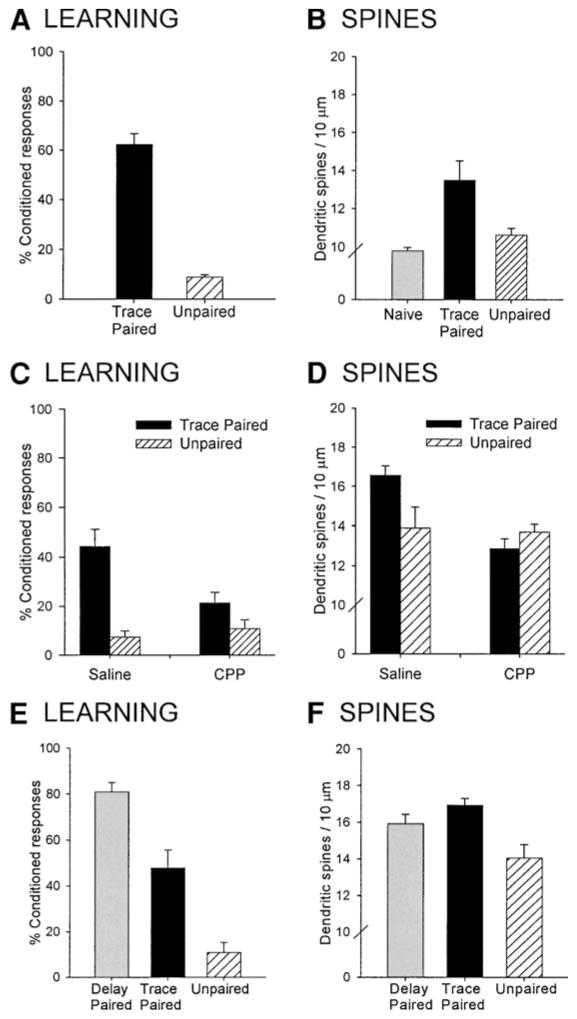
Because of these procedural advantages, we tested whether the acquisition of trace memories alters the presence of dendritic spines in the hippocampus (33). Adult male rats were trained on the trace eyeblink conditioning paradigm. Control rats were exposed to the same number of stimuli presented in an explicitly unpaired manner or were naive. Twenty-four hours following training, brain tissue was processed for Golgi impregnation and the density of dendritic spines was measured. Our data demonstrate that trace conditioning was associated with an increase in the density of dendritic spines on pyramidal cells of area CA1 of the hippocampus (see Fig. 4A,B). Since the increase was only observed in those exposed to the paired stimuli and not in those exposed to explicitly unpaired stimuli, it appears to be specific to learning a positive association between the two conditioning stimuli. However, the possibility remains that other aspects of the training experience are responsible for the alteration in spine number. To address this issue, we took advantage of previous studies demonstrating that acquisition of the conditioned eyeblink response is dependent on activation of the N-methyl-D-aspartate (NMDA) type of glutamate receptor (34,35). A competitive NMDA-receptor antagonist (CPP) was administered prior to training to determine whether changes in spine density are evident following the blockade of learning. Animals that were injected with the antagonist did not emit CRs and showed no increase in spine density suggesting that the training-induced increase in spine density appears to be specific to learning the association between the CS and the US (Fig. 4C,D). In combination, these results demonstrate that changes in spine density occur as a result of learning and not simply exposure to the training procedures.
Fig. 4.
Associative learning increases the observation of dendritic spines in the hippocampus (33). Animals that underwent trace conditioning with paired stimuli exhibited more conditioned responses than animals trained with unpaired stimuli (A). Trace conditioning was associated with an increase in the density of spines on basal dendrites of CA1 pyramidal neurons of the hippocampus (B). Administration of a competitive NMDA-receptor antagonist (CPP) prior to training blocked acquisition of the conditioned response (C) and prevented the training-induced increase in spine density on basal dendrites (D). The hippocampal-independent task of delay conditioning (E) also increased the density of spines in area CA1 (F).
These learning-associated changes in spine density were not specific to hippocampal-dependent learning but were also evident in animals trained on the hippocampal-independent task of delay conditioning in which the CS and US overlap and are thus continuous in time (Fig. 4E,F). Both trace and delay eyeblink conditioning result in increased hippocampal neuronal excitability which could influence the formation or extension of dendritic spines (36). Indeed, changes in activity have been associated with alterations in synaptic structure on cerebellar Purkinje cells after classical eyeblink conditioning (37) and recent studies indicate that activity enhances the de novo appearance of dendritic spines, at least in vitro (38,39). Furthermore, exposure to both trace- and delay-conditioning increase other measures of synaptic plasticity in area CA1 of the hippocampus, such as the binding affinity of AMPA receptors (40). These data suggest that initial acquisition of these associations affects the density of dendritic spines regardless of whether the hippocampus is necessary.
Interestingly, the effects of classical conditioning on spine density were evident on basal, and not apical, dendrites. Similarly, others have found that experiences such as environmental enrichment and stimulus exposure increase spine density on basal, and not apical dendrites, and these experiences were associated with enhanced spatial learning ability (23,25,26). Why would structural alterations related to learning preferentially occur on the basal dendrites? Relative to the apical, basal dendrites receive more contralateral input (41–43) as well as fewer inhibitory inputs from interneurons (44). There also are physiological differences between these regions, at least to the extent that the magnitude of LTP is greater when recording in stratum oriens, the area in which basal dendrites reside (45). Together, these data suggest that basal dendrites in CA1 have a high capacity for synaptic plasticity.
That classical conditioning did not affect spine density on apical dendrites is consistent with findings by Geinisman et al. (46) who reported that synapse density in the CA1 stratum radiatum was not altered in animals trained on the trace eyeblink conditioning task. This is not to say that trace conditioning does not induce structural changes in the apical dendritic region. Indeed, the area of the postsynaptic density (PSD) was increased after trace eyeblink conditioning (46). Given that the PSD contains proteins involved in signal transduction, including receptors and ion channels, an increase in PSD area may be related to an addition of these components with a resultant increase in synaptic efficacy. Trace conditioning has also been shown to increase the number of multiple synapse boutons that synapse with more than one dendritic spine (47). Together these data suggest that trace eyeblink conditioning is accompanied by a remodeling of existing synaptic contacts in stratum radiatum, and not the formation of new synaptic contacts. It should be noted that structural changes following classical eyeblink conditioning also occur in the cerebellum (37,48), another region critical for acquisition and performance of the learned response (49).
Spine Density Changes in the Cortex
In addition to the hippocampus, some cortical regions have also been shown to exhibit training-related alterations in dendritic morphology. For example, pyramidal cells in the piriform cortex possess more dendritic spines 3 d following training on an olfactory discrimination task in which rats learn to distinguish between two pairs of odors (50). Moreover, dendritic spines are more numerous in the prefrontal cortex than other cortical regions of the macaque monkey and may thus be related to the presumed increase in complexity of processing that occurs at synapses further from sensory regions (51). There is some support of this notion in that cortical regions involved in the early stages of processing (i.e., primary sensory areas) possess fewer dendritic spines than regions which play a role in later stages of processing such as the prefrontal cortex (52).
In humans, abnormalities in cortical dendritic spine formation have been associated with deficiencies in cognitive capabilities. The density of dendritic spines is decreased in persons with some types of mental retardation such as Downs syndrome. They reportedly have fewer spines on pyramidal neurons of the hippocampus and cingulate gyrus (53,54). In contrast, other types of mental retardation, such as fragile X syndrome exhibit abnormal morphology, but an increased density of spines (55). Schizophrenia is another disorder characterized by poor performance on cognitive tasks, particularly those in which the dorsolateral prefrontal cortex is important. Spine density in this region, as well as the temporal cortex, is decreased in schizophrenic subjects (56,57). Finally, reduced numbers of dendritic spines have also been observed on cortical pyramidal neurons in Alzheimer’s disease and senile dementia (58,59). Taken together, these data implicate dendritic spines as an anatomical substrate important for normal cognitive processes and suggests that a reduction in spine number may contribute to the cognitive dysfunction characteristic of these disorders. However, other interpretations are feasible and the possibility remains that changes in spine number are a consequence and not a cause of the disorder.
Additional Evidence for the Relationship Between Dendritic Spines and Learning
There is also evidence that learning and performance are related to spine number in other vertebrate species such as birds. For example, chicks can be trained to avoid pecking at beads coated with an aversive substance, and will subsequently avoid an identical but dry bead. Control chicks that are water trained but not exposed to the aversive substance continue to peck at water coated or dry beads. Using Golgi impregnation it was determined that this paradigm, known as one-trial passive-avoidance conditioning, is associated with an increase in spine density on neurons of two forebrain regions 24 h after training (60). These changes appear specific to memory formation since chicks given subconvulsive shock 5 min after training were rendered amnesic for the experience and did not express an increase in spine density. Morphological correlates of learning have also been shown to occur in other avian species. In particular, differences in the complexity of song learning leads to differences in both the amount learned as well as dendritic spine density in a specific forebrain song nucleus known as the high vocal center (61). Songbirds that were exposed to and learned large song repertoires had a greater number of dendritic spines than birds that were exposed to and learned small repertoires. These studies suggest that neurons of the bird brain are also sensitive to learning-related changes in dendritic spines.
Conclusions
How changes in spine density would alter processes involved in learning and memory remains to be determined (62). One might propose that the presence of spines enhances synaptic efficacy and thereby enhances the excitability of the network involved in the learning process. In this particular scenario, learning is not necessarily dependent on spine-density changes; rather changes in density indirectly enhance the environmental support for information processing; i.e., they provide anatomical structure for processes that are either occurring or will occur in the future. This scenario, simplistic though it may be, is consistent with data accumulated over the past several decades. In the case of estrogen for example, an increase in spine density would alter the presence of spines during proestrus, a time in the female cycle when she is most exploratory and when mate selection would produce offspring (63). In the case of stress, the increase in spine density in males persists for at least 24 h and correlates with increased learning. This is a time period when the chance of stressful encounters (with predators, for example) remains relatively high. The presence of spines may provide anatomical support for forming associations at a more rapid rate during that time period. Another example would involve the spine increase that occurs after trace- and delay-eyeblink conditioning. It has been shown in several preparations that neurons in the CA1 pyramidal cell regions are more excitable during and for some time after training (36). These cells exhibit decreases in the afterhyperpolarization (AHP) which would contribute to and possibly account for increases in excitability (64). Regardless of the exact mechanism, excitability and spine density tend to correlate under these experimental training conditions. Whether the increase in excitability results in increases in spine density or vice versa is debatable, but in either case, increased excitability in the network could thereby enhance its processing capability. This idea proposes a rather broad and nonspecific effect of training on spine formation and is thus inconsistent with the more traditional ideas about how spines may be involved in learning processes. In most, it is assumed that the changes that occur at the synaptic level are relatively specific to just a few synapses necessary to encode an association; learning would not influence most of, or even a substantial number of, cells in the network. However, the data that we have reviewed indicate that spine changes in response to experience are not relegated to just a few neurons in a network. Rather, there are 20–30% increases in spine density in area CA1 after these acute experiences. It is clear that a great number of cells are being affected by the experience, more than would be necessary to encode a single association.
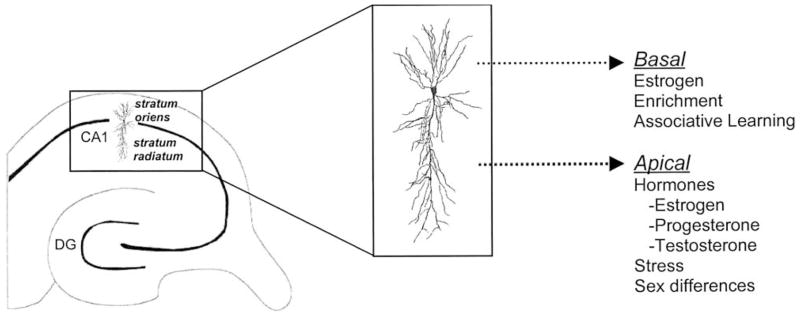
It is perhaps instructive in this regard that spines are so sensitive to changes in hormonal status. As discussed, estrogen and testosterone are potent mediators of spine density. The use of hormones to alter spine density may be one way that the nervous system alters their presence in a relatively ubiquitous fashion to then alter learning ability. In the case of proestrus, the release of estrogen would enhance density on multiple types of neurons and brain regions. In the hippocampus, apical and basal dendrites are sensitive to estrogen, and thus an estrogen-induced increase in spine density could have a number of functional consequences, only one of which may be related to learning and memory. In response to learning alone, however, it appears that spines on the basal dendrites are especially sensitive. The more selective response to learning may represent a more specific response to the formation of an associative memory. Thus, the sensitivity of spines to hormonal status may provide the opportunity for broad changes in synaptic density and efficacy, whereas the more localized response to learning may reflect more specific responses to experience (see Fig. 5).
Fig. 5.
Factors altering spine density on the apical and basal dendrites of hippocampal CA1 pyramidal neurons. Hormonal status affects dendritic spines on both apical and basal dendrites. In response to learning, spine density changes occur preferentially on basal dendrites. The sensitivity of spines to hormones may provide the opportunity for broad changes in synaptic density and efficacy, whereas the more localized response to learning may reflect more specific responses to experience.
It wasn’t too long ago that we considered dendritic spines to be relatively stable substrates of the nervous system. We now know that they are remarkably plastic, some even in constant motion (65). Thus, it is perhaps not so surprising that they could respond so rapidly and yet change persistently to new learning experiences. With new live imaging techniques, we can now visualize spines in real time and in the future will be able to observe their response to experience (66). This technology will greatly assist in the effort to understand the function of spines. Even so, it is likely that we will remain as enamored by their presence in the next 100 yr as we have in the past.
Acknowledgments
This work was supported by National Institute of Mental Health (R01-59970), National Science Foundation (IBN0217403), and the National Alliance for Research on Schizophrenia and Depression (T. J. Shors). B. Leuner was supported by a National Institute of Mental Health NRSA (MH63568). We thank N. L. Desmond for providing the electron micrograph.
References
- 1.Ramon y Cajal S. Neue Darstellung vom histologischen Bau des Centralnerven system. Arch Anat Physiol. 1893;17:319–428. [Google Scholar]
- 2.Andersen P, Balckstad TW, Lomo T. Location and identification of excitatory synapses on hippocampal pyramidal cells. Exp Brain Res. 1966;1:236–248. doi: 10.1007/BF00234344. [DOI] [PubMed] [Google Scholar]
- 3.Sorra KE, Harris KM. Overview on the structure, composition, function, development, and plasticity of hippocampal dendritic spines. Hippocampus. 2000;10:501–511. doi: 10.1002/1098-1063(2000)10:5<501::AID-HIPO1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 4.Greenough WT, Juraska JM, Volkmar FR. Maze training effects on dendritic branching in occipital cortex of adult rats. Behav Neural Biol. 1979;26:287–297. doi: 10.1016/s0163-1047(79)91278-0. [DOI] [PubMed] [Google Scholar]
- 5.Bailey CH, Kandel ER. Structural changes accompanying memory storage. Annu Rev Physiol. 1993;55:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- 6.Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woolley CS, McEwen BS. Role of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- 8.Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci. 2001;21:6292–9297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shors ATJ, Falduto J, Leuner B. The opposite effects of stress on dendritic spines in male vs. female rats are NMDA receptor-dependent. Eur J Neurosci. 2004;19:145–150. doi: 10.1046/j.1460-9568.2003.03065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2254. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klintsova A, Levy WB, Desmond NL. Astrocytic volume fluctuates in the hippocampal CA1 region across the estrous cycle. Brain Res. 1995;690:269–274. doi: 10.1016/0006-8993(95)00642-4. [DOI] [PubMed] [Google Scholar]
- 12.Leranth C, Shanabrough M, Redmond DE., Jr Gonadal hormones are responsible for maintaining the integrity of spine synapses in the CA1 hippocampal subfield of female nonhuman primate. J Comp Neurol. 2002;447:34–42. doi: 10.1002/cne.10230. [DOI] [PubMed] [Google Scholar]
- 13.Bloch GJ, Gorski RA. Estrogen/progesterone treatment in adulthood affects the size of several components of the medial preoptic area in the male rat. J Comp Neurol. 1988;275:613–622. doi: 10.1002/cne.902750409. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto A. Synaptogenic action of sex steroids in developing and adult neuroendocrine brain. Psychoneuroendocrinology. 1991;16:25–40. doi: 10.1016/0306-4530(91)90069-6. [DOI] [PubMed] [Google Scholar]
- 15.Shors TJ, Lewczyk C, Pacynski M, Matthew PR, Pickett J. Stages of estrus mediate the stress-induced impairment of associative learning in the female rat. Neuroreport. 1998;9:419–423. doi: 10.1097/00001756-199802160-00012. [DOI] [PubMed] [Google Scholar]
- 16.Wood GE, Beylin A, Shors TJ. The contribution of adrenal and reproductive hormones to the sexually opposed effects of stress on trace conditioning. Behav Neurosci. 2001;115:1–13. doi: 10.1037/0735-7044.115.1.175. [DOI] [PubMed] [Google Scholar]
- 17.Sandstrom NL, Williams CL. Memory retention is modulated by acute estradiol and progesterone replacement. Behav Neurosci. 2001;115:384–393. [PubMed] [Google Scholar]
- 18.Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci. 2003;23:1588–1592. doi: 10.1523/JNEUROSCI.23-05-01588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frye CA, Seliga AM. Testosterone increases analgesia, anxiolysis, and cognitive performance of male rats. Cogn Affect Behav Neurosci. 2001;1:371–381. doi: 10.3758/cabn.1.4.371. [DOI] [PubMed] [Google Scholar]
- 20.Cherrier MM, Anawalt BD, Herbst KL, Amory JK, Craft S, Matsumoto AM, Bremner WJ. Cognitive effects of short-term manipulation of serum sex steroids in healthy young men. J Clin Endocrinol Metab. 2001;87:3090–3096. doi: 10.1210/jcem.87.7.8570. [DOI] [PubMed] [Google Scholar]
- 21.Wood GE, Shors TJ. Stress facilitates classical conditioning in males but impairs conditioning in females through the activational influences of ovarian hormones. Proc Natl Acad Sci USA. 1998;95:4066–4071. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenough WT, Wood WE, Madden TC. Possible memory storage differences among mice reared in environments varying in complexity. Behav Biol. 1972;7:717–722. doi: 10.1016/s0091-6773(72)80078-6. [DOI] [PubMed] [Google Scholar]
- 23.Moser MB, Trommald M, Andersen P. An increase in dendritic spine density on hippocampal CA1 pyramidal cells following spatial learning in adult rats suggests the formation of new synapses. Proc Natl Acad Sci USA. 1994;91:12,673–12,675. doi: 10.1073/pnas.91.26.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rampon C, Tang YP, Goodhouse J, Shimizu E, Kyin M, Tsien JZ. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nat Neurosci. 2000;3:238–244. doi: 10.1038/72945. [DOI] [PubMed] [Google Scholar]
- 25.Moser MB, Trommald M, Egeland T, Andersen P. Spatial training in a complex environment and isolation alter the spine distribution differentially in rat CA1 pyramidal cells. J Comp Neurol. 1997;380:373–381. doi: 10.1002/(sici)1096-9861(19970414)380:3<373::aid-cne6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 26.Globus A, Rosenzweig MR, Bennett EL, Diamond MC. Effects of differential experience on dendritic spine counts in rat cerebral cortex. J Comp Physiol Psych. 1973;82:175–181. doi: 10.1037/h0033910. [DOI] [PubMed] [Google Scholar]
- 27.O’Malley A, O’Connell C, Regan CM. Ultrastructural analysis reveals avoidance conditioning to induce a transient increase in hippocampal dentate spine density in the 6 hour post-training period of consolidation. Neuroscience. 1998;87:607–613. doi: 10.1016/s0306-4522(98)00178-x. [DOI] [PubMed] [Google Scholar]
- 28.O’Malley A, O’Connell C, Murphy KJ, Regan CM. Transient spine density increases in the mid-molecular layer of hippocampal dentate gyrus accompany consolidation of a spatial learning task in the rodent. Neuroscience. 2000;99:229–232. doi: 10.1016/s0306-4522(00)00182-2. [DOI] [PubMed] [Google Scholar]
- 29.Rusakov DA, Davies HA, Harrison E, Diana G, Richter-Levin G, Bliss TVP, Stewart MG. Ultrastructural synaptic correlates of spatial learning in rat hippocampus. Neuroscience. 1997;80:69–77. doi: 10.1016/s0306-4522(97)00125-5. [DOI] [PubMed] [Google Scholar]
- 30.Solomon PR, VanderSchaaf ER, Thompson RF, Weisz DJ. Hippocampus and trace conditioning of the rabbit’s classically conditioned nictitating membrane response. Behav Neurosci. 1986;100:729–744. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- 31.Moyer JR, Jr, Deyo RA, Disterhoft JF. Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behav Neurosci. 1990;104:243–252. doi: 10.1037//0735-7044.104.2.243. [DOI] [PubMed] [Google Scholar]
- 32.Beylin AV, Gandhi CC, Wood G, Talk AC, Matzel LD, Shors TJ. The role of the hippocampus in trace conditioning: temporal discontiguity or task difficulty? Neurobio Learn Mem. 2001;76:447–461. doi: 10.1006/nlme.2001.4039. [DOI] [PubMed] [Google Scholar]
- 33.Leuner B, Faldtuo J, Shors TJ. Associative memory formation increases the observation of dendritic spines in the hippocampus. J Neurosci. 2003;23:659–665. doi: 10.1523/JNEUROSCI.23-02-00659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Servatius RJ, Shors TJ. Early acquisition, but not retention, of the classically conditioned eyeblink response is N-Methyl-D-Aspartate (NMDA) receptor dependent. Behav Neurosci. 1996;110:1040–1048. doi: 10.1037//0735-7044.110.5.1040. [DOI] [PubMed] [Google Scholar]
- 35.Thompson LT, Disterhoft JF. N-Methyl-D-Aspartate receptors in associative eye-blink conditioning: both MK-801 and Phencyclidine produce task and dose dependent impairments. J Pharmacol Exp Ther. 1997;281:928–940. [PubMed] [Google Scholar]
- 36.Berger TW, Clark GA, Thompson RF. Learning-dependent neuronal responses recorded from limbic system brain structures during classical conditioning. Physiol Psychol. 1980;8:155–167. [Google Scholar]
- 37.Anderson BJ, Relucio K, Haglund K, Logan C, Knowlton B, Thompson J, Steinmetz JE, Thompson RF, Greenough WT. Effects of paired and unpaired eye-blink conditioning on Purkinje cell morphology. Learn Mem. 1999;6:128–137. [PMC free article] [PubMed] [Google Scholar]
- 38.Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283:1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- 39.Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- 40.Tocco G, Annala AJ, Baudry M, Thompson RF. Learning of a hippocampal dependent conditioning task changes the binding properties of AMPA receptors in rabbit hippocampus. Behav Neural Biol. 1992;58:222–231. doi: 10.1016/0163-1047(92)90510-b. [DOI] [PubMed] [Google Scholar]
- 41.Swanson LW, Wyss JM, Cowan WM. An autoradiographic study of the organization of intrahippocampal association pathways in the rat. J Comp Neurol. 1978;181:681–716. doi: 10.1002/cne.901810402. [DOI] [PubMed] [Google Scholar]
- 42.Amaral DG, Witter MP. Hippocampal formation. In: Paxinos G, editor. The Rat Nervous System. Academic; San Diego, CA: 1995. pp. 443–493. [Google Scholar]
- 43.Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projection originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990;295:580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- 44.Toth K, Freund TF. Calbindin D28k-containing nonpyramidal cells in the rat hippocampus: their immunoreactivity for GABA and projection to the medial septum. Neuroscience. 1992;49:793–805. doi: 10.1016/0306-4522(92)90357-8. [DOI] [PubMed] [Google Scholar]
- 45.Kaibara T, Leung LS. Basal versus apical dendritic long-term potentiation of commissural afferents to hippocampal CA1: a current-source density study. J Neurosci. 1993;13:2391–2404. doi: 10.1523/JNEUROSCI.13-06-02391.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geinisman Y, Disterhoft JF, Gunderson JG, McEchron MD, Persina IS, Power JM, Van der Zee EA, West MJ. Remodeling of hippocampal synapses after hippocampus-dependent associative learning. J Comp Neurol. 2000;417:49–59. [PubMed] [Google Scholar]
- 47.Geinisman Y, Berry RW, Disterhoft JF, Power JM, Van der Zee EA. Associative learning elicits the formation of multiple synapse boutons. J Neurosci. 2001;21:5568–5573. doi: 10.1523/JNEUROSCI.21-15-05568.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kleim JA, Freeman JF, Bruneau R, Nolan BC, Cooper NR, Zook A, Walters D. Synapse formation is associated with memory storage in the cerebellum. Proc Natl Acad Sci USA. 2002;99:13,228–13,231. doi: 10.1073/pnas.202483399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCormick DA, Thompson RF. Cerebellum: essential involvement in the classically conditioned eyelid response. Science. 1984;223:296–299. doi: 10.1126/science.6701513. [DOI] [PubMed] [Google Scholar]
- 50.Knafo S, Grossman Y, Barkai E, Benshalom G. Olfactory learning is associated with increased spine density along apical dendrites of pyramidal neurons in the rat piriform cortex. Eur J Neurosci. 2001;13:633–638. doi: 10.1046/j.1460-9568.2001.01422.x. [DOI] [PubMed] [Google Scholar]
- 51.Elston GN. Pyramidal cells of the frontal lobe: all the more spinous to think with. J Neurosci. 2000;20:RC95. doi: 10.1523/JNEUROSCI.20-18-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jacobs B, Schall M, Prather M, Kapler E, Driscoll L, Baca S, Jacobs J, Ford K, Wainwright M, Tremi M. Regional dendritic and spine variation in human cerebral cortex: a quantitive Golgi study. Cerebral Cortex. 2001;11:558–571. doi: 10.1093/cercor/11.6.558. [DOI] [PubMed] [Google Scholar]
- 53.Purpura DP. Dendritic spine “dysgenesis” and mental retardation. Science. 1974;30:1126–1128. doi: 10.1126/science.186.4169.1126. [DOI] [PubMed] [Google Scholar]
- 54.Suetsugu M, Mehraein P. Spine distribution along the apical dendrites of the pyramidal neurons in Down’s syndrome. A quantitative Golgi study. Acta Neuropathol (Berl) 1980;50:207–210. doi: 10.1007/BF00688755. [DOI] [PubMed] [Google Scholar]
- 55.Irwin SA, Patel B, Idupulapati M, Harris JB, Crisostomo RA, Larsen BP, Kooy F, Willems PJ, Cras P, Kozlowski PB, Swain RA, Weiler IJ, Greenough WT. Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with Fragile-X syndrome. Am J Med Gen. 2001;98:161–167. doi: 10.1002/1096-8628(20010115)98:2<161::aid-ajmg1025>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 56.Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, Barnes TRE, Hirsch SR. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 1998;65:446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 58.Mehraein P, Yamada M, Tarnowska-Dziduszko E. Quantitative study on dendrites and dendritic spines in Alzheimer’s disease and senile dementia. Adv Neurol. 1975;12:453–458. [PubMed] [Google Scholar]
- 59.Catala I, Ferrer I, Galofre E, Fabregues I. Decreased numbers of dendritic spines in pyramidal neurons in dementia. A quatitatove Golgi study on biopsy samples. Human Neurbiol. 1988;6:255–259. [PubMed] [Google Scholar]
- 60.Stewart MG, Rusakov DA. Morphological changes associated with stages of memory formation in the chick following passive avoidance training. Behav Brain Res. 1995;66:21–28. doi: 10.1016/0166-4328(94)00119-z. [DOI] [PubMed] [Google Scholar]
- 61.Airey DC, Kroodsma DE, DeVoogd TJ. Differences in the complexity of song tutoring cause differences in the amount learned and in dendritic spine density in a songbird telencephalic song control nucleus. Neurobiol Learn Mem. 2000;73:274–281. doi: 10.1006/nlme.1999.3937. [DOI] [PubMed] [Google Scholar]
- 62.Lamprecht R, LeDoux J. Structural plasticity and memory. Nat Rev Neurosci. 2004;5:45–54. doi: 10.1038/nrn1301. [DOI] [PubMed] [Google Scholar]
- 63.Desmond NL, Levy WB. Ovarian steroidal control of connectivity in the female hippocampus: an overview of recent experimental findings and speculations on its functional consequences. Hippocampus. 1997;7:239–245. doi: 10.1002/(SICI)1098-1063(1997)7:2<239::AID-HIPO10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 64.Disterhoft JF, Coulter DA, Alkon DL. Conditioning-specific membrane changes of rabbit hippocampal neurons measured in vitro. Proc Natl Acad Sci USA. 1986;83:2733–2737. doi: 10.1073/pnas.83.8.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bonhoeffer T, Yuste R. Spine motility, phenomenology, mechanisms, and function. Neuron. 2002;35:1019–1027. doi: 10.1016/s0896-6273(02)00906-6. [DOI] [PubMed] [Google Scholar]
- 66.Mizrahi A, Crowley JC, Shtoyerman E, Katz LC. High resolution in vivo imaging of hippocampal dendrites and spines. J Neurosci. 2004;24:3147–3151. doi: 10.1523/JNEUROSCI.5218-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]