Abstract
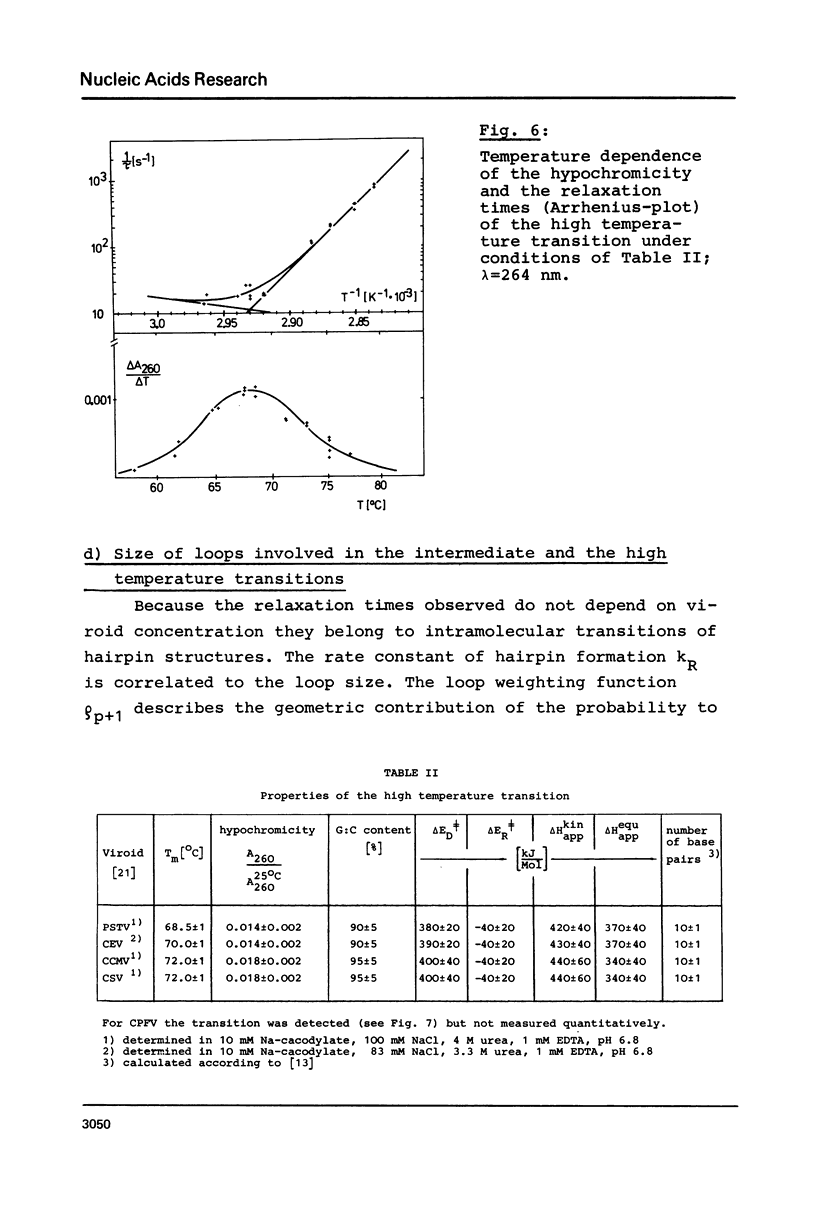
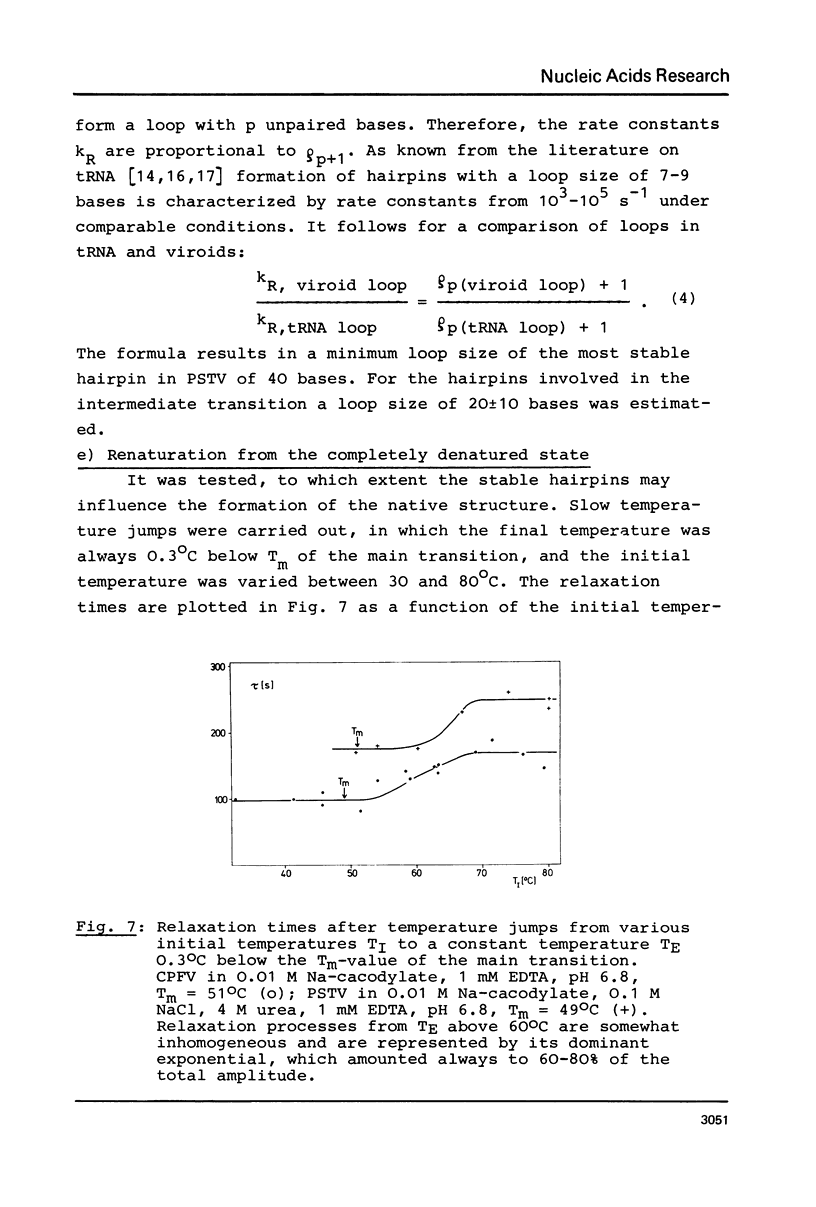
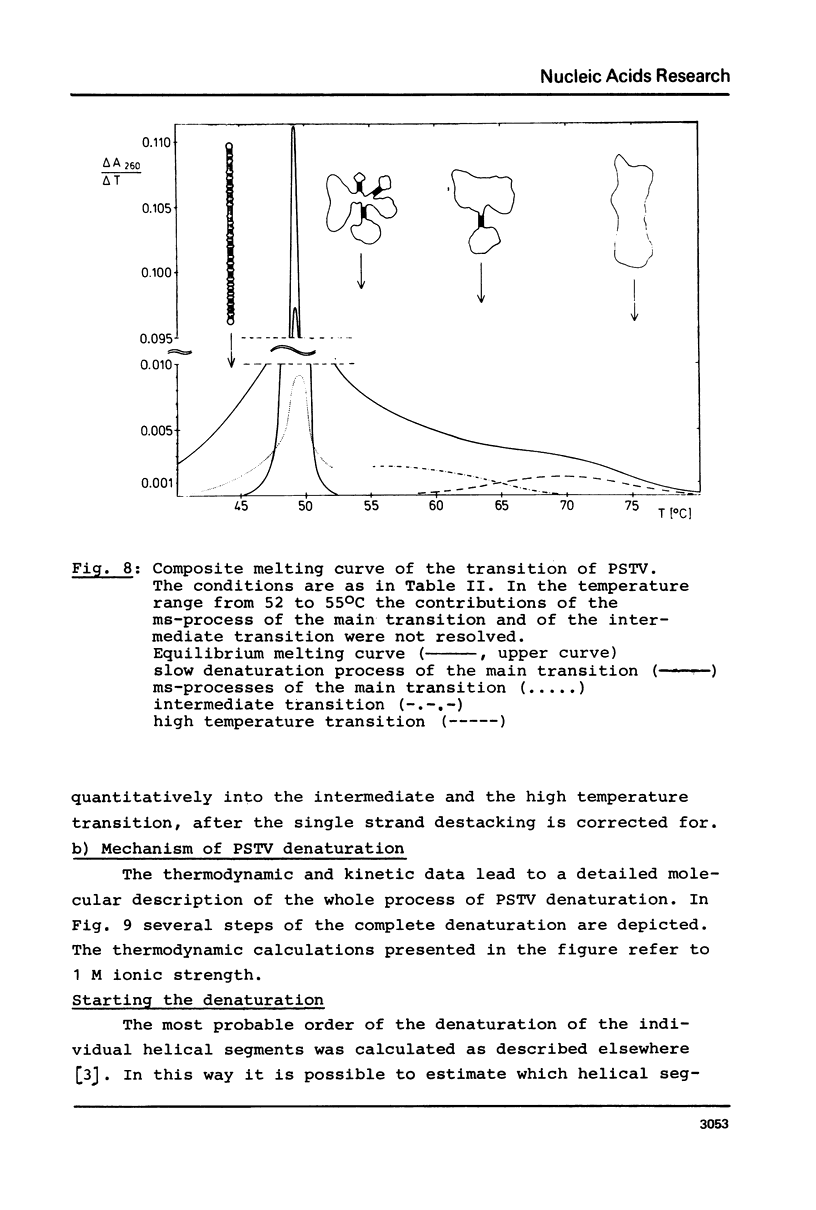
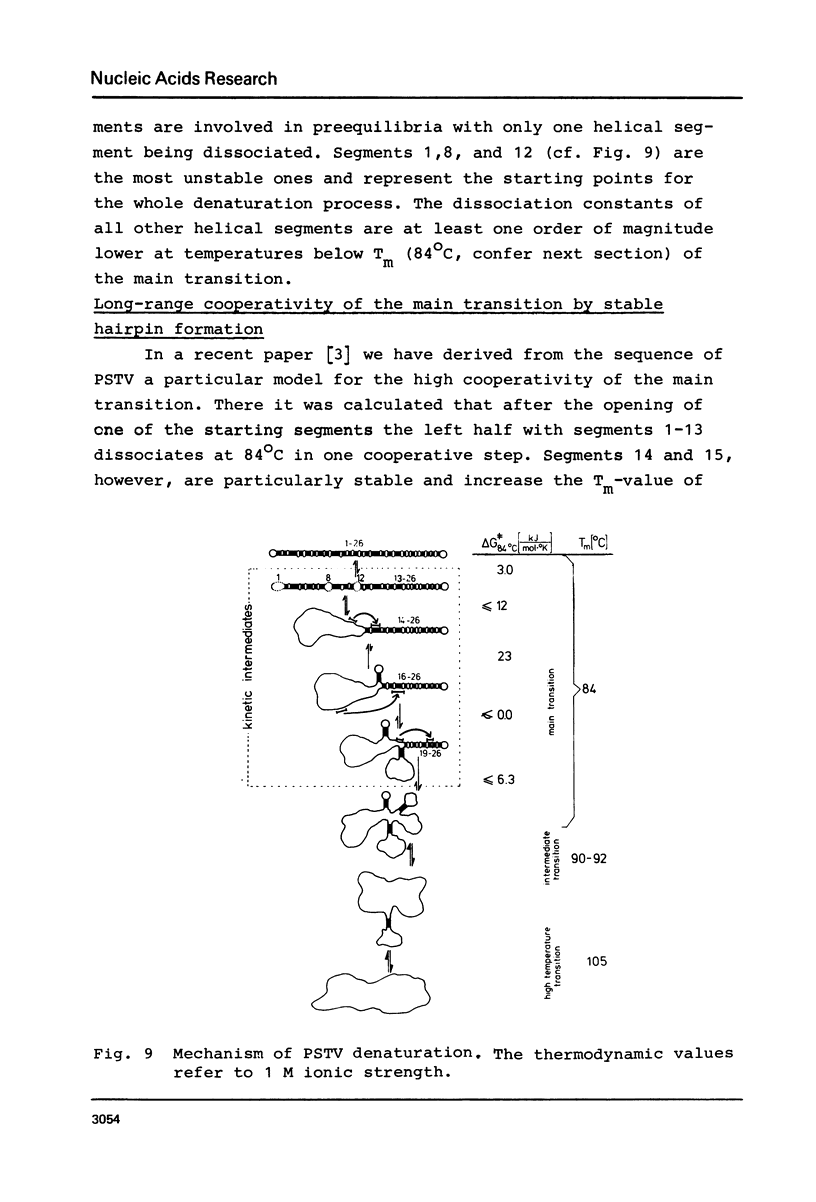
The conformational transitions of five viroid species were studied by melting analysis and by fast and slow temperature jump techniques. Experiments with the fast temperature jump technique had to be carried out in 10 mM Na-cacodylate, 0.1 M NaCl, 4 M urea, 1 mM EDTA, pH 6.8. In addition to the highly cooperative main transition (Tm between 46.5 and 49 degrees C for different viroid species [1]) all viroids show at higher temperatures an intermediate transition (Tm approximately equal to 57 degrees C) and a high temperature transition (Tm approximately equal to 68 degrees C). The maximum amplitudes of these transitions amount only to about 1% of that of the main transition. The main transition represents a net dissociation of 78 to 94 base pairs depending on the viroid species. The intermediate transition corresponds to the dissociation of two hairpins with 5-10 base pairs each, and 10-20 nucleotides in the loops. The high temperature transition corresponds to a hairpin of 9 G:C pairs and 1 A:U pair and more than 40 bases in the loop. It is shown that these stable hairpins are not part of the native structure but are newly formed during the main transition. Their formation is responsible for the extraordinary cooperativity observed in the main transition. Hairpins can be correlated to defined sequences of PSTV. Based on these studies, on the sequence of PSTV [2], and on a theoretical treatment [3] a detailed description of the whole mechanism of PSTV denaturation is given.
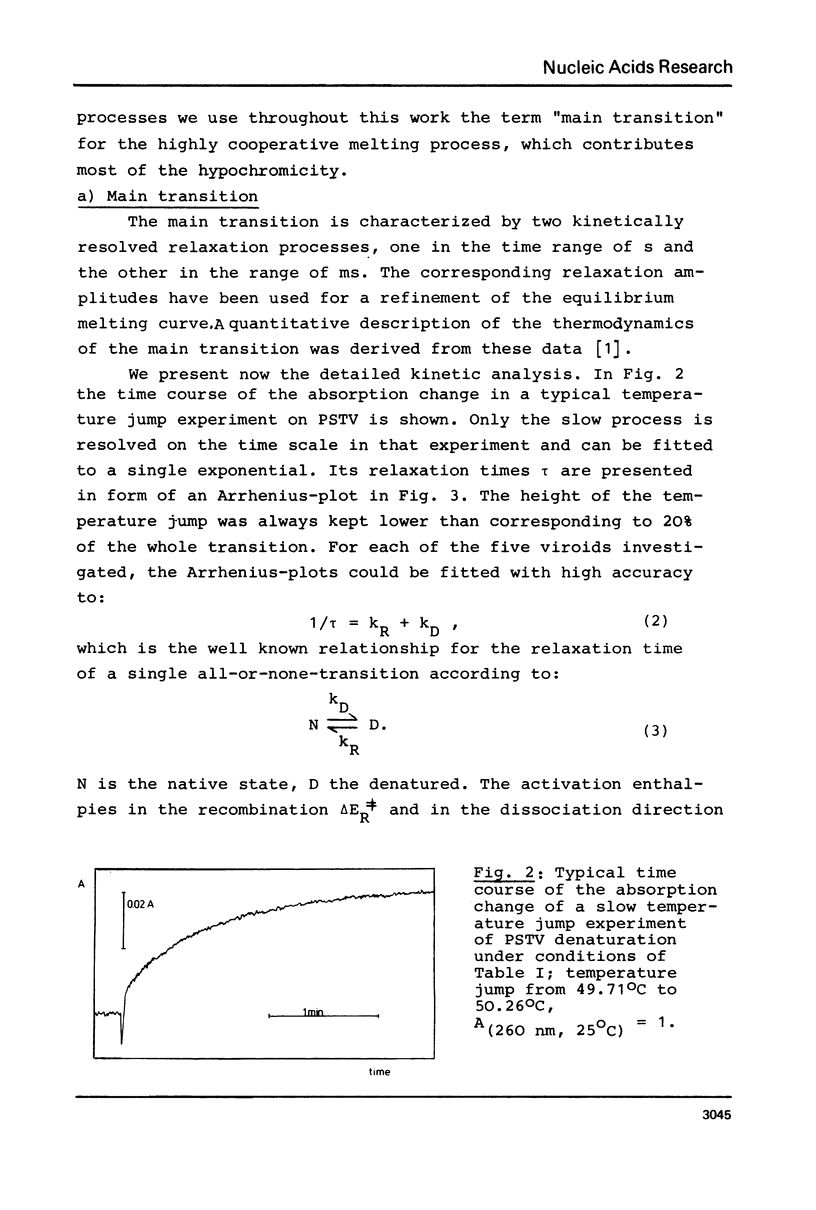
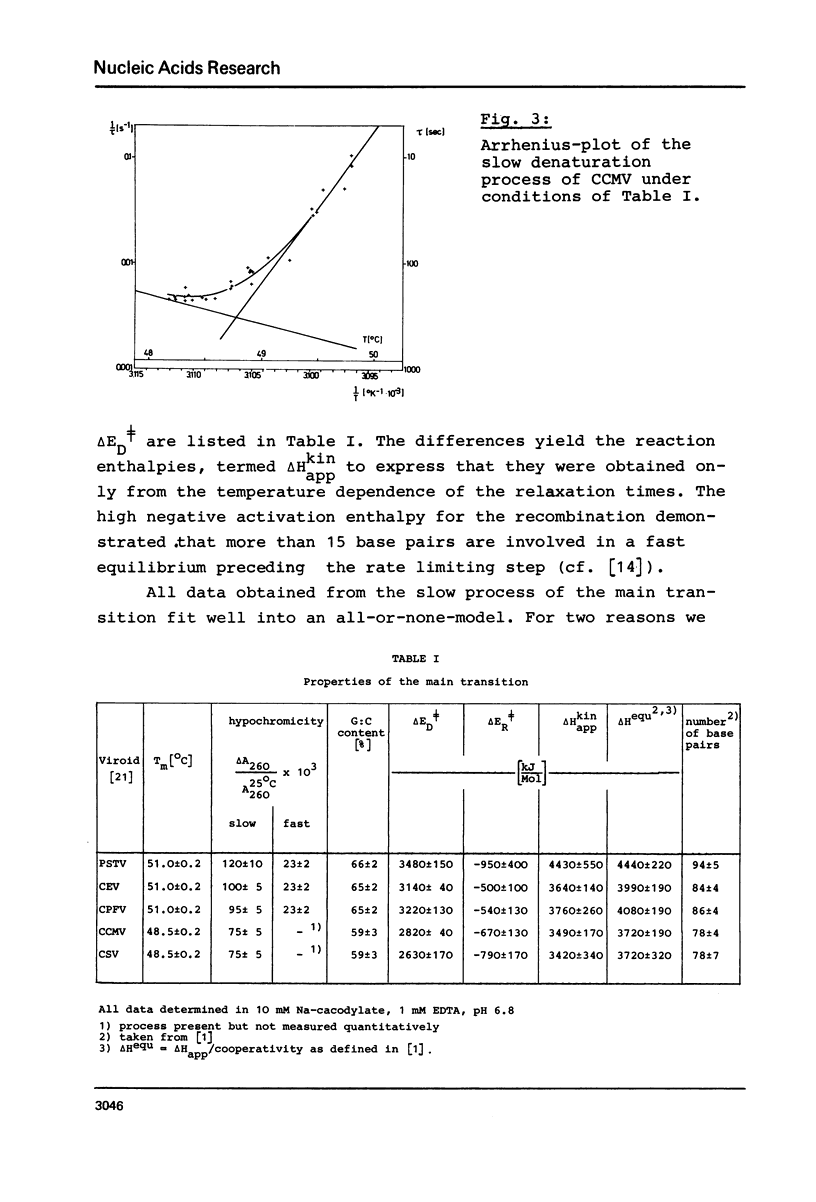
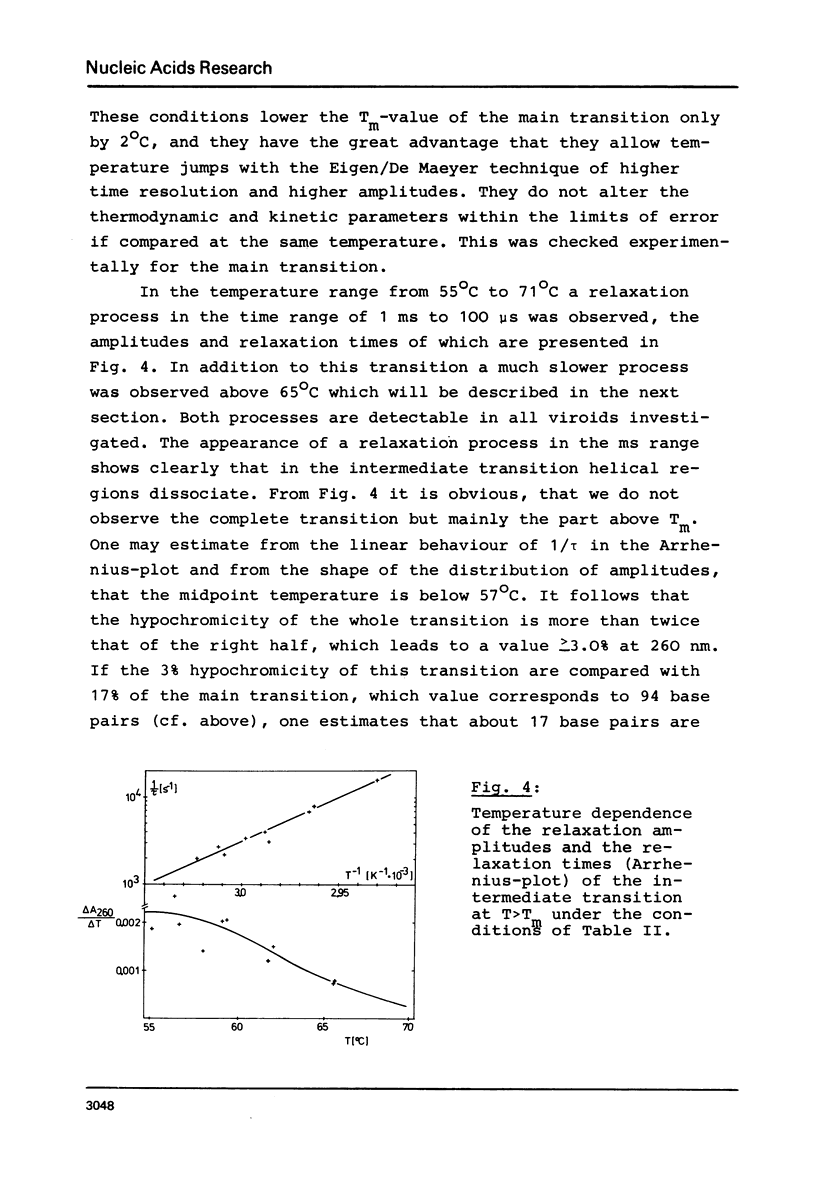
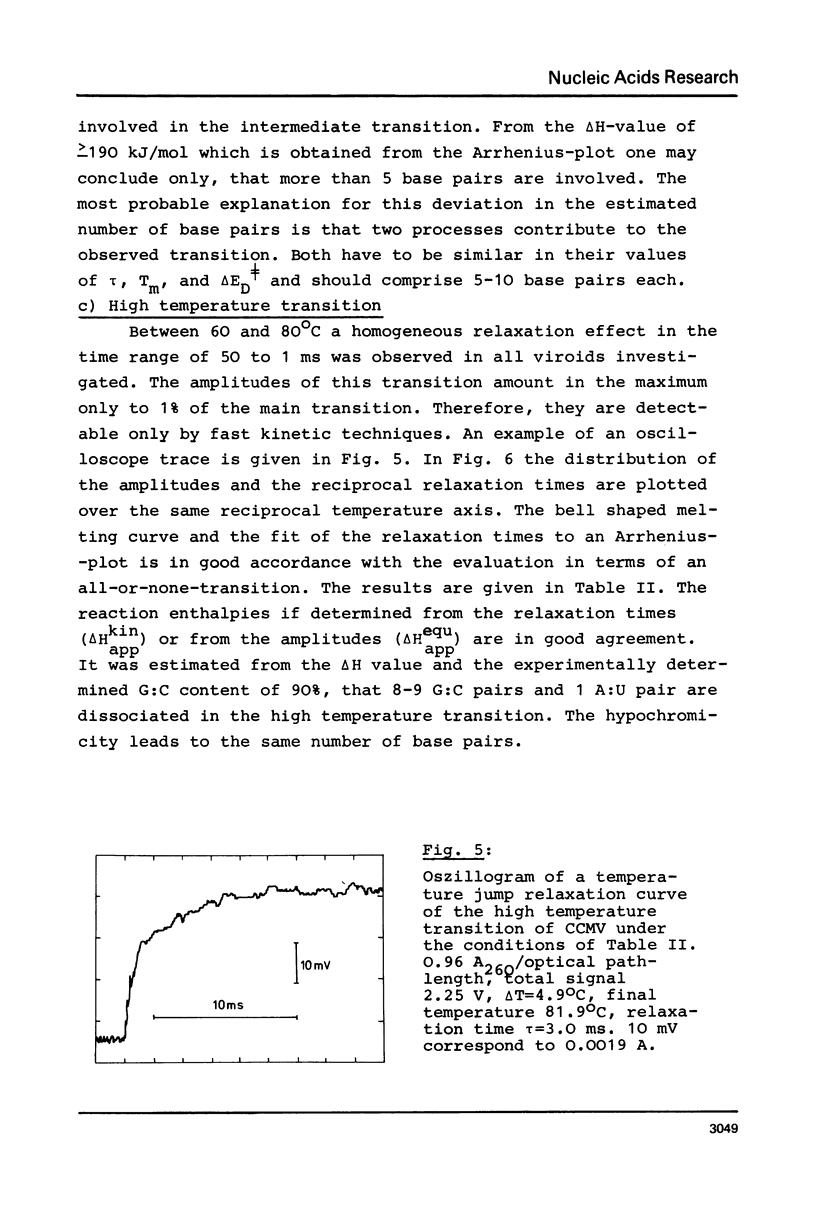
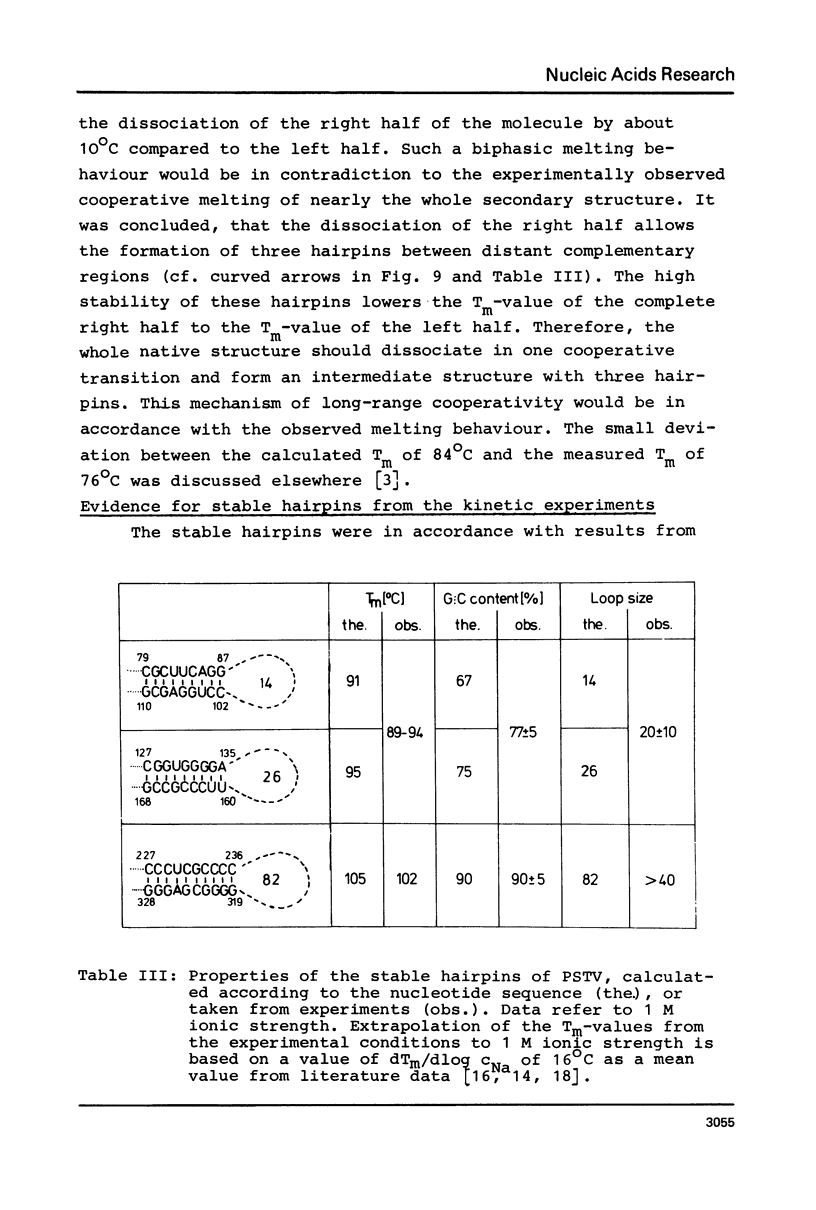
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coutts S. M. Thermodynamics and kinetics of G-C base pairing in the isolated extra arm of serine-specific transfer RNA from yeast. Biochim Biophys Acta. 1971 Feb 25;232(1):94–106. doi: 10.1016/0005-2787(71)90494-1. [DOI] [PubMed] [Google Scholar]
- Gross H. J., Domdey H., Lossow C., Jank P., Raba M., Alberty H., Sänger H. L. Nucleotide sequence and secondary structure of potato spindle tuber viroid. Nature. 1978 May 18;273(5659):203–208. doi: 10.1038/273203a0. [DOI] [PubMed] [Google Scholar]
- Gross H. J., Domdey H., Sänger H. L. Comparative oligonucleotide fingerprints of three plant viroids. Nucleic Acids Res. 1977 Jun;4(6):2021–2028. doi: 10.1093/nar/4.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henco K., Riesner D., Sanger H. L. Conformation of viroids. Nucleic Acids Res. 1977 Jan;4(1):177–194. doi: 10.1093/nar/4.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump H., Riesner D., Sänger H. L. Calorimetric studies on viroids. Nucleic Acids Res. 1978 May;5(5):1581–1587. doi: 10.1093/nar/5.5.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langowski J., Henco K., Riesner D., Sänger H. L. Common structural features of different viroids: serial arrangement of double helical sections and internal loops. Nucleic Acids Res. 1978 May;5(5):1589–1610. doi: 10.1093/nar/5.5.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F. M. Einfache Temperatursprung-Methode im Sekunden-bis Stundenbereich und die reversible denaturierung von Chymotrypsin. Eur J Biochem. 1968 Apr;4(3):373–377. doi: 10.1111/j.1432-1033.1968.tb00221.x. [DOI] [PubMed] [Google Scholar]
- Pörschke D. Model calculations on the kinetics of oligonucleotide double helix coil transitions. Evidence for a fast chain sliding reaction. Biophys Chem. 1974 Aug;2(2):83–96. doi: 10.1016/0301-4622(74)80028-1. [DOI] [PubMed] [Google Scholar]
- Riesner D., Römer R., Maass G. Kinetic study of the three conformational transitions of alanine specific transfer RNA from yeast. Eur J Biochem. 1970 Jul;15(1):85–91. doi: 10.1111/j.1432-1033.1970.tb00979.x. [DOI] [PubMed] [Google Scholar]
- Sanger H. L., Klotz G., Riesner D., Gross H. J., Kleinschmidt A. K. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3852–3856. doi: 10.1073/pnas.73.11.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz G., Engel J. Kinetics of cooperative conformational transitions of lineal biopolymers. Angew Chem Int Ed Engl. 1972 Jul;11(7):568–575. doi: 10.1002/anie.197205681. [DOI] [PubMed] [Google Scholar]
- Van N. T., Holder J. W., Woo S. L., Means A. R., O'Malley B. W. Secondary structure of ovalbumin messenger RNA. Biochemistry. 1976 May 18;15(10):2054–2062. doi: 10.1021/bi00655a005. [DOI] [PubMed] [Google Scholar]


