Abstract
Chromosomally normal first trimester fetuses with an increased nuchal translucency measurement have an elevated risk of congenital heart defect (CHD). so there is an increased demand for imaging the fetal heart during the first and early second trimesters of pregnancy.
Echocardiographic and anatomical correlations in firsttrimester fetuses show that by 11 weeks’ gestation, the position of the fetal heart within the chest is similar to that in later gestation, and the spatial relation of the great arteries and their relative sizes are similar to those on second-trimester scans by 12 weeks’ gestation.
In the first trimester during the heart analysis it’s possible value: anatomic structure (size, rate), hemodynamic development through analysis of these waveforms and flow patterns (inflow and outflow waveforms of the diastolic filling and the systolic ejection) and modification during the first trimester.
Keywords: fetal heart, function, echocardiography, first trimester
Introduction
Extensive studies have confirmed the feasibility of first trimester nuchal translucency measurements for Down syndrome screening in the general population (1, 2).
It was observed previously that chromosomally normal first trimester fetuses with an increased nuchal translucency measurement have an elevated risk of congenital heart defect (CHD) (3). CHD is the most important category of congenital anomaly, with a reported worldwide frequency of 4 to 9 of every 1000 births (4).
So the development of first-trimester screening programmes for chromosomal abnormalities based on measurements of fetal nuchal translucency at 10-14 weeks’ gestation, there is an increased demand for imaging the fetal heart during the first and early second trimesters of pregnancy.
Material and Methods
We analyzed the works, in the recent literature, on the study of the heart in the first trimester of pregnancy.
After analyzing the individual work, evaluating the diagnostic methods used, the parameters analyzed, we evaluated the results and the presence of any differences found by the individual authors.
Results
The heart rate (HR) increases between the 5th week of gestation and 9th week of gestation and after the 13th week of gestation reduces. The inflow waveform is monophasic in every case until 9+ weeks of gestation and at 10+ weeks, inflow patterns are biphasic.
The isovolumic relaxation time (IRT) decreases significantly between 6+ and 7+ weeks while isovolumic contraction time (ICT) decreases from 8+ to 9+ weeks.
The valvular regurgitation is diagnosed if it is found during at least half of the systole and with a velocity of over 60 cm/s and it’s important don’t confuse the presence of click, corresponding to the opening or closure of the valvular structures showed the presence of valve signals, with the regurgitation.
Discussion
Echocardiographic and anatomical correlations in firsttrimester fetuses show that by 11 weeks’ gestation, the position of the fetal heart within the chest is similar to that in later gestation, and the spatial relation of the great arteries and their relative sizes are similar to those on second- trimester scans by 12 weeks’ gestation (5).
Many studies have valuated the efficacies of early echocardiography and have compared these two different methods: tv and transabdominal.
Smrcek et al have demostrated that at 10th-13rd gestational week transvaginal echocardiography can better show the anatomic heart details, whereas afther the 15th gestational week the transabdominal method is more usefull. Finally at 14th gestational weeks there isn’t any different between these two different approaches (6).
Heart is a vital organ of a human. Cardiovascular development in a human embryo occurs between 3 and 6 weeks after ovulation. Cardiac function is the first sign of independent cardiac activity that can be explored with non-invasive techniques such as Doppler ultrasound (5).
Heart and placenta are the main organs determining fetal hemodynamics. Two types of circulation unite these organs: on one side, the venous circulation, and on the other, the arterial circulation. Both circulations can be altered by changes at the placental, cardiac, central nervous system, and peripheric levels.
At the end of the 4th week of gestation, the heartbeats of the embryo begin.
The heart, whose development starts at the 3rd week of gestation, has rapid and irregular contractions capable of pumping the blood inside the vessels.
At this period, the developing circulatory system allows maternal- embryonic nutritive and gaseous changes at the chorionic villi. It is well documented in the literature that, in healthy fetuses, the heart rate (HR) increases from 110 bpm at the 5th week of gestation to 170 bpm at the 9th week of gestation. From then on, there is a gradual reduction in the HR that reaches a mean value of 150 bpm at the 13th week of gestation.
The initial elevation of the HR coincides with the morphological development of the heart, and the subsequent decline can result from the functional maturation of the parasympathetic nervous system (7).
The fetal circulation works in parallel with the dominant right ventricle, ejecting approximately 60% of the combined ventricular output. Three important communications exist between the two circulations (oval foramen and the arterial and venous ducts) that influence loading conditions. Pulmonary venous return contributes only a small proportion to left ventricular preload because of the relatively low pulmonary blood flow in fetal life (8).
Left ventricular filling depends predominantly on patency of the oval foramen to allow the relatively oxygen-rich blood returning from the placental circulation, via the umbilical vein and venous duct, to stream through the right atrium and enter the left side of the heart (9).
The right ventricle fills from mostly upper body systemic venous return and the majority of its output is diverted away from the pulmonary circulation, via the arterial duct, to the descending aorta and thence to the placenta via the umbilical arteries. Hence the fetal right ventricle pumps against the systemic pressures of the lower fetal body and placental impedance, while the left ventricle ejects against the relatively high impedance of the fetal brain and upper body.
Not surprisingly, ventricular loading is affected by the maturation of structures such as the placenta and changes in the impedance of the cerebral and placental vascular beds in response to hypoxaemia, as well as circulatory adaptations made by the fetus in response to intracardiac malformations (10).
The cardiac flow velocities are influenced by preload, contractile function, afterload and heart rate. The cardiac functional development in early pregnancy is not well know so,
Doppler techniques is able to study early human fetal cardiac function indirectly, and it have made it possible to examine the human fetal heart and vessel non invasively and to determine normal and abnormal cardiovascular in early phases of pregnancy (11).
The heart undergoes a unique combination of morphological and physiological changes to reach an anatomical maturity while maintaining the circulatory needs of the developing embryo. Therefore, it is not surprising that early cardiac and valvular functions in the fetus are characterized by changes in blood velocities within the heart (12).
Fetal cardiovascular performance is dependent on physiological determinants of cardiac function: systolic function, primarily determinants by the amount of blood distending the ventricles before contraction (preload), the combined resistance of the blood, ventricular mass, and central and peripheral vascular beds (afterload), the intrinsic ability of the myocardial fibres to contract (contractility) and rate of contraction (heart rate) (5).
The cardiac cycle is composed by:
systolic ejection;
isovolumic relaxation, it’s the time from the end of ventricular ejection to the onset of ventricular filling (IRT);
diastolic filling;
isovolumic contraction, it’s the interval between the cessation of diastolic filling and the onset of ventricular ejection (ICT).
During the pregnancy there’s a modification of the different phases of cardiac cycle (11).
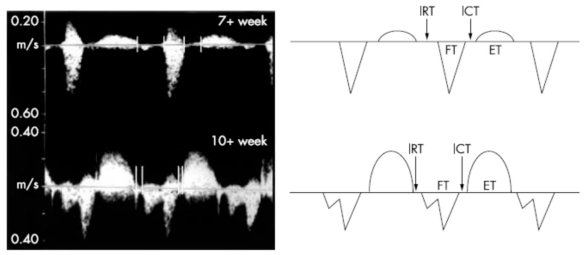
The inflow waveform is monophasic in every case until 9+ weeks of gestation and at 10+ weeks, inflow patterns are biphasic, in fact in early gestation, the rapid filling portion of diastole (E wave) is not present (12) (Fig. 1).
Figure 1.
- The upper panel also illustrates the presence of the isovolumic contraction time (ITC) and the isovolumic relaxation time (IRT) at 6 weeks of gestation. The image below detained at 12 weeks demonstrates the presence of biphasic atrial (A and E waves) and ventricular contraction velocities.
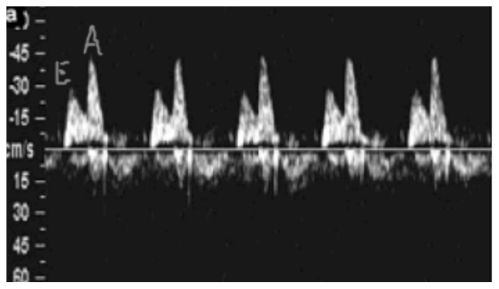
When the waveform was biphasic the E (early ventricular filling) and A (filling during atrial contraction) wave peak velocities and time-velocity integrals were obtained, and their A to E ratios were calculated (13) (Fig. 2).
Figure 2.
- Flow across the atrial-ventricular valves: E, velocity during early diastole; A, velocity in late diastole (atrial contraction).
In the fetus the peak velocity during early diastole (peak E velocity) is significantly lower than the peak velocity in late diastole (peak A velocity), in contrast with the adult heart (11).
This modification in waveform depends by better empyting of the ventricle, thus lowering ventricular pressure at the beginning of diastole and allowing passive filling to occur in early diastole (13).
The tricuspid peak E and A velocities systematically exceeded the mitral peak E and A velocities.
The A/E ratio tendes to decrease during the gestational age, for the tricuspid valve the decreasing A/E ratio is caused by an increase in peak E velocity while for the mitral valve is caused by a decrease in A wave velocity.
A indirectly evaluating cardiac contractility is the assessment of diastolic function in the fetus.
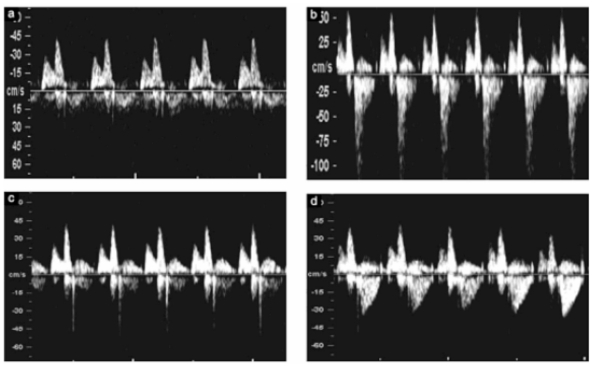
Doppler blood flow velocities across the tricuspid and mitral valve have been used as indicators of ventricular filling (11) (Fig. 3).
Figure 3.
- Doppler flow profile in the tricuspid valve with non regulation during systole (a). Regurgitation during approximately half of systole and with a velocity more than 60 cm/s (b). The short reverse ‘spike’ generated by closure of the valve cusp (c). And the jet produced by aortic or pulmonary arterial blood flow, which at this gestation can produce a maximum velocity of 50 cm/s (d).
IRT describes the time needed for the ventricle to drop its pressure from a systemic to an atrial level. IRT% can be used to describe diastolic function of the heart, especially during the early part of diastole. IRT decreases significantly between 6+ and 7+ weeks and the improvement in the cardiac diastolic function in early pregnancy is important for the fetal heart to adapt to an increased volume blood flow (13).
ICT is the time interval needed for the ventricle to increase its pressure to the systemic blood pressure level, thus giving information about ventricular contractility and pressure generation during the early part of systole. ICT% decreases from 8+ to 9+ weeks so it’s possible that a improved pressure generation of the ventricle enables better emptying of the ventricle, thus lowering ventricular pressure at the beginning of diastole and allowing passive filling to occur in early diastole (13).
Another important element that it’s possible study is the presence of valve regurgitation during systole.
AVVR begins when ventricular pressure exceeds the atrial pressure and it lasts until atrial pressure again exceeds ventricular pressure. Thus, the regurgitation jet is present before ventricular ejection occurs. In addition, the maximum velocity of AVVR is higher than that of ventricular ejection because the systemic pressure is much greater than the atrial pressure (13).
The regurgitation is diagnosed if it is found during at least half of the systole and with a velocity of over 60 cm/s, since aortic or pulmonary arterial blood flow at this gestation can produce a maximum velocity of 50 cm/s.
It’s important don’t confuse the presence of click, corresponding to the opening or closure of the valvular structures showed the presence of valve signals, with the regurgitation.
It’s possible examine the flow velocities in the umbilical artery and fetal aorta and the analysis of waveform. In the first trimester there is a high pulsatility and absence of end-diastolic flow velocities in both umbilical arteries and reflecting the downstream impedance at the fetal placental level.
There is a slight increase in PI values from 7 weeks until 11-12 weeks followed by a decrease afterwards. This reduction may be explained by a drop in umbilical-placental resistance that coincides in the maternal side with a resurgence of endovascular trophoblast migration with a second wave of cells moving into the muscular layer of spiral arteries and a process of angiogenesis in the placenta.
An initially high resistance territory is converted in a low pressure conductance system that is ready to accommodate the increased blood flow volume of the developing fetus (11).
This low resistance circuit is pivotal to permit appropriate fetal growth as the right ventricle (unlike the left) responds to increasing afterload with a fall in ventricular output (10).
References
- 1.Snijders RJM, Noble P, Sebire N, Souka A, Nicolaides KH. UK multicenter project on assessment of risk of trisomy 21 by maternal age and fetal nuchal translucency thickness at 10-14 weeks of gestation. Lancet. 1998;351:343–6. doi: 10.1016/s0140-6736(97)11280-6. [DOI] [PubMed] [Google Scholar]
- 2.Wapner R, Thom E, Simpson JL, Pergament E, Silver R, Filkins K, et al. First- trimester screening for trisomies 21 and 18. N Engl J Med. 2003;349:1405–13. doi: 10.1056/NEJMoa025273. [DOI] [PubMed] [Google Scholar]
- 3.Hyett AJ, Perdu M, Sarland G, Snijders R, Nicolaides K. Using fetal nuchal translucency to screen for major cardiac defects at 10-14 weeks of gestation: population based cohort study. BMJ. 1999;318:81–5. doi: 10.1136/bmj.318.7176.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell S. Isolated major congenital heart disease. Ultrasound Obstet Gynecol. 2001;17:370–9. doi: 10.1046/j.1469-0705.2001.00439.x. [DOI] [PubMed] [Google Scholar]
- 5.Carvalho J S, Moscoso G, Ville Y. First-trimester transabdominal fetal echocardiography. Lancet. 1998;351:1023–27. doi: 10.1016/S0140-6736(97)08406-7. [DOI] [PubMed] [Google Scholar]
- 6.Smrcej IM, Berg C, Geipel A, et al. Early fetal echocardiography. Heart biometry and visualization of cardiac structures between 10 and 15 weeks’ gestation. J Ultrasound Med. 2006;25:173–182. doi: 10.7863/jum.2006.25.2.173. [DOI] [PubMed] [Google Scholar]
- 7.Murta CGV, Moron AF, Pinto de Avila MA. Detection of Functional Changes of the Fetal Heart in the First Trimester of Gestation. Arq Bras Cardiol. 1999;72(6):745–750. doi: 10.1590/s0066-782x1999000600009. [DOI] [PubMed] [Google Scholar]
- 8.Rasanen J, Wood DC, Weiner S, et al. Role of the pulmonary circulation in the distribution of human fetal cardiac output during the second half of pregnancy. Circulation. 1996;94:1068–73. doi: 10.1161/01.cir.94.5.1068. [DOI] [PubMed] [Google Scholar]
- 9.Edelstone DI, Rudolph AM. Preferential streaming of ductus venosus blood to the brain and heart in fetal lambs. Am J Physiology. 1979;237:H724–9. doi: 10.1152/ajpheart.1979.237.6.H724. [DOI] [PubMed] [Google Scholar]
- 10.Gardiner H.M. Response of the fetal heart to changes in load from hyperplasia to heart failure. Heart. 2005;91:871–873. doi: 10.1136/hrt.2004.047399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matias A., Montenegro N., Arelas JC., Leite LP. Haemodynamic evaluation of the first trimester fetus with special emphasis on venous return. Human Reproduction Update. 2000;6(2):177–189. doi: 10.1093/humupd/6.2.177. [DOI] [PubMed] [Google Scholar]
- 12.Leiva M. C., Tolosa J. E., Binotto C.N., Weiner S, Huppert L, Denis A.L, Huhta J.C. Fetal cardiac development and hemodynamics in the first trimester. Ultrasound Obstet Gynecol. 1999;14:169–174. doi: 10.1046/j.1469-0705.1999.14030169.x. [DOI] [PubMed] [Google Scholar]
- 13.Ma¨kikallio K, Jouppila P, Räsänen. J. Human fetal cardiac function during the first trimester of Pregnancy. Heart. 2005;91:334–338. doi: 10.1136/hrt.2003.029736. [DOI] [PMC free article] [PubMed] [Google Scholar]