Abstract
Preeclampsia (PE) is one of the most common diseases worldwide, complicating ~5% of all pregnancies.
Although no major progress has been achieved in the treatment of PE, our ability to identify women at highrisk has increased considerably during the past decade.
The early identification of patients with an increased risk for preeclampsia is therefore one of the most important goals in obstetrics. Today, several markers may offer the potential to be used, most likely in a combinatory analysis, as predictors or diagnostic tools. We present here the current knowledge on the biology of preeclampsia and review several biochemical markers which may be used to monitor preeclampsia in a future, that, we hope, is not to distant from today.
Keywords: preeclampsia, early diagnosis, hypertension, flt-1, sEng, P-selectin, effDNA, ADAM12, PP-13, PTX3, PAPP-A
Introduction
Preeclampsia occurs in 2–5% of pregnancies in the Occident, but it complicates up to 10% of pregnancies in the developing countries, where emergency care is often inadequate or lacking. Therefore we are in need of a widely applicable and affordable test that could permit presymptomatic diagnosis in order to identify and monitor the patients at risk and thus provide the best prenatal care for these women and their child. Such a test would also be of benefit to confirm a confounding clinical diagnosis and for future studies investigating prophylactic treatments or temporizing therapies.
To be effective a screening test need to be sufficiently sensitive and specific and must provide an adequate positive predictive value. Today, several promising markers have been described, alone or in combination, that might fulfill these criteria. However, these data came often from small case studies with selected populations.
Therefore, there is a need for worldwide large scale prospective studies to confirm the sensitivity and specificity of these promising markers and assess their utility in different subtypes of preeclampsia before they could serve in clinically useful screening tests.
Preeclampsia
Preeclampsia is a multi-system disorder of pregnancy, which is characterized by new onset hypertension (systolic and diastolic blood pressure of ≥ 140 and 90 mm Hg, respectively, on two occasions, at least 6 hours apart) and proteinuria (protein excretion of ≥ 300 mg in a 24 h urine collection, or a dipstick of ≥ 2+), that develop after 20 weeks of gestation in previously normotensive women (1, 2).
Dependent on the systemic involvement, several other symptoms, such as edema, disturbance of haemostasis, renal or liver failure, and the HELLP syndrome (hemolysis, elevated liver enzymes, and low platelet counts) also complicate the clinical picture. Preeclampsia can have an early onset (preeclampsia starting before 34 weeks of gestation) or late onset (preeclampsia starting after 34 weeks of gestation), can show mild or severe symptoms (systolic blood pressure ≥ 160 mmHg or diastolic blood pressure ≥ 110 mmHg, proteinuria >5 g/24 hours, oliguria, neurological symptoms, other clinical symptoms such as deranged liver function, thrombocytopenia < 100 000 mm3, HELLP syndrome), and can evolve in eclampsia in the most severe cases. In addition, it can manifest as a maternal disorder only, with an appropriate fetal growing, or it can present itself with a growth restricted fetus (intrauterine growth restriction (IUGR)) or sudden fetal distress.
Pathophysiology
The precise origin of preeclampsia remains elusive, but it is believed to be likely multifactorial. A certainty is the central role played by the placenta in its pathology (3, 4, 5, 6).
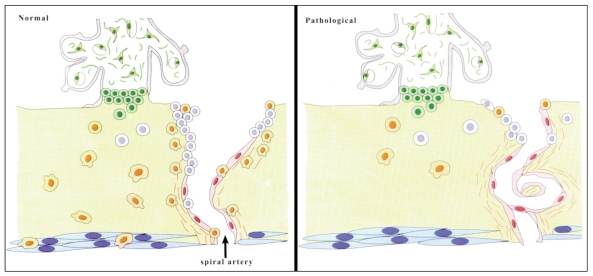
A long standing hypothesis has been that preeclampsia develops as a consequence of some kind of immune maladaptation between the mother and the fetus during the very first weeks of pregnancy, leading to a 2-step disorder progression that can be summarized as following: in a first – asymptomatic – step, local aberrant fetomaternal immune interactions within the uterine wall lead to impaired tissue and arterial invasion by trophoblast cells. This results in failed transformation of the uterine spiral arteries and subsequently worsened placental perfusion. Chronic hypoxia or alternate periods of hypoxia/re-oxygenation within the intervillous space is expected to trigger tissue oxidative stress and increase placental apoptosis and necrosis (7, 8) (Fig. 1).
Figure 1.
The clinical disorder arises, in a second step, when the maternal vascular and immune systems cannot handle any longer the increased shedding of placental-produced debris and the aberrant expression of pro-inflammatory, anti-angiogenic and angiogenic factors, leading to a systemic endothelial cell dysfunction and an exaggerated inflammatory response (1, 9, 10). Recently, this hypothesis has been challenged (11).
It was proposed instead that intrinsic failure in trophoblast differentiation at different time points of ontogeny may lead to either a mild disorder with late-onset appearance, or IUGR complicated or not with the maternal symptoms.
However, the origin of preeclampsia might not be restricted to an alteration of trophoblast differentiation, but may also in some cases depend on an underlying maternal constitutional factors such as genetic, obesity, dysfunctional maternal clearance or inflammatory systems (12).
Material and Methods
Since many years, different biophysical and biochemical markers have been investigated, based on pathophysiological observations that have been noted in case of preeclampsia, such as placental dysfunction, a generalized inflammatory response, endothelial dysfunction and activation of the coagulation system.
Using a series of keywords, we reviewed electronic databases (Medline, Elsevir) reporting the performance of biological markers to predict preeclampsia, both single markers and combinations of markers.
Biomarkers
Angiogenic factors
Angiogenesis requires the complex interplay between the pro-angiogenic factors vascular endothelial growth factor (VEGF) and placental growth factor (PlGF) with their cognate receptors VEGF receptor-1 (VEGFR-1, which is alternatively called fms-like tyrosine kinase (flt)-1) and VEGFR-2 [for a review on the function of these factors (13)].
Interestingly, the placenta is a rich source of these factors (14, 15).
In addition to regulating blood vessel homeostasis, VEGF, PlGF and the flt-1 receptor have been shown to be key components in regulating trophoblast cell survival and function (14, 16).
Placental cells also secrete a soluble isoform of flt-1, which is generated through alternative splicing of the messenger RNA and acts as an anti-angiogenic factor by interacting with, and thereby neutralizing, PlGF and VEGF (17).
There is strong evidence for the occurrence of higher placental expression of sflt-1 and repeated findings of elevated circulating levels of sflt-1 and reduced free bioactive PlGF and VEGF in preeclamptic patients (9, 18, 19, 20, 21).
It was thus suggested that a part of this excess of circulatory sflt- 1 may stem from the placenta. Maternal blood levels of sflt-1 were shown to correlate with the severity of preeclampsia, whereas, in an opposite manner, the quantities of bioactive VEGF and PlGF were further decreased in patients with severe symptoms compared to normal pregnant women or preeclamptic patients with mild symptoms (9, 18, 22). Alterations in sflt-1 and PlGF are also more pronounced in early onset in comparison to late onset preeclampsia (22, 23).
However, it was also shown that increased levels of sflt-1 were also associated with IUGR (24).
Remarkably, when introduced into pregnant rats, exogenous sflt-1 triggers hypertension and proteinuria, symptoms akin to those in preeclampsia (9).
According to some studies, the presymptomatic alterations in sflt-1 levels appeared to be specific for preeclampsia as no changes are detected in women who later deliver SGA neonate or whose pregnancies are complicated by IUGR, compared to women with normal pregnancy outcome (25, 26).
However, others found that in a selected group of patients with abnormal uterine perfusion, similar alterations in sflt-1 and PlGF levels could be detected during the second trimester in cases with subsequent IUGR 27.
Nevertheless, owing to the evolving unbalance of angiogenic factors after 25 weeks of gestation in women with subsequent preeclampsia, the ratio sflt-1/PlGF has been advocated to be a reliable marker of overall preeclampsia risk. As a matter of fact, soluble flt-1 and PlGF have been launched by Roche as a screening test for preeclampsia in the second trimester in Europe and is expected to be submitted to the FDA soon.
It has recently been reported that patients with pree - clampsia have lower plasma concentrations of soluble VEGF-R2 (28).
However, this biomarker may not be specific for preeclampsia as an equivalent decrease was observed in patients with SGA babies in the absence of preeclampsia.
Soluble Endoglin
Endoglin (Eng) is a co-receptor for transforming growth factor (TGF)-β1 and TGF-β3 that is highly expressed on cellular membranes of the vascular endothelium and on the syncytiotrophoblast (29, 30).
It functions as a modulator of TGF-β signaling and is involved in angiogenesis and the regulation of the vascular tone (31, 32).
A circulatory form of endoglin, which consists of the extra cellular part of the molecule that may be produced through the proteocleavage of the placental membranebound form, has been identified in normal pregnancy and in preeclampsia.
In vitro, sEng acts as a negative regulator of angiogenesis by competitive interaction with TGF-β, thereby impairing capillary formation by endothelial cells. Furthermore, it induces high arterial pressure and vascular permeability in pregnant rats in which the protein was over-expressed. Very interestingly, the combined introduction of sEng and sflt-1 in the pregnant animals induced renal, placental and hepatic changes reminiscent of the HELLP syndrome (33).
Soluble Eng is present in substantial excess in preeclamptic patients compared to normotensive controls, and its concentrations appear to increase with the severity of the symptoms and are the highest in preeclampsia complicated by the HELLP symptom (24, 33, 34).
Pregnancies with IUGR without the maternal syndrome may also be characterized by elevated levels of sEng, suggesting that this factor is not specific for preeclampsia, but may be a marker for clinical conditions associated with an underlying placental pathology (24, 34).
However, these results remain conflicting as others demonstrated no association between IUGR and the levels of sEng. Moreover, a pilot study has suggested that sEng may prove useful in differentiating preeclampsia from other hypertensive diseases of pregnancy, such as gestational or chronic hypertension (35).
Large scale studies will be needed in order to clarify these important issues. Like sflt-1, sEng concentrations raise during the last 2 months of normal pregnancy. In pregnancies ending with preeclampsia, this increase occurs earlier and is steeper (26, 36, 37).
The distinction becomes significant starting 9 - 11 weeks before the clinical symptoms, for both early and late onset preeclampsia, but is more prominent for preterm preeclampsia or in women in whom preeclampsia is complicated with SGA. Altered levels of this factor throughout gestation are also associated with SGA pregnancies without the maternal symptoms (26, 38).
Thus, a specific prediction cannot be achieved with this analyses alone.
Several longitudinal case-control studies have therefore evaluated the potential of sEng in combination with the pro- and anti-angiogenic factors PlGF and sflt-1 for the prediction of preeclampsia (26, 36).
The studies reported that the pattern of changes in the ratio of different combinations of these factors (PlGF/sEng; (sflt- 1+sEng)/PlGF; etc), collected at 13 weeks and around 20 weeks, was more informative than the individual biomarkers at single time-point screening. One study suggested that a rigorous monitoring of the sequential changes in the profile of these three biomarkers between the first and the second trimesters permits sensitive and specific risk assessment (38).
A change in PlGF/sEng ratio that was below the median slope for controls conferred an odds ratio of 7.68 for the development of pre-term preeclampsia, and 2.46 for the development of term preeclampsia, and discriminated SGA pregnancies from preeclampsia. Further studies with large number of patients will be required to confirm these very promising preliminary results and assess the utility of analyzing these biomarkers in the clinical routine.
P-Selectin
P-selectin is a member of the selectin family of cell surface adhesion molecules. It is expressed by platelets and endothelial cells upon activation and plays crucial roles in inflammatory reactions by supporting the recruitment and activation of circulating leucocytes, and in coagulation through the generation of leukocyte-derived “blood borne” tissue factor (39, 40).
P-selectin is rapidly shed from the cellular membrane of activated platelets and this release is suggested to contribute to most of the soluble isoform of the molecule that is found in the plasma (41).
Preeclampsia is associated with extensive platelet activation (42).
P-selectin-exposing micro particles with procoagulant activity, released from activated platelets, have been detected in the peripheral blood of preeclamptic women (43, 44). In addition, soluble P-selectin has been repeatedly, though not constantly, observed in higher amounts in serum or plasma of patients with this disorder (45, 46).
Interestingly, it has recently been shown that alterations in the levels of soluble P-selectin before 20 weeks of gestation antedate the symptoms (47,48). This early upregulation of soluble P-selectin has been suggested to reflect the early but still asymptomatic disturbances of the maternal vascular system. In one of these studies, P-selectin was identified as the marker with the highest discriminatory ability among three biomolecules evaluated between gestational weeks 11 to 15 (47).
However, the combination of P-selectin with the two other markers, namely Activin A and VEGFR, showed a detection rate of only 59% (with a false-positive rate of 5%), which is not sufficient for a possible routine clinical implementation as a screening test.
Cell-free fetal DNA
Since its detection in maternal plasma many approaches have been tested to use cell free fetal DNA for non-invasive diagnostic approaches. These include qualitative analyses like fetal sex analysis (49), determination of the fetal Rhesus status (50, 51) or the analysis of fetal point mutations (52) as well as the quantitative analysis as an indicator for several fetal anomalies, e.g. fetal growth restriction (53), polyhydramnios (54), trisomy (55) or preterm labor (56).
The value of cffDNA in maternal plasma as an indicator for preeclampsia has first been reported by Lo et al. in a small scale study in the plasma of 20 preeclamptic women and 20 gestational age matched controls in the third trimester, where cffDNA was increased approximately 5-fold in women with preeclampsia (57). The same effect was observed in the second trimester in a study by Zhong et al. in 10 preeclamptic women and 40 controls (58). The so far biggest study in that field was conducted by Levine et al. with 120 preeclamptic women and 120 controls: A two- to five-fold increase of cffDNA levels was monitored starting from week 17 until three weeks before the onset of preeclampsia (59).
As the amount of fetal DNA is routinely determined by quantifying Y-chromosome specific sequences, e.g. SRY (sex determining region Y) and DYS (60), alternative approaches have been tested to overcome this limitation:
An increase of total cell free DNA was observed in women with preeclampsia at term (61, 62, 63) and before the onset of preeclampsia (63). Furthermore, approaches to analyze cffDNA independent from fetal sex, using epigenetic differences between maternal and fetal DNA have been developed, e.g. the use of the maspin gene, which is hypomethylated in fetal tissue (64) or the hypermethylated fetal promoter sequence of RASSF1A (65).
Although these approaches are promising, only one study quantifying cffDNA with the RASFF1A approach in 10 women with preeclampsia and 20 controls has been published (66). cffDNA has shown some predictive value for the prediction of preeclampsia between 20–25 weeks of gestation, however, higher sensitivities and specificities can be obtained by combining several markers as has been shown in a nested case-control study for cell free DNA combined with Inhibin A in the second (n = 15 at risk for PE), n = 68 controls) and third trimester (n = 34 preeclampsia, n = 44 controls) (67).
Currently, several multicenter studies are being performed to confirm the predictive value of cffDNA to predict and monitor preeclampsia in combination with other potential markers, e.g. P-selectin, PAPP-A, PP-13, sflt-1, sEng, PlGF).
ADAM12
ADAM12 (a disintegrin and metalloprotease 12) is a membrane bound zinc dependant protease and belongs to the ADAM protein family, a group of proteins involved in cell-cell and cell-matrix interactions in fertilization, muscle development and neurogenesis (68, 69). For this gene, two alternatively spliced transcripts are known, a short secreted form and a long membrane-bound form (70). The plasma concentration of ADAM12 has been found to be altered in several pregnancy related disorders. Several studies have demonstrated that the plasma level of ADAM12 is decreased in women carrying a fetus with trisomy 21 and trisomy 18 (71, 72, 73). It has also been shown that the ADAM12 concentration is decreased in women with other aneuploidies and in women with low for gestational age birth weights (74).
The first connection of ADAM12 serum levels to preeclampsia was demonstrated by Laigaard et al. in a study with 160 women with preeclampsia and 324 healthy controls in the first trimester (75). The serum concentration of ADAM12 was significantly decreased in women that later developed preeclampsia. These results were confirmed by Spencer et al. in a study with two groups (1. n = 64 PE, n = 240 controls, 2: n = 24 cases, n = 144 controls) (76). However another study failed to confirm these promising results but concluded that measurement of ADAM12 does not provide useful prediction of SGA, preeclampsia, or spontaneous preterm delivery (77).
PP-13
Placental protein 13 (PP-13, galectin-13) was first isolated in 1983 by Bohn et al. (78, 79). It is a relatively small protein with 139 amino acids (16,118 kDa) which is highly homologous (69%) to the human eosinophil Charcot-Leyden Crystal protein, a phospholipase that belongs to the beta-galactoside binding S-type animal lectin super family. The homodimer which is linked by disulfide bonds probably has special haemostatic and immunobiological functions at the feto-maternal interface or a developmental role in the placenta (80). The 600 bp mRNA transcript is only detectable in placental tissue but not in any other fetal or adult tissue (78, 79, 81, 82).
The serum levels of PP-13 slowly increase during a normal pregnancy but abnormally low levels of PP-13 were detected in first trimester serum samples of women subsequently developing fetal growth restriction and preeclampsia, in particular cases with early onset (83, 84).
Elevated serum concentrations of PP-13 have been found in the second and third trimester in women with preeclampsia, IUGR and in preterm delivery (85). For this study 514 controls, 69 cases with preeclampsia, 69 cases with IUGR, 52 cases with preterm delivery and 24 cases with preeclampsia developing before 34 weeks of gestation have been included.
Another study concluded that first-trimester serum levels of PP-13 may serve as a suitable marker for preterm preeclampsia but are weak for the prediction of severe preeclampsia and ineffective for mild preeclampsia at term (86). Here again the combination of several diagnostic tools results in improved predictive power as was shown by combined measuring of first trimester serum PP-13 levels and median uterine artery pulsatility index by ultrasound. This combination achieved a detection rate for preeclampsia of 90% with a false positive rate of 6% (87). However, this combination of serum PP-13 levels and uterine artery pulsatility index loses its predictive power when late second trimester (22–24 weeks of gestation) serum is analyzed (88).
Currently a commercial PP-13 test kit is developed for the first trimester screening for preeclamp sia by Diagnostic Technologies, Haifa. The test has already been approved in Europe and approval in the United States is expected in the near future.
PTX3
Pentraxin 3 [PTX3, tumor necrosis factor stimulated gene- 14 (89)] belongs to the same family as C-reactive protein (CRP) or serum amyloid P component (SAP) and consists of 381 amino acids. The C terminus is highly homologous to SAP and CRP whereas the N-terminus doesn’t show any homology to other proteins. The according gene is organized into three exons (90) and is extremely evolutionarily conserved from horseshoe crab to human (91).
Responding to proinflammatory stimuli CRP, SAP and PTX3 are produced by various tissues. It is also expressed in tissues undergoing cell death. PTX3 then interacts with several growth factors, extra cellular matrix components and certain pathogens but is also involved in the activation of the complement system (92) and facilitates pathogen recognition by phagocytes (93).
During pregnancy, PTX3 is increasingly expressed in amniotic epithelium, chorionic mesoderm, trophoblast terminal villi, and perivascular stroma of placentae (93).
Cetin et al. and Rovere-Querini et al. showed that in case of a future preeclampsia and IUGR the PTX3 plasma levels are even more increased in all three trimesters (94, 95).
So far no studies that combine PTX3 with other potential markers have been performed.
PAPP-A
PAPP-A (pregnancy-associated plasma protein A, pappalysin 1, insulin-like growth factor binding protein-4 protease, EC 3.4.24.79) is a disulfide bond linked homodimeric peptidase of 1628 amino acids and a mass of 400 kDa 96. It can be detected during pregnancy in maternal circulation mainly as a complex with the proform of the eosinophil major basic protein, an inhibitor of PAPP-A (97).
Although the reaction products are not identified yet, insulin-like growth factor binding proteins are substrates for the hydrolytic activity of PAPP-A (98). PAPP-A is supposedly involved in local proliferative processes, for example bone remodeling (99).
In the recent years decreased plasma levels of PAPP-A have been reported in all trimesters in women with preeclampsia (100, 101, 102, 103).
Discussion
Regardless of the lack of existing prophylactic and therapeutic means against preeclampsia, the search for noninvasive, blood-borne or urinary biomarkers that could predict the development or assist in the detection of this life-threatening pregnancy disorder is still of utmost importance. The availability of such markers could have decisive impact on the medical management of pregnant women and their child (e.g. refer to a tertiary centre) but also on the health costs associated with this poor medical condition.
So, early identification of pregnant women at risk for preeclampsia is a priority to implement preventive measures.
Conclusion
Despite there exists many different potential markers for preeclampsia, the reliability of these markers in predicting preeclampsia has been inconsistent between different studies. Furthermore, preeclampsia is a multifaceted disorder, certain say it is not one but several diseases. Therefore, there is a need for high quality, large scale multicenter trials which enroll patients with different risks of developing the syndrome and throughout multiethnical background, in order to assess the predictive value of different markers and finally propose the best marker combination for a routine use in clinical settings.
Those biomarkers parameters have shown promising predictive performance, but so far there is no clinically validated screening procedure.
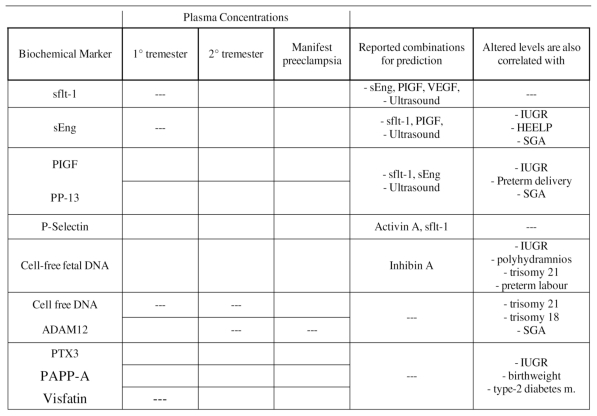
Below is a table about summary of potential biochemical markers for the prediction (1° and 2° trimester) or the detection (manifest preeclampsia) of preeclampsia in maternal peripheral blood (Table 1).
Table 1.
- Today the use of biomarkers in combination with uterine artery Doppler screening is promising as a potential screening tool.
References
- 1.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 2.Sibai B, Dekker G, Kupferminc M: Pre-eclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 3.Hahn S, Gupta AK, Troeger C, Rusterholz C, Holzgreve W. Disturbances in placental immunology: ready for therapeutic interventions? Springer Semin Immunopathol. 2006;27:477–493. doi: 10.1007/s00281-006-0016-5. [DOI] [PubMed] [Google Scholar]
- 4.Jauniaux E, Poston L, Burton GJ. Placental-related diseases of pregnancy: Involvement of oxidative stress and implications in human evolution. Hum Reprod Update. 2006;12:747–755. doi: 10.1093/humupd/dml016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saito S, Shiozaki A, Nakashima A, Sakai M, Sasaki Y. The role of the immune system in preeclampsia. Mol Aspects Med. 2007;28:192–209. doi: 10.1016/j.mam.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Wen SW, Chen XK, Rodger M, White RR, Yang Q, Smith GN, Sigal RJ, Perkins SL, Walker MC. Folic acid supplementation in early second trimester and the risk of preeclampsia. Am J Obstet Gynecol. 2008;198:45–47. doi: 10.1016/j.ajog.2007.06.067. [DOI] [PubMed] [Google Scholar]
- 7.Hung TH, Skepper JN, Charnock-Jones DS, Burton GJ. Hypoxiareoxygenation: a potent inducer of apoptotic changes in the human placenta and possible etiological factor in preeclampsia. Circ Res. 2002;90:1274–1281. doi: 10.1161/01.res.0000024411.22110.aa. [DOI] [PubMed] [Google Scholar]
- 8.Soleymanlou N, Jurisica I, Nevo O, Ietta F, Zhang X, Zamudio S, Post M, Caniggia I. Molecular evidence of placental hypoxia in preeclampsia. J Clin Endocrinol Metab. 2005;90:4299–4308. doi: 10.1210/jc.2005-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–1204. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 11.Huppertz B. Placental origins of preeclampsia: challenging the current hypothesis. Hypertension. 2008;51:970–975. doi: 10.1161/HYPERTENSIONAHA.107.107607. [DOI] [PubMed] [Google Scholar]
- 12.Roberts JM, Hubel CA. The two stage model of preeclampsia: variations on the theme. Placenta. 2009;30(Suppl A):S32–S37. doi: 10.1016/j.placenta.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tjwa M, Luttun A, Autiero M, Carmeliet Carmeliet. P: VEGF and PlGF: two pleiotropic growth factors with distinct roles in development and homeostasis. Cell Tissue Res. 2003;314:5–14. doi: 10.1007/s00441-003-0776-3. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed A, Li XF, Dunk C, Whittle MJ, Rushton DI, Rollason T. Colocalisation of vascular endothelial growth factor and its Flt-1 receptor in human placenta. Growth Factors. 1995;12:235–243. doi: 10.3109/08977199509036883. [DOI] [PubMed] [Google Scholar]
- 15.Khaliq A, Li XF, Shams M, Sisi P, Acevedo CA, Whittle MJ, Weich H, Ahmed A. Localisation of placenta growth factor (PIGF) in human term placenta. Growth Factors. 1996;13:243–50. doi: 10.3109/08977199609003225. [DOI] [PubMed] [Google Scholar]
- 16.Crocker IP, Strachan BK, Lash GE, Cooper S, Warren AY, Baker PN. Vascular endothelial growth factor but not placental growth factor promotes trophoblast syncytialization in vitro. J Soc Gynecol Investig. 2001;8:341–346. [PubMed] [Google Scholar]
- 17.Clark DE, Smith SK, He Y, Day KA, Licence DR, Corps AN, Lammoglia R, Charnock-Jones DS. A vascular endothelial growth factor antagonist is produced by the human placenta and released into the maternal circulation. Biol Reprod. 1998;59:1540–1548. doi: 10.1095/biolreprod59.6.1540. [DOI] [PubMed] [Google Scholar]
- 18.Chaiworapongsa T, Romero R, Espinoza J, Bujold E, Mee KY, Goncalves LF, Gomez R, Edwin S. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am J Obstet Gynecol. 2004;190:1541–1547. doi: 10.1016/j.ajog.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 19.Tsatsaris V, Goffin F, Munaut C, Brichant JF, Pignon MR, Noel A, Schaaps JP, Cabrol D, Frankenne F, Foidart JM. Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences. J Clin Endocrinol Metab. 2003;88:5555–5563. doi: 10.1210/jc.2003-030528. [DOI] [PubMed] [Google Scholar]
- 20.Chung JY, Song Y, Wang Y, Magness RR, Zheng J : Differential expression of vascular endothelial growth factor (VEGF), endocrine gland derived-VEGF, and VEGF receptors in human placentas from normal and preeclamptic pregnancies. J Clin Endocrinol Metab. 2004;89:2484–2490. doi: 10.1210/jc.2003-031580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine RJ, Karumanchi SA. Circulating angiogenic factors in preeclampsia. C. lin Obstet Gynecol. 2005;48:372–386. doi: 10.1097/01.grf.0000160313.82606.d7. [DOI] [PubMed] [Google Scholar]
- 22.Robinson CJ, Johnson DD, Chang EY, Armstrong DM, Wang W. Evaluation of placenta growth factor and soluble Fms-like tyrosine kinase 1 receptor levels in mild and severe preeclampsia. Am J Obstet Gynecol. 2006;195:255–259. doi: 10.1016/j.ajog.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 23.Wikstrom AK, Larsson A, Eriksson UJ, Nash P, Norden-Linderberg S, Olovsson M. Placental growth factor and soluble FMS-like tyrosine kinase-1 in early-onset and late-onset preeclampsia. Obstet Gynecol. 2007;109:1368–1374. doi: 10.1097/01.AOG.0000264552.85436.a1. [DOI] [PubMed] [Google Scholar]
- 24.Stepan H, Kramer T, Faber R. Maternal plasma concentrations of soluble endoglin in pregnancies with intrauterine growth restriction. J Clin Endocrinol Metab. 2007;92:2831–2834. doi: 10.1210/jc.2006-2774. [DOI] [PubMed] [Google Scholar]
- 25.Wathen KA, Tuutti E, Stenman UH, Alfthan H, Halmesmaki E, Finne P, Ylikorkala O, Vuorela P : Maternal serum-soluble vascular endothelial growth factor receptor-1 in early pregnancy ending in preeclampsia or intrauterine growth retardation. J Clin Endocrinol Metab. 2006;91:180–184. doi: 10.1210/jc.2005-1076. [DOI] [PubMed] [Google Scholar]
- 26.Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, Kusanovic JP, Gotsch F, Erez O, Mazaki-Tovi S, Gomez R, Edwin S, Chaiworapongsa T, Levine RJ, Karumanchi SA. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. 2008;21:9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stepan H, Unversucht A, Wessel N, Faber R. Predictive value of maternal angiogenic factors in second trimester pregnancies with abnormal uterine perfusion. Hypertension. 2007;49:818–824. doi: 10.1161/01.HYP.0000258404.21552.a3. [DOI] [PubMed] [Google Scholar]
- 28.Chaiworapongsa T, Romero R, Gotsch F, Espinoza J, Nien JK, Goncalves L, Edwin S, Kim YM, Erez O, Kusanovic JP, Pineles BL, Papp Z, Hassan S. Low maternal concentrations of soluble vascular endothelial growth factor receptor-2 in preeclampsia and small for gestational age. J Matern Fetal Neonatal Med. 2008;21:41–52. doi: 10.1080/14767050701831397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheifetz S, Bellon T, Cales C, Vera S, Bernabeu C, Massague J, Letarte M. Endoglin is a component of the transforming growth factor- beta receptor system in human endothelial cells. J Biol Chem. 1992;267:19027–19030. [PubMed] [Google Scholar]
- 30.St-Jacques S, Forte M, Lye SJ, Letarte M. Localization of endoglin, a transforming growth factor-beta binding protein, and of CD44 and integrins in placenta during the first trimester of pregnancy. Biol Reprod. 1994;51:405–413. doi: 10.1095/biolreprod51.3.405. [DOI] [PubMed] [Google Scholar]
- 31.Duff SE, Li C, Garland JM, Kumar S. CD105 is important for angiogenesis: evidence and potential applications. FASEB J. 2003;17:984–992. doi: 10.1096/fj.02-0634rev. [DOI] [PubMed] [Google Scholar]
- 32.Toporsian M, Gros R, Kabir MG, Vera S, Govindaraju K, Eidelman DH, Husain M, Letarte M. A role for endoglin in coupling eNOS activity and regulating vascular tone revealed in hereditary hemorrhagic telangiectasia. Circ Res. 2005;96:684–692. doi: 10.1161/01.RES.0000159936.38601.22. [DOI] [PubMed] [Google Scholar]
- 33.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, D'Amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Letarte M, Karumanchi SA. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 34.Jeyabalan A, McGonigal S, Gilmour C, Hubel CA, Rajakumar A. Circulating and placental endoglin concentrations in pregnancies complicated by intrauterine growth restriction and preeclampsia. Placenta. 2008;29:555–563. doi: 10.1016/j.placenta.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salahuddin S, Lee Y, Vadnais M, Sachs BP, Karumanchi SA, Lim KH. Diagnostic utility of soluble fms-like tyrosine kinase 1 and soluble endoglin in hypertensive diseases of pregnancy. Am J Obstet Gynecol. 2007;197:28–6. doi: 10.1016/j.ajog.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Robinson CJ, Johnson DD. Soluble endoglin as a second-trimester marker for preeclampsia. Am J Obstet Gynecol. 2007;197:174–175. doi: 10.1016/j.ajog.2007.03.058. [DOI] [PubMed] [Google Scholar]
- 37.Stepan H, Geipel A, Schwarz F, Kramer T, Wessel N, Faber R. Circulatory soluble endoglin and its predictive value for preeclampsia in second-trimester pregnancies with abnormal uterine perfusion. Am J Obstet Gynecol. 2008;198:175–176. doi: 10.1016/j.ajog.2007.08.052. [DOI] [PubMed] [Google Scholar]
- 38.Erez O, Romero R, Espinoza J, Fu W, Todem D, Kusanovic JP, Gotsch F, Edwin S, Nien JK, Chaiworapongsa T, Mittal P, Mazaki-Tovi S, Than NG, Gomez R, Hassan SS : The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and smallfor- gestational age. J Matern Fetal Neonatal Med. 2008;21:279–287. doi: 10.1080/14767050802034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andre P. P-selectin in haemostasis. Br J Haematol. 2004;126:298–306. doi: 10.1111/j.1365-2141.2004.05032.x. [DOI] [PubMed] [Google Scholar]
- 40.Polgar J, Matuskova J, Wagner DD. The P-selectin, tissue factor, coagulation triad. J Thromb Haemost. 2005;3:1590–1596. doi: 10.1111/j.1538-7836.2005.01373.x. [DOI] [PubMed] [Google Scholar]
- 41.Dunlop LC, Skinner MP, Bendall LJ, Favaloro EJ, Castaldi PA, Gorman JJ, Gamble JR, Vadas MA, Berndt MC. Characterization of GMP 140 (P-selectin) as a circulating plasma protein. J Exp Med. 1992;175:1147–1150. doi: 10.1084/jem.175.4.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holthe MR, Staff AC, Berge LN, Lyberg T. Different levels of platelet activation in preeclamptic, normotensive pregnant, and nonpregnant women. Am J Obstet Gynecol. 2004;190:1128–1134. doi: 10.1016/j.ajog.2003.10.699. [DOI] [PubMed] [Google Scholar]
- 43.Bretelle F, Sabatier F, Desprez D, Camoin L, Grunebaum L, Combes V, D'Ercole C, gnat-George F. Circulating microparticles: a marker of procoagulant state in normal pregnancy and pregnancy complicated by preeclampsia or intrauterine growth restriction. Thromb Haemost. 2003;89:486–492. [PubMed] [Google Scholar]
- 44.Lok CA, Nieuwland R, Sturk A, Hau CM, Boer K, Vanbavel E, Vanderpost JA : Microparticle-associated P-selectin reflects platelet activation in preeclampsia. Platelets. 2007;18:68–72. doi: 10.1080/09537100600864285. [DOI] [PubMed] [Google Scholar]
- 45.Aksoy H, Kumtepe Y, Akcay F, Yildirim AK. Correlation of Pselectin and lipoprotein(a), and other lipid parameters in preeclampsia. Clin Exp Med. 2002;2:39–43. doi: 10.1007/s102380200005. [DOI] [PubMed] [Google Scholar]
- 46.Heyl W, Handt S, Reister F, Gehlen J, Schroder W, Mittermayer C, Rath W. Elevated soluble adhesion molecules in women with pre-eclampsia. Do cytokines like tumour necrosis factoralpha and interleukin- 1beta cause endothelial activation. Eur. J Obstet Gynecol Reprod Biol. 1999;86:35–41. doi: 10.1016/s0301-2115(99)00042-1. [DOI] [PubMed] [Google Scholar]
- 47.Banzola I, Farina A, Concu M, Sekizawa A, Purwosunu Y, Strada I, Arcelli D, Simonazzi G, Caramelli E, Rizzo N : Performance of a panel of maternal serum markers in predicting preeclampsia at 11–15 weeks' gestation. Prenat Diagn. 2007;27:1005–1010. doi: 10.1002/pd.1821. [DOI] [PubMed] [Google Scholar]
- 48.Chavarria ME, Lara-Gonzalez L, Garcia-Paleta Y, Vital-Reyes VS, Reyes A. Adhesion molecules changes at 20 gestation weeks in pregnancies complicated by preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2008;137:157–164. doi: 10.1016/j.ejogrb.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 49.Lo YM, Corbetta N, Chamberlain PF, Rai V, Sargent IL, Redman CW, Wainscoat JS. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350:485–487. doi: 10.1016/S0140-6736(97)02174-0. [DOI] [PubMed] [Google Scholar]
- 50.Hromadnikova I, Vechetova L, Vesela K, Benesova B, Doucha J, Kulovany E, Vlk R. Non-invasive fetal RHD exon 7 and exon 10 genotyping using real-time PCR testing of fetal DNA in maternal plasma. Fetal Diagn Ther. 2005;20:275–280. doi: 10.1159/000085085. [DOI] [PubMed] [Google Scholar]
- 51.Finning K, Martin P, Daniels G. A clinical service in the UK to predict fetal Rh (Rhesus) D blood group using free fetal DNA in maternal plasma. Ann N Y Acad Sci. 2004;1022:119–123. doi: 10.1196/annals.1318.019. [DOI] [PubMed] [Google Scholar]
- 52.Li Y, Holzgreve W, Hahn S. Size fractionation of cellfree DNA in maternal plasma and its application in noninvasive detection of fetal single gene point mutations. Methods Mol Biol. 2008;444:239–251. doi: 10.1007/978-1-59745-066-9_19. [DOI] [PubMed] [Google Scholar]
- 53.Sekizawa A, Jimbo M, Saito H, Iwasaki M, Matsuoka R, Okai T, Farina A. Cell-free fetal DNA in the plasma of pregnant women with severe fetal growth restriction. Am J Obstet Gynecol. 2003;188:480–484. doi: 10.1067/mob.2003.27. [DOI] [PubMed] [Google Scholar]
- 54.Zhong XY, Holzgreve W, Li JC, Aydinli K, Hahn S. High levels of fetal erythroblasts and fetal extracellular DNA in the peripheral blood of a pregnant woman with idiopathic polyhydramnios: case report. Prenat Diagn. 2000;20:838–841. doi: 10.1002/1097-0223(200010)20:10<838::aid-pd911>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 55.Vodicka R, Vrtel R, Dusek L, Prochazka M, Schneiderova E, Vrbicka D, Krejcirikova E, Dhaifalah I, Santava A, Santavy J: Refined fluorescent STR quantification of cell-free fetal DNA during pregnancy in physiological and Down syndrome fetuses. Prenat Diagn. 2008;28:425–433. doi: 10.1002/pd.1996. [DOI] [PubMed] [Google Scholar]
- 56.Farina A, LeShane ES, Romero R, Gomez R, Chaiworapongsa T, Rizzo N, Bianchi DW. High levels of fetal cell-free DNA in maternal serum: a risk factor for spontaneous preterm delivery. Am J Obstet Gynecol. 2005;193:421–425. doi: 10.1016/j.ajog.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 57.Lo YM, Leung TN, Tein MS, Sargent IL, Zhang J, Lau TK, Haines CJ, Redman CW. Quantitative abnormalities of fetal DNA in maternal serum in preeclampsia. Clin Chem. 1999;45:184–188. [PubMed] [Google Scholar]
- 58.Zhong XY, Holzgreve W, Hahn S. The levels of circulatory cell free fetal DNA in maternal plasma are elevated prior to the onset of preeclampsia. Hypertens Pregnancy. 2002;21:77–83. doi: 10.1081/PRG-120002911. [DOI] [PubMed] [Google Scholar]
- 59.Levine RJ, Qian C, LeShane ES, Yu KF, England LJ, Schisterman EF, Wataganara T, Romero R, Bianchi DW. Two-stage elevation of cell-free fetal DNA in maternal sera before onset of preeclampsia. Am J Obstet Gynecol. 2004;190:707–713. doi: 10.1016/j.ajog.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 60.Zimmermann BG, Maddocks DG, Avent ND. Quantification of circulatory fetal DNA in the plasma of pregnant women. Methods Mol Biol. 2008;444:219–229. doi: 10.1007/978-1-59745-066-9_17. [DOI] [PubMed] [Google Scholar]
- 61.Farina A, Sekizawa A, Iwasaki M, Matsuoka R, Ichizuka K, Okai T. Total cell-free DNA (beta-globin gene) distribution in maternal plasma at the second trimester: a new prospective for preeclampsia screening. Prenat Diagn. 2004;24:722–726. doi: 10.1002/pd.973. [DOI] [PubMed] [Google Scholar]
- 62.Sekizawa A, Farina A, Koide K, Iwasaki M, Honma S, Ichizuka K, SaitoH SaitoH, Okai T. Beta-globin DNA in maternal plasma as a molecular marker of pre-eclampsia. Prenat Diagn. 2004;24:697–700. doi: 10.1002/pd.965. [DOI] [PubMed] [Google Scholar]
- 63.Swinkels DW, de Kok JB, Hendriks JC, Wiegerinck E, Zusterzeel PL, Steegers EA. Hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome as a complication of preeclampsia in pregnant women increases the amount of cell-free fetal and maternal DNA in maternal plasma and serum. Clin Chem. 2002;48:650–653. [PubMed] [Google Scholar]
- 64.Chim SS, Tong YK, Chiu RW, Lau TK, Leung TN, Chan LY, Oudejans CB, Ding C, Lo YM. Detection of the placental epigenetic signature of the maspin gene in maternal plasma. Proc Natl Acad Sci USA. 2005;102:14753–14758. doi: 10.1073/pnas.0503335102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chan KC, Ding C, Gerovassili A, Yeung SW, Chiu RW, Leung TN, Lau TK, Chim SS, Chung GT, Nicolaides KH, Lo YM. Hypermethylated RASSF1A in maternal plasma: A universal fetal DNA marker that improves the reliability of noninvasive prenatal diagnosis. Clin Chem. 2006;52:2211–2218. doi: 10.1373/clinchem.2006.074997. [DOI] [PubMed] [Google Scholar]
- 66.Tsui DW, Chan KC, Chim SS, Chan LW, Leung TY, Lau TK, Lo YM, Chiu RW. Quantitative aberrations of hypermethylated RASSF1A gene sequences in maternal plasma in preeclampsia. Prenat Diagn. 2007;27:1212–1218. doi: 10.1002/pd.1897. [DOI] [PubMed] [Google Scholar]
- 67.Diesch CH, Holzgreve W, Hahn S, Zhong XY. Comparison of activin A and cell-free fetal DNA levels in maternal plasma from patients at high risk for preeclampsia. Prenat Diagn. 2006;26:1267–1270. doi: 10.1002/pd.1606. [DOI] [PubMed] [Google Scholar]
- 68.White ADAMs. Mmodulators of cell-cell and cellmatrix interactions. Curr Opin Cell Biol. 2003;15:598–606. doi: 10.1016/j.ceb.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 69.Yang P, Baker KA, Hagg T. A disintegrin and metalloprotease 21 (ADAM21) is associated with neuro genesis and axonal growth in developing and adult rodent CNS. J Comp Neurol. 2005;490:163–179. doi: 10.1002/cne.20659. [DOI] [PubMed] [Google Scholar]
- 70.Gilpin BJ, Loechel F, Mattei MG, Engvall E, Albrechtsen R, Wewer UM. A novel, secreted form of human ADAM 12 (meltrin alpha) provokes myogenesis in vivo. J Biol Chem. 1998;273:157–166. doi: 10.1074/jbc.273.1.157. [DOI] [PubMed] [Google Scholar]
- 71.Laigaard J, Spencer K, Christiansen M, Cowans NJ, Larsen SO, Pedersen BN, Wewer UM. ADAM 12 as a first-trimester maternal serum marker in screening for Down syndrome. Prenat Diagn. 2006;26:973–979. doi: 10.1002/pd.1540. [DOI] [PubMed] [Google Scholar]
- 72.Laigaard J, Christiansen M, Frohlich C, Pedersen BN, Ottesen B, Wewer UM. The level of ADAM12-S in maternal serum is an early first-trimester marker of fetal trisomy 18. Prenat Diagn. 2005;25:45–46. doi: 10.1002/pd.1029. [DOI] [PubMed] [Google Scholar]
- 73.Spencer K, Cowans NJ. ADAM12 as a marker of trisomy 18 in the first and second trimester of pregnancy. J Matern Fetal Neonatal Med. 2007;20:645–650. doi: 10.1080/14767050701483389. [DOI] [PubMed] [Google Scholar]
- 74.Spencer K, Cowans NJ, Stamatopoulou A. Maternal serum ADAM12s as a marker of rare aneuploidies in the first or second trimester of pregnancy. Prenat Diagn. 2007;27:1233–1237. doi: 10.1002/pd.1885. [DOI] [PubMed] [Google Scholar]
- 75.Laigaard J, Sorensen T, Placing S, Holck P, Frohlich C, Wojdemann KR, Sundberg K, Shalmi AC, Tabor A, Norgaard-Pedersen B, Ottesen B, Christiansen M, Wewer UM. Reduction of the disintegrin and metalloprotease ADAM12 in preeclampsia. Obstet Gynecol. 2005;106:144–149. doi: 10.1097/01.AOG.0000165829.65319.65. [DOI] [PubMed] [Google Scholar]
- 76.Spencer K, Cowans NJ, Stamatopoulou A. ADAM12s in maternal serum as a potential marker of preeclampsia. Prenat Diagn. 2008;28:212–216. doi: 10.1002/pd.1957. [DOI] [PubMed] [Google Scholar]
- 77.Poon LC, Chelemen T, Granvillano O, Pandeva I, Nicolaides KH. First-trimester maternal serum a disintegrin and metalloprotease 12 (ADAM12) and adverse pregnancy outcome. Obstet Gynecol. 2008;112:1082–1090. doi: 10.1097/AOG.0b013e318188d6f9. [DOI] [PubMed] [Google Scholar]
- 78.Bohn H, Kraus W, Winckler W. Purification and characterization of two new soluble placental tissue proteins (PP13 and PP17) Oncodev Biol Med. 1983;4:343–350. [PubMed] [Google Scholar]
- 79.Visegrady B, Than NG, Kilar F, Sumegi B, Than GN, Bohn H. Homology modelling and molecular dynamics studies of human placental tissue protein 13 (galectin-13) Protein Eng. 2001;14:875–880. doi: 10.1093/protein/14.11.875. [DOI] [PubMed] [Google Scholar]
- 80.Than NG, Pick E, Bellyei S, Szigeti A, Burger O, Berente Z, Janaky T, Boronkai A, Kliman H, Meiri H, Bohn H, Than GN, Sumegi B. Functional analyses of placental protein 13/galectin-13. Eur J Biochem. 2004;271:1065–1078. doi: 10.1111/j.1432-1033.2004.04004.x. [DOI] [PubMed] [Google Scholar]
- 81.Than NG, Sumegi B, Than GN, Berente Z, Bohn H. Isolation and sequence analysis of a cDNA encoding human placental tissue protein 13 (PP13), a new lysophospholipase, homologue of human eosinophil Charcot-Leyden Crystal protein. Placenta. 1999;20:703–710. doi: 10.1053/plac.1999.0436. [DOI] [PubMed] [Google Scholar]
- 82.Sekizawa A, Purwosunu Y, Yoshimura S, Nakamura M, Shimizu H, Okai T, Rizzo N, Farina A. PP13 mRNA expression in trophoblasts from preeclamptic placentas. Reprod Sci. 2009;16:408–413. doi: 10.1177/1933719108328615. [DOI] [PubMed] [Google Scholar]
- 83.Huppertz B, Sammar M, Chefetz I, Neumaier-Wagner P, Bartz C, Meiri H. Longitudinal determination of serum placental protein 13 during development of preeclampsia. Fetal Diagn Ther. 2008;24:230–236. doi: 10.1159/000151344. [DOI] [PubMed] [Google Scholar]
- 84.Chafetz I, Kuhnreich I, Sammar M, Tal Y, Gibor Y, Meiri H, Cuckle H, Wolf M. First-trimester placental protein 13 screening for preeclampsia and intrauterine growth restriction. Am J Obstet Gynecol. 2007;197:35–37. doi: 10.1016/j.ajog.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 85.Burger O, Pick E, Zwickel J, Klayman M, Meiri H, Slotky R, Mandel S, Rabinovitch L, Paltieli Y, Admon A, Gonen R. Placental protein 13 (PP-13): effects on cultured trophoblasts, and its detection in human body fluids in normal and pathological pregnancies. Placenta. 2004;25:608–622. doi: 10.1016/j.placenta.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 86.Romero R, Kusanovic JP, Than NG, Erez O, Gotsch F, Espinoza J, Edwin S, Chefetz I, Gomez R, Nien JK, Sammar M, Pineles B, Hassan SS, Meiri H, Tal Y, Kuhnreich I, Papp Z, Cuckle HS. First-trimester maternal serum PP13 in the risk assessment for preeclampsia. Am J Obstet Gynecol. 2008;199((2)):122. doi: 10.1016/j.ajog.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nicolaides KH, Bindra R, Turan OM, Chefetz I, Sammar M, Meiri H, Tal J, Cuckle HS. A novel approach to first-trimester screening for early pre-eclampsia combining serum PP-13 and Doppler ultrasound. Ultrasound Obstet Gynecol. 2006;27:13–17. doi: 10.1002/uog.2686. [DOI] [PubMed] [Google Scholar]
- 88.Spencer K, Cowans NJ, Chefetz I, Tal J, Kuhnreich I, Meiri H. Second- trimester uterine artery Doppler pulsatility index and maternal serum PP13 as markers of pre-eclampsia. Prenat Diagn. 2007;27:258–263. doi: 10.1002/pd.1664. [DOI] [PubMed] [Google Scholar]
- 89.Souza DG, Soares AC, Pinho V, Torloni H, Reis LF, Teixeira MM, Dias AA. Increased mortality and inflammation in tumor necrosis factor-stimulated gene-14 transgenic mice after ischemia and reperfusion injury. Am J Pathol. 2002;160:1755–1765. doi: 10.1016/s0002-9440(10)61122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Breviario F, d'Aniello EM, Golay J, Peri G, Bottazzi B, Bairoch A, Saccone S, Marzella R, Predazzi V, Rocchi M. Interleukin-1-inducible genes in endothelial cells. Cloning of a new gene related to C-reactive protein and serum amyloid P component. J Biol Chem. 1992;267:22190–22197. [PubMed] [Google Scholar]
- 91.Alles VV, Bottazzi B, Peri G, Golay J, Introna M, Mantovani A. Inducible expression of PTX3, a new member of the pentraxin family, in human mononuclear phagocytes. Blood. 1994;84:3483–3493. [PubMed] [Google Scholar]
- 92.Inforzato A, Peri G, Doni A, Garlanda C, Mantovani A, Bastone A, Carpentieri A, Amoresano A, Pucci P, Roos A, Daha MR, Vincenti S, Gallo G, Carminati P, De SR, Salvatori G. Structure and function of the long pentraxin PTX3 glycosidic moiety: fine-tuning of the interaction with C1q and complement activation. Biochemistry. 2006;45:11540–11551. doi: 10.1021/bi0607453. [DOI] [PubMed] [Google Scholar]
- 93.Popovici RM, Krause MS, Jauckus J, Germeyer A, Brum IS, Garlanda C, Strowitzki T, von WM. The long pentraxin PTX3 in human endometrium: regulation by steroids and trophoblast products. Endocrinology. 2008;149:1136–1143. doi: 10.1210/en.2007-1302. [DOI] [PubMed] [Google Scholar]
- 94.Cetin I, Cozzi V, Pasqualini F, Nebuloni M, Garlanda C, Vago L, Pardi G, Mantovani A. Elevated maternal levels of the long pentraxin 3 (PTX3) in preeclampsia and intrauterine growth restriction. Am J Obstet Gynecol. 2006;194:1347–1353. doi: 10.1016/j.ajog.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 95.Rovere-Querini P, Antonacci S, Dell'Antonio G, Angeli A, Almirante G, Cin ED, Valsecchi L, Lanzani C, Sabbadini MG, Doglioni C, Manfredi AA, Castiglioni MT. Plasma and tissue expression of the long pentraxin 3 during normal pregnancy and preeclampsia. Obstet Gynecol. 2006;108:148–155. doi: 10.1097/01.AOG.0000224607.46622.bc. [DOI] [PubMed] [Google Scholar]
- 96.Lawrence JB, Bale LK, Haddad TC, Clarkson JT, Conover CA. Characterization and partial purification of the insulin-like growth factor (IGF)-dependent IGF binding protein-4-specific protease from human fibroblast conditioned media. Growth Horm IGF Res. 1999;9:25–34. doi: 10.1054/ghir.1998.0083. [DOI] [PubMed] [Google Scholar]
- 97.Chen BK, Overgaard MT, Bale LK, Resch ZT, Christiansen M, Oxvig C, Conover CA. Molecular regulation of the IGF-binding protein- 4 protease system in human fibroblasts: identification of a novel inducible inhibitor. Endocrinology. 2002;143:1199–1205. doi: 10.1210/endo.143.4.8729. [DOI] [PubMed] [Google Scholar]
- 98.Chelius D, Conover CA, Baldwin MA, Spencer EM. Characterization of the enzymatic specificity of the IGF-dependent insulin- like growth factor binding protein- 4 (IGFBP-4) protease. Growth Horm IGF Res. 2000;10:360–366. doi: 10.1054/ghir.2000.0177. [DOI] [PubMed] [Google Scholar]
- 99.Miller BS, Bronk JT, Nishiyama T, Yamagiwa H, Srivastava A, Bolander ME, Conover CA. Pregnancy fracture healing in mice. J Endocrinol. 2007;192:505–513. doi: 10.1677/JOE-06-0011. [DOI] [PubMed] [Google Scholar]
- 100.Cowans NJ, Spencer K. First-trimester ADAM12 and PAPP-A as markers for intrauterine fetal growth restriction through their roles in the insulin-like growth factor system. Prenat Diagn. 2007;27:264–271. doi: 10.1002/pd.1665. [DOI] [PubMed] [Google Scholar]
- 101.Spencer K, Cowans NJ, Chefetz I, Tal J, Meiri H. Firsttrimester maternal serum PP-13, PAPP-A and second-trimester uterine artery Doppler pulsatility index as markers of preeclampsia. Ultrasound Obstet Gynecol. 2007;29:128–134. doi: 10.1002/uog.3876. [DOI] [PubMed] [Google Scholar]
- 102.Spencer K, Cowans NJ, Nicolaides KH. Low levels of maternal serum PAPP-A in the first trimester and the risk of preeclampsia. Prenat Diagn. 2008;28:7–10. doi: 10.1002/pd.1890. [DOI] [PubMed] [Google Scholar]
- 103.Spencer K, Cowans NJ, Molina F, Kagan KO, Nicolaides KH. Firsttrimester ultrasound and biochemical markers of aneuploidy and the prediction of preterm or early preterm delivery. Ultrasound Obstet Gynecol. 2008;31:147–152. doi: 10.1002/uog.5163. [DOI] [PubMed] [Google Scholar]
- 104.Yves Giguère Charland, Emmanuel Bujold, Nathalie Bernard, Sonya Grenier, Francǫis Rousseau, Julie Lafond, France Forest. Combining Biochemical and Ultrasonographic Markers in Predicting Preeclampsia: A Systematic Review. Clinical Chemistry. 2010;56(3):361–374. doi: 10.1373/clinchem.2009.134080. [DOI] [PubMed] [Google Scholar]