Abstract
Previous research showed that axonal inputs to both anterior and posterior subdivisions of the medial amygdala from the main and accessory olfactory bulbs of female mice, respectively, process volatile and non-volatile pheromonal signals from male conspecifics. In the present study we found that bilateral electrolytic lesions that included posterior portions, but not the anterior subdivision alone of the medial amygdala (Me) blocked the preference of estrous female mice to investigate volatile urinary odors from testes-intact vs. castrated males. Similar results were obtained in separate tests in which nasal contact with urinary stimuli was permitted. In addition, total time investigating volatile urinary stimuli was reduced in subjects with posterior Me lesions. Subjects were able to discriminate volatile urinary odors from testes-intact vs. castrated male mice, suggesting that this disruption of odor preference did not result from the inability of females given amygdaloid lesions to discriminate these male urinary odors. Bilateral lesions of the Me that were either restricted to the anterior or posterior subdivisions, or included areas of both regions, caused significant reductions in the display of lordosis behavior in estrous female mice. Our results suggest that the Me is a critical segment of the olfactory circuit that controls both mate recognition and mating behavior in the female mouse.
Keywords: Sexual behavior, partner preference, estradiol, progesterone
1. Introduction
In rodents both appetitive and consumatory behaviors concerned with reproduction are mediated in large measure by chemosignals released from opposite sex conspecifics. These chemosignals are detected and processed by two anatomically distinct olfactory systems, the main (MOS) and the accessory (AOS) olfactory system. High molecular weight, non-volatile odors important for reproduction are detected by the vomeronasal organ (VNO) and processed by the AOS whereas low molecular weight, volatile odors are detected by the main olfactory epithelium (MOE) and processed by the MOS [1–3]. Mitral cell efferents from the accessory olfactory bulb (AOB), part of the AOS, project directly to the `vomeronasal' amygdala, which includes the medial amygdala (Me) and the posterior medial cortical amygdala (PMCO). These nuclei send projections to the ventromedial hypothalamus (VMH), bed nucleus of the stria terminalis (BNST), and medial preoptic area (MPA), hypothalamic regions critically important for mediating the effects of chemosignals on neuroendocrine as well as behavioral aspects of reproduction [4,5]. The Me also receives direct inputs from a subset of mitral/tufted projection neurons of the main olfactory bulb (MOB) [6,7]. As a result, volatile opposite-sex chemosignals can potentially gain direct access to the Me. Consequently, there may be considerable functional overlap between the MOS and AOS in both sexes [7].
A recent study [8] reported that the male mouse pheromone ESP1, found in lacrimal gland secretions, enhances lordosis behavior after its detection by the VNO receptor, V2Rp5, and subsequent processing along the AOS. This pheromone-induced enhancement of lordosis was correlated with an increase in immediate early gene (c-fos) expression in the Me, PMCO, and several other forebrain areas of female mice. Several additional studies have shown that exposure to urine and/or soiled bedding (both non-volatile as well as volatile components) from male mice also increased Fos protein induction in neurons from the vomeronasal amygdala and other forebrain nuclei [7,9–12]. Female mice show a hard-wired preference to investigate non-volatile urinary odors from testes-intact vs. castrated male conspecifics, and this preference is blocked by surgical removal of the VNO [13]. Likewise, female mice prefer to approach volatile urinary odors from testes-intact vs. castrated male mice, and this preference was blocked by chemical lesioning of the MOE [14]. Thus both the AOS and the MOS may contribute to heterosexual mate recognition and/or the motivation of female mice to seek out a male partner.
As already explained, murine chemosignals detected by either the VNO or MOE can be conveyed to the Me, which includes anterior and posterior subdivisions. These subnuclei of the Me are functionally connected [15, 16], and it has been suggested that they play distinct roles in the processing of body chemosignals. Thus, in male Syrian hamsters, the anterior Me has been implicated in the transmission of sexual odor information by acting as a “chemosensory filter” that distinguishes between opposite-sex and same-sex odorants [17]. By contrast, the posterior Me, which includes a dense population of steroid receptor-expressing neurons [18, 19] may enhance subjects' motivation to seek out opposite-sex body odorants [15]. We extended this hypothesis to female mice by testing the effect of bilateral electrolytic lesions aimed at the anterior or posterior Me on subjects' preference to investigate either volatile or non-volatile urinary odors from testes-intact as opposed to castrated male mice, and to discriminate the urinary volatiles of males in these two endocrine states. Finally, we assessed the effects of different amygdaloid lesions on the expression of sexual receptivity (lordosis) in female mice. In addition to mice in which lesions were restricted to posterior or anterior Me subdivisions, a third group was analyzed that included subjects in which lesion damage occurred along the anterior-posterior Me border (AP-Me lesions).
2. Materials and methods
2.1. Subjects
All procedures were approved by the Boston University Charles River Campus Institutional Animal Care and Use Committee. Eighty-four female and 12 male (stimulus animals for lordosis testing) Swiss Webster mice (Charles River Laboratories, Wilmington, MA, USA) were purchased at 5–6 weeks of age and maintained on a reversed 12 : 12 h light:dark cycle with food and water available ad libitum. The policy at Charles River Farms for Swiss Webster mice is to remove the male from the cage of the pregnant female well before parturition occurs. Therefore the females used in the present study had never had direct nasal access to breeding males prior to their arrival at Boston University. Females were housed 4 per cage until 48 hours prior to the start of behavioral testing, whereupon they were housed individually. Stimulus males were housed individually after receiving mating experience with an estrous stimulus female. All behavioral testing was conducted under red light during the dark phase of the photoperiod and all behavioral tests were video-recorded. Five days after arrival in the animal colony, female subjects underwent bilateral ovariectomy under 2% isoflurane anesthesia and were allowed 1 week to recover. Subjects were given injections of analgesic (carprofen, 5 mg/kg, s.c.) for two days after surgery. In preparation for induction of lordosis in later behavioral testing, females were implanted subcutaneously at the back of the neck with polymeric silicone SILASTIC capsules (inner diameter, 1.57 mm; outer diameter, 2.41 mm; length, 5 mm) packed with estradiol (E2; diluted 1:1 with cholesterol) at either the time of ovariectomy or 1 week later, upon the completion of electrolytic lesion or sham surgery.
2.2. Urine Collection
Urine used for odor preference and odor discrimination testing was collected from individual donor mice (testes Intact and castrated male mice; 4–6 animals per group) using a metabolic cage collection system. In preparation for urine collection, four group-housed male mice underwent bilateral castration under 2% isoflurane anesthesia and were allowed 3 weeks to recover to ensure that circulating androgens had dissipated before urine was collected. Intact male urine was collected from 6 testes-intact, group-housed male mice. The pooled urine was then aliquotted into 1 ml vials according to endocrine status and stored at −80°C.
2.3. Me Lesion Procedure
Female subjects were assigned to one of three groups: Sham lesion (n=17), bilateral anterior medial amygdala lesion (Ant-Me; n=7), or bilateral posterior medial amygdala lesion (Post-Me; n=13). For some subjects, lesions aimed at either the anterior or posterior Me were later discovered to have been placed along the border of these sites such that damage included portions of both areas bilaterally; these subjects were placed in a fourth group (AP-Me; n=9).
Subjects were anesthetized with ketamine (100 mg/kg) and xylazine (20 mg/kg) and the head was secured in a sterotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA). 2% isoflurane was given continuously to ensure that animals remained under anesthesia for the duration of the surgery. The skull was exposed and a small hole was drilled over each lesion site bilaterally. Lateral coordinates were 2.0 mm for all lesions. Due to variability in the location of bregma observed in this outbred strain, A-P coordinates were calculated using the interaural line as a reference point: Ant-Me lesion, +3.2 mm from the interaural line; Post-Me lesion, +3.0 mm from the interaural line. A tungsten electrode (FHC, Bowdoin, ME, USA) was lowered into the brain to a depth of 4.95 mm from the dura. The electrode was left in place for 3 min before delivering a 200 μA, 1 min cathodal lesion (Grass Instruments, Quincy, MA, USA). After passing current, the electrode was left in place for 2 min before being slowly retracted. Sham animals received identical treatment except that the electrode was lowered to a depth of 4.25 mm and left in place for 5 min with no current being passed. A-P coordinates for shams were either +3.2 mm (n=9) or +3.0 mm (n=7) from the interaural line. Holes in the skull were plugged with bone wax and the incision was closed by suture. Animals were given an injection of carprofen (5 mg/kg, s.c.) immediately following surgery and allowed 1 week recovery before behavioral testing.
2.4. Odor Preference
Beginning one week after lesion or sham surgery, subjects were given two 5-min odor preference tests (separated by four days) conducted in their homecage. Testing began 6 hr into the subjects' dark cycle. To induce a behavioral condition in which females are most responsive to the gonadal status of male subjects, an injection of progesterone (500 μg, s.c.) was administered 4 h before odor preference testing to subjects that were previously implanted with E2 capsules. Ten minutes prior to testing, food and water were removed from the animal's homecage and the food hopper was cleaned with 70% ethanol. A video camera with a night-vision function was used to record behavioral tests conducted in the dark under dim red light illumination. Two separate two-choice odor preference test in which castrated and intact male urine were presented simultaneously were given. In the first test (non-contact), 20 μl of testes-intact male urine or castrated male urine was pipetted onto filter paper secured to a small plastic weigh boat, and the two stimuli were then placed 10 cm apart in the food hopper. Direct nasal contact was prevented by placing wire mesh between the odor stimulus and the subject, thus allowing access to only volatile urinary stimuli. Time spent sniffing each stimulus was recorded using a stopwatch by an observer blind to the lesion status of the subject. Olfactory investigation was scored whenever the subject sniffed actively with its nose within 1 cm of the stimulus. A second test, conducted four days later, was carried out in a similar manner except that the wire barrier was removed, thus providing direct nasal access to the two stimuli. Subjects again received progesterone (500 ug, s.c.) 4 hr prior to testing. Two analyses were performed. First, time spent investigating intact male urine minus the time spent investigating castrated male urine was determined for each test, and one-sample t-tests were used to determine whether these difference scores were significantly different from zero. One-way ANOVAs (followed by Fisher's LSD post hoc tests) were then used to assess treatment group effects on these difference scores for the nasal contact and non-contact tests. A second analysis (one-way ANOVAs followed by Fisher's LSD post hoc tests) examined whether the total time spent investigating either stimulus differed between treatment groups for the nasal contact and non-contact tests. For two subjects, non-contact odor preference data were lost due to equipment error, yielding a final sample size for the non-contact preference test of n=15 (Sham), n=10, (Ant-Me), n=15, (Post-Me), n=8 (AP-Me) and n=16 (Sham), n=10 (Ant-Me), n=16 (Post-Me) and n=8 (AP-Me) for the nasal contact preference test.
2.5. Odor Discrimination
To verify that subjects could discriminate between testes-intact and castrated male urinary volatiles, subjects underwent a home-cage habituation/dishabituation test as previously described [20, 21]. Subjects did not receive progesterone prior to these tests.
2.6. Lordosis
Two weeks after the odor discrimination test, female-typical lordosis behavior was observed in four separate tests that took place every 4 days with a different testes-intact, sexually experienced stimulus male mouse. On the day of each test, progesterone (500 μg, s.c) was administered to subjects 4 h before being introduced into the home cage of a stimulus male. Mice were observed for 20 min or until 10 mount attempts were received from the stimulus male, and the number of times the female subject showed lordosis (defined by the display of an upward pointing snout and arched back posture upon being mounted by the male) was scored. A lordosis quotient (number of lordosis responses shown divided by the total number of male mounting attempts) was calculated for each subject. Lordosis quotients were averaged over the four tests and group means were compared using a one-way ANOVA (followed by the Fisher LSD post hoc test). Five of 84 mice (Sham = 2, Ant-Me = 1, Post-Me = 1, Ant-Post Me = 1) did not receive lordosis testing because they died prior to the beginning of testing.
2.7. Histology
Two to four days following the conclusion of behavioral testing, subjects were anesthetized with sodium pentobarbital (100 mg/kg) and underwent transcardiac perfusion with 0.1 M phosphate-buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde in 0.1 M PBS. Brains were removed, post-fixed in paraformaldehyde for 2 h, and subsequently cryoprotected in 30% sucrose in 0.1 M PBS for 24 h. Forebrains were isolated, frozen in OCT (Tissue-Tek; Sakura Finetek USA Inc., Torrance, CA, USA) and stored at −80°C. Coronal (30 μm) sections were collected using a cryostat and stored free-floating in PBS at 4°C until mounting on gelatin-coated slides. Sections were stained with cresyl violet and coverslipped with Permount (Fisher Scientific, Pittsburgh, PA, USA), and the size, extent and location of lesion damage for each subject was determined with the aid of a mouse atlas [22].
3. Results
3.1. Odor Preference tests
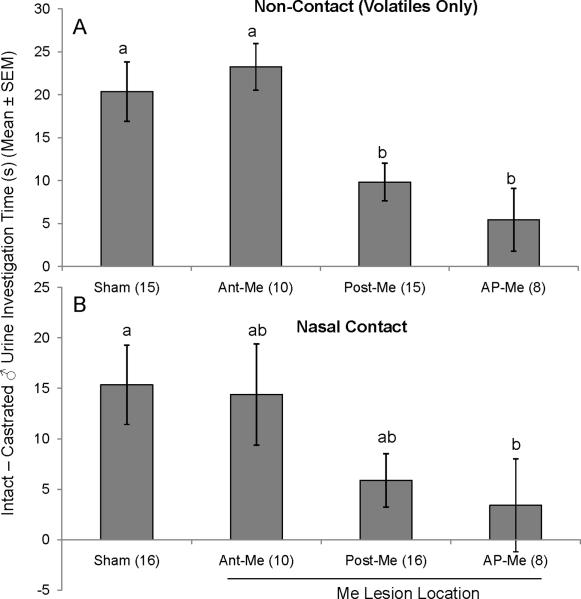
A preference for urinary volatiles from intact vs. castrated males was displayed by Sham (t(14)=5.9, p < 0.001), Ant-Me (t(6)=8.5, p < 0.001) and Post-Me (t(14)=5.9, p < 0.001) subjects, whereas AP-Me females showed no preference (t(8)=1.5, p > 0.05) (Fig 1A). Differences in time investigating volatiles from intact as opposed to castrated male urine were significantly affected by lesion group (F(3,40) = 6.099; p < 0.05); specifically, Post-Me and AP-Me females displayed reduced preferences for intact male urine in comparison to Sham and Ant-Me groups (Fisher post hoc test, p < 0.05).
Fig. 1.
A, B, Effect of bilateral Me lesions (Ant-Me, Post-Me, AP-Me) on the preference of ovariectomized, estradiol and progesterone-primed female mice to investigate urinary odors from castrated male versus testes-intact male mice presented simultaneously in the subjects' home cage. A, Subjects' preference for volatile urinary odors presented outside the home cage (Non-contact) and B, subjects' preference for volatile + non-volatile urinary odors presented inside the home cage (Nasal Contact). Data are represented as the average (± SEM) time spent sniffing intact male urine minus the time spent sniffing castrated male urine for each group. Different letters above columns in each group indicate statistically significant differences from each other (1-way ANOVA followed by Fisher LSD test). The number of subjects in each group is given in parentheses.
Similar results were obtained when mice were allowed physical contact with urinary stimuli (Fig 1B). Statistically significant preferences were seen for intact over castrated male urine for Sham (t(15)=3.9, p < 0.001), Ant-Me (t(6)=2.9, p < 0.05) and Post-Me (t(12)=2.2, p < 0.05) groups, but not for AP-Me females (t(8)=1.3, p > 0.05). One-way ANOVA indicated that the degree of preference depended on lesion group (F(3,41) = 2.861; p < 0.05). Although mean differences in intact vs. castrated male investigation times were reduced relative to Shams in both Post-Me and AP-Me groups, post hoc analysis determined that this reduction was significant only for AP-Me (p < 0.05).
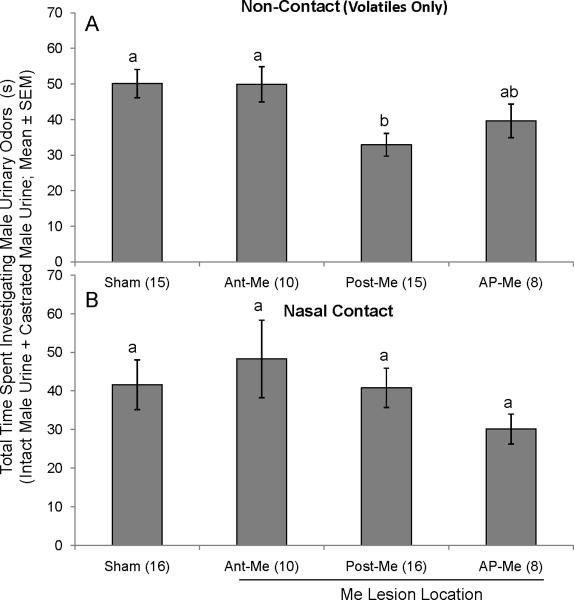
In general, total time investigating urinary stimuli was not affected by lesion status, with one exception. For the volatiles-only test (Fig 2A), overall investigation time was significantly reduced in Post-Me mice in comparison to Shams (F(3,40) = 4.453; p < 0.05; Fisher post hoc test, p < 0.05). There were no group differences in total investigation times in tests where nasal contact with the stimulus was permitted (F(3,40) = 0.915; p > 0.05) (Fig 2B).
Fig. 2.
A, B, Effect of bilateral Me lesions (Ant-Me, Post-Me, AP-Me) on the total amount of time ovariectomized, estradiol and progesterone-primed female mice spent investigating male urinary odors. A, total amount of time subjects spent investigating intact male + castrated male urinary volatiles (non-contact). B, total amount of time subjects spent investigating intact male + castrated male urinary volatiles and nonvolatiles (nasal contact). Data are represented as the average (± SEM) time spent sniffing intact male urine + the average time spent sniffing castrated male urine for each group. Different letters above columns in each group indicate statistically significant differences from each other (1-way ANOVA followed by Fisher LSD test). The number of subjects in each group is given in parentheses.
3.2. Odor Discrimination
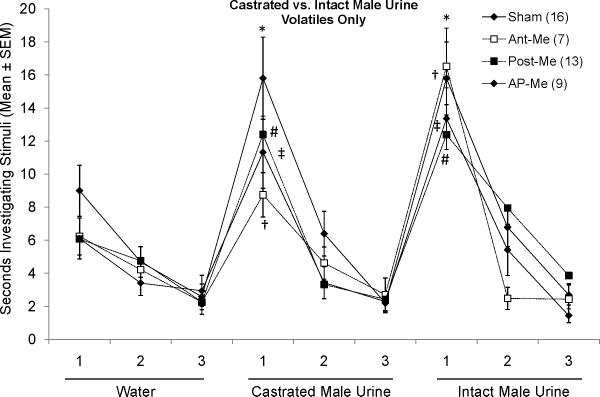
To control for the possibility that lack of preference for any odor was due to deficits in olfactory discrimination, subjects underwent habituation/dishabituation testing for urinary volatiles (i.e. presented outside the home cage; Fig 3). Subjects in all groups successfully dishabituated from the final presentation of water to the first presentation of castrated male urinary volatiles as well as from the final presentation of castrated male urine to the first presentation of intact male urine. To examine whether there were group differences in level of investigation of different urinary cues, one way ANOVAs were performed on the first dishabituation score for each urinary stimulus. No significant group differences were found (F(3,42) = 1.412, p > 0.05 for castrated male urine; F(3,42) = 0.780, p > 0.05 for intact male urine). Thus, an intact Me is not required for females to distinguish between urinary volatiles of males in different endocrine states.
Fig. 3.
Effect of bilateral Me lesions (Ant-Me, Post-Me, AP-Me) on the ability of ovariectomized, estradiol-treated female mice to discriminate between volatile urinary odors (presented outside the home cage) from castrated and testes-intact male mice, respectively. *, #, †, ‡ p < 0.003, Student's t-test comparisons with the third presentation of the previous stimulus for each of the respective groups. The number of subjects in each group is given in parentheses.
3.3. Lordosis
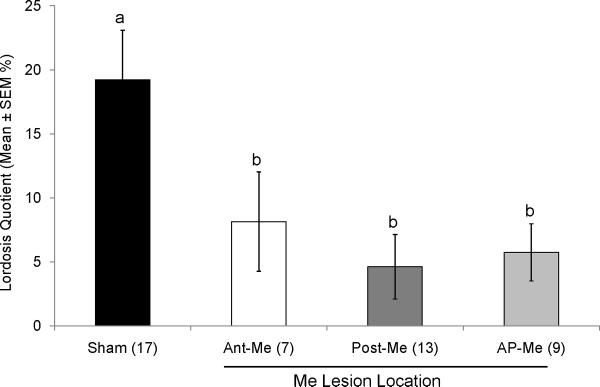
Lordosis quotients averaged over 4 separate tests were significantly reduced by Me lesions (F(3,42) = 4.616; p < 0.05; Fig 4). Post hoc tests revealed that all Me lesion groups differed significantly from Shams but not from each other.
Fig. 4.
Effect of bilateral Me lesions (Ant-Me, Post-Me, AP-Me) on lordosis quotients in ovariectomized, estradiol and progesterone-primed female mice. Different letters above columns in each group indicate statistically significant differences from each other (1-way ANOVA followed by Fisher LSD test). The number of subjects in each group is given in parentheses.
3.4. Confirmation of Lesions
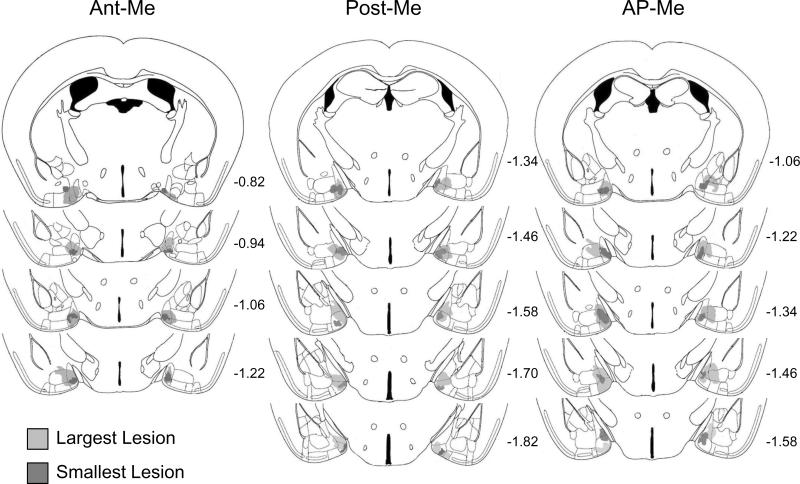
Subjects were used in this study only if the appropriate regions of the Me were damaged by lesions bilaterally (Fig 5). For some subjects in the Ant-Me and Post-Me groups (n=2 and n=3, respectively), lesion damage was found to extend into both Me subdivisions in one hemisphere. Subjects remained in the study in these cases because damage to the anterior and posterior regions was unilateral. Similarly, there were some subjects in which lesions extended beyond the anterior and posterior Me. Such subjects were included only if damage to any of these adjacent regions was minimal and unilateral. Animals that sustained appreciable bilateral damage to any of the adjacent amygdaloid nuclei were excluded from the study, as were several animals that sustained bilateral damage to the ventral surface of the brain.
Fig. 5.
Schematic reconstruction of coronal sections of the largest (light gray) and smallest (dark grey) extent of lesion damage in female mice given bilateral medial amygdala lesions of the anterior (Ant-Me; n=10) or posterior (Post-Me; n=16) subdivisions, or along the anterior-posterior border (AP-Me; n=9). Sections are ordered sequentially from anterior (top) to posterior (bottom), with the numbers shown representing the distance in mm posterior to bregma for each section (adapted from Franklin and Paxinos, 2008).
4. Discussion
Bilateral lesions that included the posterior Me (Post-Me and AP-Me groups) significantly reduced the typical preference shown by estrous female mice for investigating volatile urinary odors from breeding, as opposed to castrated males, with similar trends being seen in tests where nasal contact with stimuli was permitted. In addition, the total amount of time spent investigating either stimulus in the two-choice test of urinary volatiles was reduced in mice that received posterior Me lesions. In contrast, lesions restricted to the anterior Me failed to disrupt either females' preference for investigating intact male urinary odors, or the total amount of time spent in nasal investigation of urinary cues. In all three lesions groups (Ant-Me, Post-Me and AP-Me), however, display of lordosis was significantly reduced compared to sham females. These observations suggest that the posterior, but not the anterior, Me may be critical for enabling female mice to show preferences for urinary volatiles from males in breeding condition, whereas both Me subdivisions are essential for lordosis.
Estrous females' preference for testes intact over castrated male urinary odors suggests that there are changes in the chemical profile of urine after castration. While to our knowledge, a full characterization of urine from testes intact and castrated male mice has not been published, there is some evidence that differences exist. For example, the putative male urinary pheromone (methylthio) methanethiol (MTMT) is innately attractive to female mice, is found in high concentrations in breeding (testes-intact) male urine, and is not present after castration [23]. Two known volatile constituents of male urine, 2-(sec-butyl)-4,5-dihydrothiazole and dehydro-exo-brevicomin are found only in testes-intact male urine, their production is androgen dependent, and they have been shown to promote the Whitten effect (induction of estrous cycle in females) [24,25]. Both Isoamylamine and trimethylamine, which are ligands for trace amine-associated receptors (TAARs), have been found in high concentrations only in testes-intact male mice, and isoamylamine can accelerate puberty onset in female mice [26,27]. Meanwhile, there have been no reports that castration results in an increase in any chemical constituents relative to gonadally intact males.
The finding that lesions of the posterior Me decreased both the preference for volatiles from intact vs. castrated male urine as well as total investigation time suggests that an assessment and/or an establishing of the salience of urinary volatiles requires the posterior Me. Although it has been known for many years that the vomeronasal projection pathway that responds to non-volatile chemosignals passes through the Me en route to hypothalamic targets, until recently it had not been clearly demonstrated that the rodent Me receives direct projections from mitral cells in the main olfactory bulb [6, 7]. In addition, in mice, Me-projecting mitral cells showed selective activation by opposite-, as opposed to same-sex urinary volatiles [7], suggesting that targets in the Me receive particular patterns of activation depending on the urinary cue. More work will be required to determine if preference for the urinary volatiles of testes-intact vs. castrated male mice involves differences in the activation of Me-projecting mitral cells.
Examination of the projections from the anterior and posterior portions of the Me suggest that differential processing of olfactory information by these structures may be due in part to differences in their downstream targets. A report by Choi and others [5] described the differential expression of LIM homeodomain genes in different subnuclei of the Me. The transcription factor Lhx6 was found to be expressed in neurons located primarily in the posterodorsal portion of the Me, and these neurons projected to targets in the hypothalamus involved in reproduction (e.g., ventrolateral part of the VMH and the medial preoptic nucleus) [5, 28]. Meanwhile Lhx9-expressing neurons were found in the posteroventral Me and projected to sites in the hypothalamus that are linked to defensive behaviors (e.g., dorsomedial part of the VMH and the anterior hypothalamic nucleus) [5, 29]. Anterior Me neurons that express Lhx5 were also found to project to these “defensive” hypothalamic nuclei [5]. Future studies will seek to determine if subnuclei in the Me also differentially project to ventral striatal regions involved in reward.
It is noteworthy that the female mice used in the present study had not previously had nasal contact with a sexually mature male prior to first being exposed to volatile male urinary odors. The fact that sham-operated as well as Ant-Me females showed a robust preference for volatile urinary odors from a testes-intact vs. a castrated male suggests that this preference was expressed in the absence of any previous association with non-volatile urinary odors otherwise detected by the VNO-accessory olfactory system. As such, our results differ from observations [30–32] that volatile male-derived olfactory signals are not intrinsically attractive to females but instead become attractive only after being paired with non-volatile male pheromones that are processed via the VNO-accessory olfactory pathway.
In general, the present data corroborate previous reports of olfactory deficits after Me lesions in female hamsters [33] and rats [34]. Male hamsters that received lesions targeted at either the anterior or posterior Me showed a reduced preference for volatiles from female-soiled over male-soiled bedding in Y-maze tests [17]. By contrast, male hamsters that received anterior Me lesions spent more time than sham-operated controls investigating both odors. It was concluded that such high levels of investigation toward both stimuli reflected males' inability to categorize the relevance of odor stimuli. Furthermore, it has been observed in rats that lesions of the entire Me diminished the preference for testes-intact over castrated male volatiles in females in which air was blown over a stimulus male into the female compartment [34]. In the present study, female mice that received anterior Me lesions did not spend more time investigating both odors when compared to sham females. In addition, they did not show any deficits in their preference for testes-intact over castrated male urine, regardless of whether or not they had direct nasal access to the stimuli. These differences may reflect species and/or sex differences in the functional anatomy of the anterior Me. Alternative explanations are that these differences could be due to the disparity in the type of odor stimuli used (bedding vs. urine) or the types of odors presented (same vs. opposite sex bedding; testes-intact vs. castrated male urine).
When nasal contact with urine was permitted, both sham-operated and Ant-Me females showed a robust preference for urinary odors from a testes-intact as opposed to a castrated male; conversely, lesions along the anterior-posterior Me border significantly reduced this preference, and a similar, though non-significant trend was observed in females given lesions of the posterior Me. Previous studies have shown that lesions of the VNO [13] or of the AOB [35] eliminated the preference of female mice to investigate non-volatile urinary odors and/or soiled bedding odors from testes-intact vs. castrated males. Our results imply that some AOB mitral cells target sites along the anterior-posterior border of the Me, and that processing of these inputs plays an essential role in females' decision to seek out a reproductively active male. It is unlikely that the absence of a preference to investigate either volatile or non-volatile urinary odors from testes-intact males in AP-Me and/or Post-Me females reflects an inability to discriminate these odors from those emitted by a castrated male because females in all four treatment groups showed robust, significant dishabituation responses towards urinary volatiles from castrated males followed, later, towards urinary volatiles from testes-intact males. Nevertheless, we cannot exclude the possibility that detection of certain subsets of odors was selectively affected by lesions.
Bilateral lesions located in any of the three regions of the Me caused a significant reduction in lordosis responses in ovariectomized female subjects previously primed with ovarian hormones. Indeed, several other studies have shown that either VNO lesions [13] or AOB lesions [20] significantly reduced lordosis behavior in estrous female mice. It should be noted, however, that chemical lesions of the MOE, which spared VNO sensory neurons, also caused significant reductions in the lordosis responses of estrous female mice [13]. It was recently suggested [8] that the male tear pheromone, ESP1, augments lordotic responsiveness in female mice following its detection by the VNO and processing along the AOS input pathway to the hypothalamus. In so far as mitral cells in the both the AOB and the MOB target several subregions of the Me in mice [7, 36], it is possible that both systems detect the relevant pheromonal stimuli (including ESP1) and provide widespread inputs to the Me, all of which are required for the normal ability of estrous female mice to display lordosis in response to male mounts. Presumably these olfactory inputs sensitize females to the lordosis-inducing effects of flank stimulation received at the time of mounting. Future research will specify sites in the nervous system where these olfactory, hormonal, and somatosensory inputs are integrated to facilitate the expression of lordosis.
Highlights
We show that lesions that include the posterior medial amygdala disrupt females' ability to prefer urinary volatiles from breeding males
Lesions of anterior or posterior portions of the medial amygdala disrupt females' lordosis response to male mounts
Females show an olfactory preference for breeding male urinary volatiles despite having no prior olfactory experience with males
Acknowledgments
Some of these findings were presented at the 2010 Association for Chemoreception Sciences (AChemS) meeting. We thank Barbara Juarez for help with behavioral testing. This work was supported by NIH grant DC008962 awarded to JAC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Meredith M. Sensory processing in the main and accessory olfactory systems: comparisons and contrasts. J Steriod Biochem Mol Biol. 1991;39(4B):601–14. doi: 10.1016/0960-0760(91)90258-7. [DOI] [PubMed] [Google Scholar]
- [2].Restrepo D, Arellano J, Oliva AM, Schaefer ML, Lin W. Emerging views on the distinct but related roles of the main and accessory olfactory systems in responsiveness to chemosensory signals in mice. Horm Behav. 2004;46(3):247–56. doi: 10.1016/j.yhbeh.2004.02.009. [DOI] [PubMed] [Google Scholar]
- [3].Baum MJ, Kelliher KR. Complementary roles of the main and accessory olfactory systems in mammalian mate recognition. Annu Rev Physiol. 2009;71:141–60. doi: 10.1146/annurev.physiol.010908.163137. [DOI] [PubMed] [Google Scholar]
- [4].Kevetter GA, Winans SS. Connections of the corticomedial amygdala in the golden hamster. I. Efferents of the “vomeronasal amygdala”. J Comp Neurol. 1981;197(1):81–98. doi: 10.1002/cne.901970107. [DOI] [PubMed] [Google Scholar]
- [5].Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46(4):647–60. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- [6].Pro-Sistiaga P, Mohedano-Moriano A, Ubeda-Banon I, Del Mar Arroyo-Jiminez M, Marcos P, Artacho-Perula E, Crespo C, Insausti R, Martinez-Marcos A. Convergence of oflactory and vomeronasal projections in the rat basal telencephalon. J Comp Neurol. 2007;504(4):346–62. doi: 10.1002/cne.21455. [DOI] [PubMed] [Google Scholar]
- [7].Kang N, Baum MJ, Cherry JA. A direct main olfactory bulb projection to the `vomeronasal' amygdala in female mice selectively responds to volatile pheromones from males. Eur J Neurosci. 2009;29(3):624–34. doi: 10.1111/j.1460-9568.2009.06638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Haga S, Hattori T, Sato T, Sato K, Matsuda S, Kobayakawa R, Sakano H, Yoshihara Y, Kikusui T, Touhara K. The male mouse pheromone ESP1 enhances female sexual receptive behaviour through a specific vomeronasal receptor. Nature. 2010;466(7302):118–22. doi: 10.1038/nature09142. [DOI] [PubMed] [Google Scholar]
- [9].Halem HA, Cherry JA, Baum MJ. Vomeronasal neuroepithelium and forebrain Fos responses to male pheromones in male and female mice. J Neurobiol. 1999;39(2):249–63. [PubMed] [Google Scholar]
- [10].Moncho-Bogani J, Martinez-Garcia F, Novejarque A, Lanuza E. Attraction to sexual pheromones and associated odorants in female mice involves activation of the reward system and basolateral amygdala. Eur J Neurosci. 2005;21(8):2186–98. doi: 10.1111/j.1460-9568.2005.04036.x. [DOI] [PubMed] [Google Scholar]
- [11].Martel KL, Baum MJ. Sexually dimorphic activation of the accessory, but not the main, olfactory bulb in mice by urinary volatiles. Eur J Neurosci. 2007;26(2):463–75. doi: 10.1111/j.1460-9568.2007.05651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Martel KL, Keller M, Douhard Q, Bakker J, Baum MJ. Comparison of urinary odor-induced glomerular activation in the main olfactory bulb of aromatase knock-out and wild type female mice. Neurosci Lett. 2007;421(2):101–5. doi: 10.1016/j.neulet.2007.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Keller M, Pierman S, Douhard Q, Baum MJ, Bakker J. The vomeronasal organ is required for the expression of lordosis behaviour, but not sex discrimination in female mice. Eur J Neurosci. 2006;23(2):521–30. doi: 10.1111/j.1460-9568.2005.04589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Keller M, Douhard Q, Baum MJ, Bakker J. Destruction of the main olfactory epithelium reduces female sexual behavior and olfactory investigation in female mice. Chem Senses. 2006;31(4):315–23. doi: 10.1093/chemse/bjj035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Maras PM, Petrulis A. The anterior medial amygdala transmits sexual odor information to the posterior medial amygdala and related forebrain nuclei. Eur J Neurosci. 2010;32(3):469–82. doi: 10.1111/j.1460-9568.2010.07289.x. [DOI] [PubMed] [Google Scholar]
- [16].Maras PM, Petrulis A. Anatomical connections between the anterior and posterodorsal sub-regions of the medial amygdala: integration of odor and hormone signals. Neuroscience. 2010;170(2):610–22. doi: 10.1016/j.neuroscience.2010.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Maras PM, Petrulis A. Chemosensory and steroid-responsive regions of the medial amygdala regulate distinct aspects of opposite-sex odor preference in male Syrian hamsters. Eur J Neurosci. 2006;24(12):3541–52. doi: 10.1111/j.1460-9568.2006.05216.x. [DOI] [PubMed] [Google Scholar]
- [18].Wood RI, Brabec RK, Swann JM, Newman SW. Androgen and estrogen concentrating neurons in chemosensory pathways of the male Syrian hamster brain. Brain Res. 1992;596(1–2):89–98. doi: 10.1016/0006-8993(92)91536-n. [DOI] [PubMed] [Google Scholar]
- [19].Wood RI, Newman SW. Mating activates androgen receptor-containing neurons in chemosensory pathways of the male Syrian hamster brain. Brain Res. 1993;614:65–77. doi: 10.1016/0006-8993(93)91019-o. [DOI] [PubMed] [Google Scholar]
- [20].Martel KL, Baum MJ. Adult testosterone treatment but not surgical disruption of vomeronasal function augments male-typical sexual behavior in female mice. J Neurosci. 2009;29(24):7658–66. doi: 10.1523/JNEUROSCI.1311-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Baum MJ, Keverne EB. Sex difference in attraction thresholds for volatile odors from male and estrous female mouse urine. Horm Behav. 2002;41(2):213–19. doi: 10.1006/hbeh.2001.1749. [DOI] [PubMed] [Google Scholar]
- [22].Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. 3rd ed Academic Press; California: 2008. [Google Scholar]
- [23].Lin D, Zhang S-Z, Block E, Katz L. Encoding social signals in the mouse main olfactory bulb. Nature. 2005;434(7032):470–7. doi: 10.1038/nature03414. [DOI] [PubMed] [Google Scholar]
- [24].Marsden HM, Bronson FH. Estrous synchrony in mice: alteration by exposure to male urine. Science. 1964;144:1469. doi: 10.1126/science.144.3625.1469. [DOI] [PubMed] [Google Scholar]
- [25].Jemiolo B, Harvey S, Novotny M. Promotion of the whitten effect in female mice by synthetic analogs of male urinary constituents. Proc Natl Acad Sci USA. 1986;83(12):4576–9. doi: 10.1073/pnas.83.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Liberles SD, Buck LB. A second class of chemosensory receptrs in the olfactory epithelium. Nature. 2006;442(7103):645–50. doi: 10.1038/nature05066. [DOI] [PubMed] [Google Scholar]
- [27].Nishimura K, Utsumi K, Yuhara M, Fujitani Y, Iritani A. Identification of puberty-accelerating pheromones. J Exp. Zool. 1989;251:300–5. doi: 10.1002/jez.1402510306. [DOI] [PubMed] [Google Scholar]
- [28].Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann. N Y Acad. Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- [29].Canteras NS. The medial hypothalamic defensive system: hodological organization and functional implications. Pharmacol Biochem Behav. 2002;71:481–491. doi: 10.1016/s0091-3057(01)00685-2. [DOI] [PubMed] [Google Scholar]
- [30].Martinez-Ricos J, Agustin-Pavon C, Lanuza E, Martinez-Garcia F. Intraspecific communication through chemical signals in female mice: reinforcing properties of involatile male sexual pheromones. Chem Senses. 2007;32(2):139–48. doi: 10.1093/chemse/bjl039. [DOI] [PubMed] [Google Scholar]
- [31].Martinez-Garcia F, Martinez-Ricos J, Agustin-Pavon C, Martinez-Hernandez J, Novejarque A, Lanuza E. Refining the dual olfactory hypothesis: pheromone reward and odour experience. Behav Brain Res. 2009;200(2):277–86. doi: 10.1016/j.bbr.2008.10.002. [DOI] [PubMed] [Google Scholar]
- [32].Ramm SA, Cheetham SA, Hurst JL. Encoding choosiness: female attraction requires prior physical contact with indivudual male scents in mice. Proc R Soc B. 2008;275:1727–35. doi: 10.1098/rspb.2008.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Petrulis A, Johnston RE. Lesions centered on the medial amygdala impair scent-marking and sex-odor recognition but spare discrimination of individual odors in female golden hamsters. Behav Neurosci. 1999;113(2):345–57. doi: 10.1037//0735-7044.113.2.345. [DOI] [PubMed] [Google Scholar]
- [34].Kondo Y, Sakuma Y. The medial amygdala controls the coital access of female rats: a possible involvement of emotional responsiveness. Jpn J Physiol. 2005;55(6):345–53. doi: 10.2170/jjphysiol.RP001105. [DOI] [PubMed] [Google Scholar]
- [35].Martinez-Ricos J, Agustin-Pavon C, Lanuza E, Martinez-Garcia F. Role of the vomeronasal system in intersexual attraction in female mice. Neuroscience. 2008;153(2):383–95. doi: 10.1016/j.neuroscience.2008.02.002. [DOI] [PubMed] [Google Scholar]
- [36].Kang N, Baum MJ, Cherry JA. Different profiles of main and accessory olfactory bulb mitral/tufted cell projections revealed in mice using an anterograde tracer and a whole-mount, flattened cortex preparation. Chem Senses. 2011;36(3):251–60. doi: 10.1093/chemse/bjq120. [DOI] [PMC free article] [PubMed] [Google Scholar]