Abstract
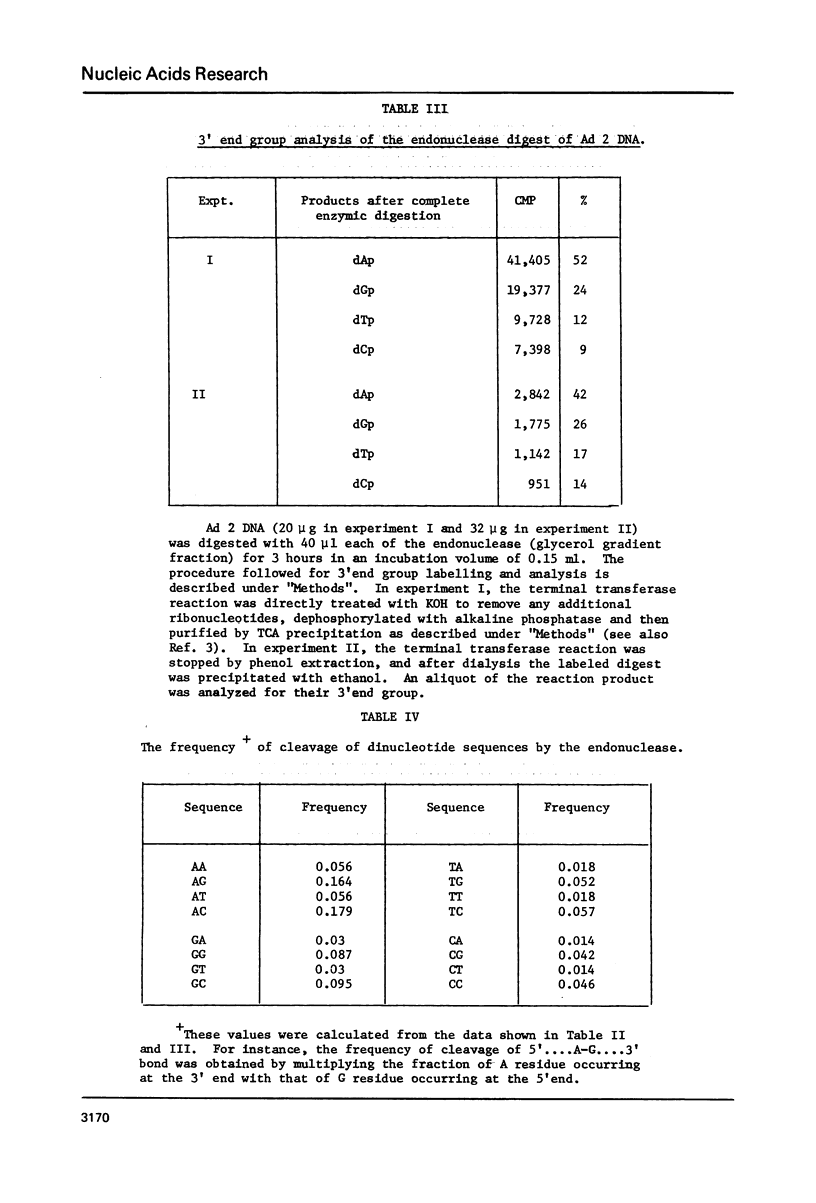
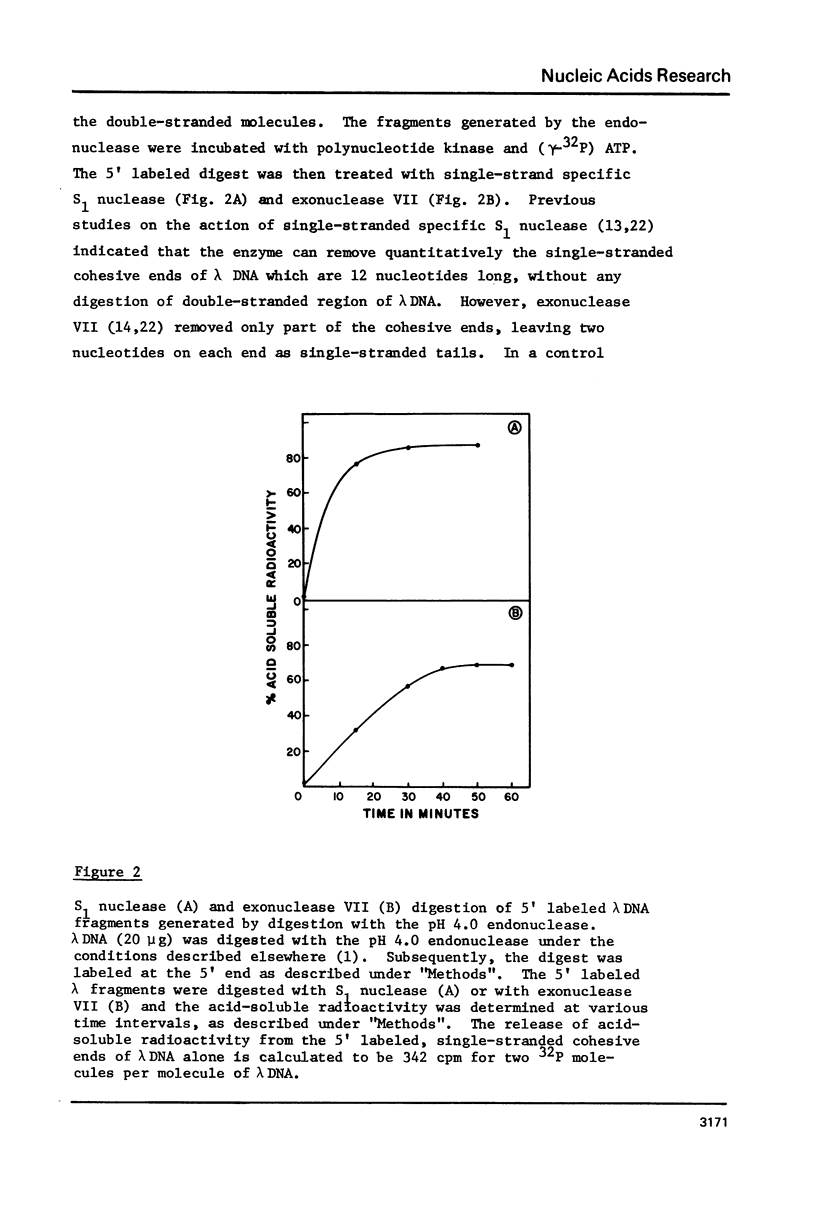
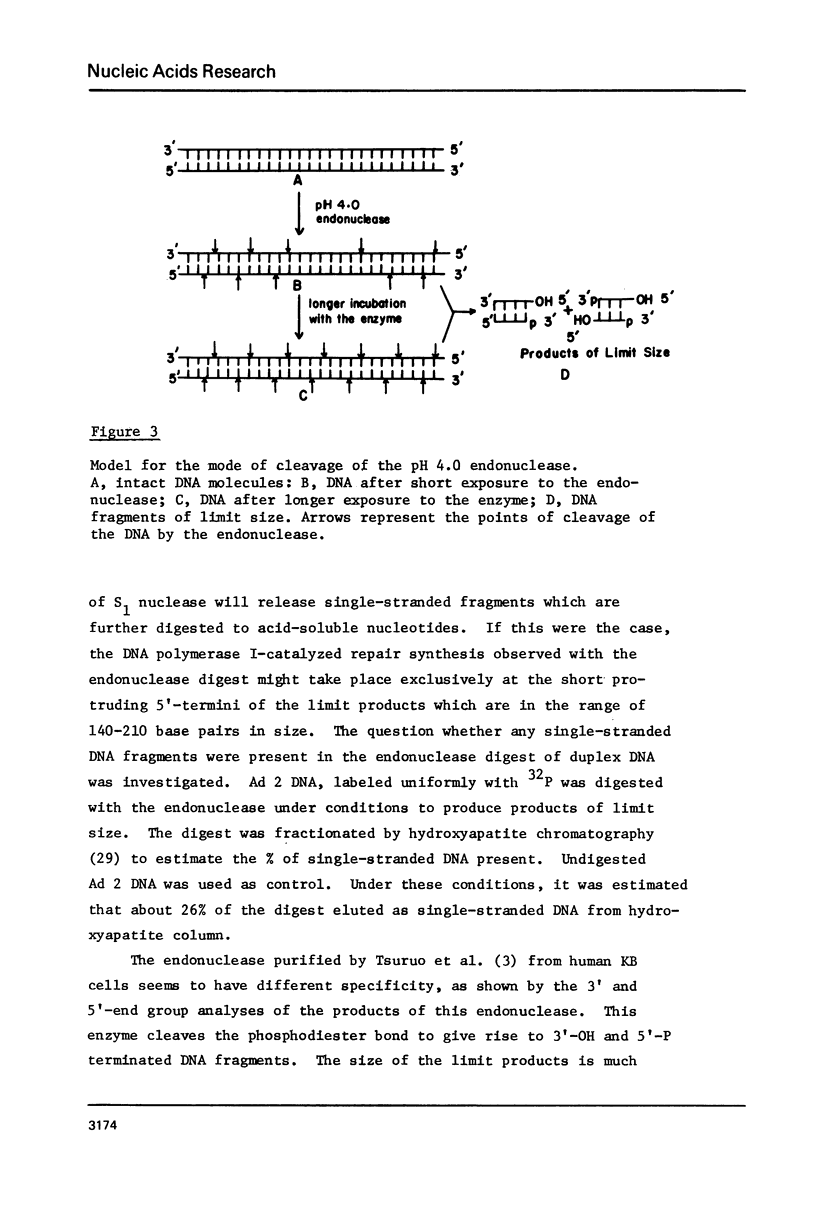
Adenovirus type 2 or lambda DNA was digested with the pH 4.0 endonuclease, purified from adenovirus 2-infected KB cells. The enzyme produces a limit digest of approximate size in the range of 140-210 base pairs long. The termini of the DNA fragments generated by the endonuclease digestion had 3'-P and 5'-OH groups. The 3' and 5' end groups of the products were analyzed. Our data indicate that 3' end group was a purine (68-76%), dA occuring about twice the frequency of dG. The 5' end group was either dG or dC with equal frequency. Data obtained by treatment of the 5' labeled endonuclease product of lambda DNA with single-strand specific S1 nuclease from Asperigillus oryzae or exonuclease VII from Escherichia coli indicated that the majority of the products had a short 5' protruding ends. The mode of cleavage of this endonuclease seems to be through initial formation of several single-strand breaks with some base specificity. If these breaks are at close proximity on opposite strands, double-stranded fragments with protruding ends are generated.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beard P., Morrow J. F., Berg P. Cleavage of circular, superhelical simian virus 40 DNA to a linear duplex by S1 nuclease. J Virol. 1973 Dec;12(6):1303–1313. doi: 10.1128/jvi.12.6.1303-1313.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlingham B. T., Doerfler W. An endonuclease in cells infected with adenovirus and associated with adenovirions. Virology. 1972 Apr;48(1):1–13. doi: 10.1016/0042-6822(72)90108-0. [DOI] [PubMed] [Google Scholar]
- Burlingham B. T., Doerfler W., Pettersson U., Philipson L. Adenovirus endonuclease: association with the penton of adenovirus type 2. J Mol Biol. 1971 Aug 28;60(1):45–64. doi: 10.1016/0022-2836(71)90446-3. [DOI] [PubMed] [Google Scholar]
- Burton W. G., Roberts R. J., Myers P. A., Sager R. A site-specific single-strand endonuclease from the eukaryote Chlamydomonas. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2687–2691. doi: 10.1073/pnas.74.7.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajean-Feroldi C., Chardonnet Y., Chantepie-Auray J. Deoxyribonuclease activity associated with adenovirus 5 and 7. Eur J Biochem. 1977 Apr 15;74(3):457–462. doi: 10.1111/j.1432-1033.1977.tb11412.x. [DOI] [PubMed] [Google Scholar]
- Chase J. W., Richardson C. C. Exonuclease VII of Escherichia coli. Purification and properties. J Biol Chem. 1974 Jul 25;249(14):4545–4552. [PubMed] [Google Scholar]
- Doerfler W., Lundholm U., Hirsch-Kauffmann M. Intracellular forms of adenovirus deoxyribonucleic acid. I. Evidence for a deoxyribonucleic acid-protein complex in baby hamster kidney cells infected with adenovirus type 12. J Virol. 1972 Feb;9(2):297–308. doi: 10.1128/jvi.9.2.297-308.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund P. T. Analysis of nucleotide sequences at 3' termini of duplex deoxyribonucleic acid with the use of the T4 deoxyribonucleic acid polymerase. J Biol Chem. 1971 May 25;246(10):3269–3276. [PubMed] [Google Scholar]
- Germond J. E., Vogt V. M., Hirt B. Characterization of the single-strand-specific nuclease S1 activity on double-stranded supercoiled polyoma DNA. Eur J Biochem. 1974 Apr 16;43(3):591–600. doi: 10.1111/j.1432-1033.1974.tb03446.x. [DOI] [PubMed] [Google Scholar]
- Ghangas G. S., Wu R. Specific hydrolysis of the cohesive ends of bacteriophage lambda DNA by three single strand-specific nucleases. J Biol Chem. 1975 Jun 25;250(12):4601–4606. [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M. E., Yarmolinsky M. B. Integration-negative mutants of bacteriophage lambda. J Mol Biol. 1968 Feb 14;31(3):487–505. doi: 10.1016/0022-2836(68)90423-3. [DOI] [PubMed] [Google Scholar]
- Hayward G. S. Gel electrophoretic separation of the complementary strands of bacteriophage DNA. Virology. 1972 Jul;49(1):342–344. doi: 10.1016/s0042-6822(72)80042-4. [DOI] [PubMed] [Google Scholar]
- Marusyk R. G., Morgan A. R., Wadell G. Association of endonuclease activity with serotypes belonging to the three subgroups of human adenoviruses. J Virol. 1975 Aug;16(2):456–458. doi: 10.1128/jvi.16.2.456-458.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méchali M., de Recondo A. M., Girard M. Action of the S1 endonuclease from Aspergillus oryzae on simian virus 40 supercoiled component I DNA. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1306–1320. doi: 10.1016/0006-291x(73)91130-3. [DOI] [PubMed] [Google Scholar]
- Reif U. M., Winterhoff U., Lundholm U., Philipson L., Doerfler W. Purification of an endonuclease from adenovirus-infected KB cells. Eur J Biochem. 1977 Mar 1;73(2):313–325. doi: 10.1111/j.1432-1033.1977.tb11321.x. [DOI] [PubMed] [Google Scholar]
- Reif U., Winterhoff U., Doerfler W. Characterization of the pH 4.0 endonuclease from adenovirus-type-2-infected KB cells. Eur J Biochem. 1977 Mar 1;73(2):327–333. doi: 10.1111/j.1432-1033.1977.tb11322.x. [DOI] [PubMed] [Google Scholar]
- Rhoades M., MacHattie L. A., Thomas C. A., Jr The P22 bacteriophage DNA molecule. I. The mature form. J Mol Biol. 1968 Oct 14;37(1):21–40. doi: 10.1016/0022-2836(68)90071-5. [DOI] [PubMed] [Google Scholar]
- Roychoudhury R., Jay E., Wu R. Terminal labeling and addition of homopolymer tracts to duplex DNA fragments by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 1976 Jan;3(1):101–116. doi: 10.1093/nar/3.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Steenbergh P. H., Sussenbach J. S., Roberts R. J., Jansz H. S. The 3'-terminal nucleotide sequences of adenovirus types 2 and 5 DNA. J Virol. 1975 Feb;15(2):268–272. doi: 10.1128/jvi.15.2.268-272.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter D., Westphal M., Doerfler W. Patterns of integration of viral DNA sequences in the genomes of adenovirus type 12-transformed hamster cells. Cell. 1978 Jul;14(3):569–585. doi: 10.1016/0092-8674(78)90243-x. [DOI] [PubMed] [Google Scholar]
- Tsuruo T., Arens M., Padmanabhan R., Green M. An endodeoxyribonuclease of human KB cells. Purification and properties of the enzyme. J Biol Chem. 1978 May 25;253(10):3400–3407. [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Wiegand R. C., Godson G. N., Radding C. M. Specificity of the S1 nuclease from Aspergillus oryzae. J Biol Chem. 1975 Nov 25;250(22):8848–8855. [PubMed] [Google Scholar]
- Wu R., Kaiser A. D. Mapping the 5'-terminal nucleotides of the DNA of bacteriophage lambda and related phages. Proc Natl Acad Sci U S A. 1967 Jan;57(1):170–177. doi: 10.1073/pnas.57.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R. Nucleotide sequence analysis of DNA. I. Partial sequence of the cohesive ends of bacteriophage lambda and 186 DNA. J Mol Biol. 1970 Aug;51(3):501–521. doi: 10.1016/0022-2836(70)90004-5. [DOI] [PubMed] [Google Scholar]
- Wu R., Taylor E. Nucleotide sequence analysis of DNA. II. Complete nucleotide sequence of the cohesive ends of bacteriophage lambda DNA. J Mol Biol. 1971 May 14;57(3):491–511. doi: 10.1016/0022-2836(71)90105-7. [DOI] [PubMed] [Google Scholar]