Abstract
Objective
To assess practitioners’ referral patterns and knowledge of palliative radiotherapy (PRT).
Design
A 23-item questionnaire.
Setting
Northern Alberta and parts of British Columbia, Saskatchewan, the Northwest Territories, and Nunavut.
Participants
A total of 1360 health practitioners, including primary care physicians and nurse clinicians in rural, remote, or far northern regions; FP-oncologists working in community cancer centres; palliative care (PC) specialists; and medical oncologists.
Main outcome measures
Survey respondents rated how much certain factors influenced their decisions to refer patients for PRT and estimated their knowledge of PRT. Descriptive and summary statistics were compiled.
Results
The overall eligible response rate was 31.8% (412 of 1294); 85.4% of respondents were FPs, 65.3% were men, and 44.9% practised in rural settings. A total of 81.8% of respondents sometimes or often provided PC and 71.0% had referred patients for PRT. Main factors taken into account when referring patients were functional status (93.1%; 349 of 375), histology (75.4%; 285 of 378), and concern about side effects (75.3%; 281 of 373). Half of respondents considered wait times for PRT delivery important. Self-rated knowledge of PRT was poor for 74.0% of respondents, fair for 24.5%, and good for 1.5%. Actual knowledge scores were poor for 46.6% of respondents, fair for 36.7%, and good for 16.7%. Respondents who referred patients for PRT had been in practice longer, saw more cancer patients per month, provided PC more frequently, had higher self-rated PRT knowledge, and had better actual PRT knowledge.
Conclusion
Disease- and patient-related factors outweighed concerns about wait times. Although referring practitioners are better informed than they believe themselves to be, further improvements in their knowledge could increase referrals of appropriate patients for PRT.
Résumé
Objectif
Déterminer comment les médecins dirigent les patients en radiothérapie palliative (RTP) et ce qu’ils connaissent de ce traitement.
Type d’étude
Un questionnaire de 23 items.
Contexte
Le nord de l’Alberta et certaines parties de la Colombie-Britannique, de la Saskatchewan, des Territoires du Nord-Ouest et du Nunavut.
Participants
Un total de 1360 membres du personnel de la santé, incluant des médecins de première ligne et des infirmières praticiennes oeuvrant dans des régions rurales et des régions éloignées ou du Grand-Nord; des MF-oncologistes travaillant dans des centres de cancer communautaires; des spécialistes des soins palliatifs (SP); et des oncologistes médicaux.
Principaux paramètres à l’étude
Les répondants à l’enquête ont comparé l’importance de certains facteurs qui influençaient leur décision d’orienter des patients en RTP et estimé leur connaissance de la RTP. On a calculé les statistiques descriptives et sommaires.
Résultats
Le taux global de réponses admissibles était de 31,8 % (412 sur 1294); 85,4 % des répondants étaient des médecins de famille, 65,3 % étaient des hommes et 44,9 % pratiquaient en région rurale. Au total, 81,8 % des répondants prodiguaient occasionnellement ou fréquemment des SP et 71,0 % avaient orienté des patients en RTP. Les principaux facteurs considérés au moment de diriger les patients étaient l’état fonctionnel (93,1 %; 349 sur 375), les do^nnées histologiques (75,4 %; 285 sur 378) et la crainte d’effets indésirables (75,3 %; 281 sur 373). La moitié des répondants considéraient que le temps d’attente pour la RTP était aussi un facteur important. À l’auto-évaluation, 74,0 % des répondants jugeaient que leur connaissance de la RTP était mauvaise, 24,5 % la jugeaient passable et 1,5 % la jugeaient bonne. En réalité, les scores obtenus pour la connaissance étaient mauvais pour 46,6 % des répondants, passables pour 36,7 % et bons pour 16,7 %. Les répondants qui dirigeaient des patients en RTP avaient plus d’années de pratique, voyaient plus de cancéreux par mois, prodiguaient plus souvent des SP, avaient une meilleure évaluation de leur connaissance de la RTP et avaient une meilleure connaissance réelle de la RTP.
Conclusion
Les facteurs liés au patient et à la maladie étaient plus importants que le temps d’attente. Même si les médecins sont mieux informés qu’ils ne le pensent, une amélioration de leurs connaissances pourrait augmenter le nombre de patients dirigés en RTP de façon appropriée.
Approximately 50% of all patients with cancer will eventually develop metastases, at which point their disease generally becomes incurable.1 Treatment focus then shifts to symptom control and quality of life. Some patients might receive palliative systemic therapy under the care of medical oncologists (MOs), and others might be followed by palliative care (PC) practitioners. Commonly, after patients have completed active treatment at the tertiary cancer centre, they are discharged to the care of their community FPs,2–4 which is advantageous in terms of accessibility, personalized care, and resource use.5
It is presumed that these practitioners will refer patients to radiation oncologists (ROs) as necessary for palliative radiotherapy (PRT)5 to ameliorate local symptoms such as pain, bleeding, or obstruction.6–11 Palliative radiotherapy is usually simple to administer, with few side effects,12 but is generally underused.13 This might be owing, in part, to referring practitioners’ uncertainty about its efficacy and indications.5,14 Patients with symptoms amenable to PRT will not have this opportunity unless they are referred to an RO.5,14,15
The sole radiotherapy referral centre in northern Alberta is the Cross Cancer Institute (CCI) in Edmonton, which serves approximately 1.5 million people. The catchment region encompasses parts of British Columbia, Saskatchewan, the Northwest Territories, and Nunavut. Within this region, FPs provide much of the end-of-life care for cancer patients, especially in rural or remote areas.
The objective of this study was to assess practitioners’ referral patterns and their knowledge of common indications for PRT.
METHODS
A 4-page, 23-item questionnaire was adapted with permission from Samant et al.14 The original development panel included a survey expert, 2 ROs, and a PC physician.14 It was pilot-tested on FPs attending an oncology update meeting to establish face validity, ease of completion, content, value, relevance, and completion time.14 Sections included respondent demographics, previous experience with CCI radiation oncology, factors influencing the decision to refer, and PRT knowledge. Answers were recorded on an 11-point numeric rating scale, verbal rating scales, or by marking tick boxes.
Knowledge of PRT was measured in 2 ways: self-rated and actual. Self-rated knowledge was assessed directly from answers to 4 questions on a 0-to-3 scale (0 = very little knowledge to 3 = extremely knowledgeable) for a total of 12 possible points. A score of 5 or less was considered a poor level of knowledge, between 6 and 8 was considered fair knowledge, and 9 or greater was considered good knowledge.
Actual knowledge was assessed by the number of correct responses to 10 questions about indications for PRT (scale: not effective to very effective), together with the minimum life span required for PRT delivery (total of 11 possible points). The correct answer was somewhat or very effective for airway obstruction, bleeding, brain metastases, dysphagia, and superior vena cava obstruction; very effective for bone metastases and spinal cord compression (SCC); and not effective for febrile neutropenia, lymphedema, and hypercalcemia. A total of 5 or fewer correct answers was considered poor actual knowledge, between 6 and 8 fair knowledge, and 9 or greater good knowledge.
Questionnaires were distributed based on the Dillman method, which traditionally calls for 3 mailings and a reminder.16 Owing to budgetary constraints, the relatively low response to the second mailing, and concerns about the extended time the survey would be open to accommodate 3 mailings, we performed an initial mailing followed by a reminder with the entire questionnaire. There was no prenotification of potential respondents. Our survey was pilot-tested on an unselected group of FP residents for ease of completion, clarity, and time (10 to 20 minutes).
Practitioners from the following groups were targeted: primary care physicians and nurse clinicians in rural, remote, or far northern regions; FP-oncologists working in community cancer centres; PC specialists; and MOs. All rural practitioners and a random sample of urban practitioners were invited to participate. Rural and urban division was based on number of postal codes (4 postal codes or less was considered rural).
The sampling frame was compiled from a number of sources, including directories of licensing bodies (eg, College of Family Physicians of Canada), provincial cancer agencies, the Canadian Hospice Palliative Care Association and provincial associations, the Canadian Medical Directory, and local resources. Exclusion criteria included those without current contact information, retirees, and trainees. A total of 1360 practitioners (1174 in Alberta, 49 in British Columbia, 99 in Saskatchewan, 32 in the Northwest Territories, and 6 in Nunavut) were initially contacted. Responses were collected over a 10-month period. Institutional review board approval was secured, with informed consent indicated by return of the questionnaire.
Anonymized responses were collated and data analyzed using SPSS, version 15. Descriptive statistics were compiled as proportions and medians (ranges) for categorical variables and means (standard deviations) for continuous variables. The χ2 test was used for unordered categorical variables, and the Student t test was used for continuous variables. Logistic regression analysis was used for dichotomous outcomes and multiple linear regression analysis was used for continuous variables. Standard model-building strategy was used to identify the best-fit model for logistic and linear regression analysis. Univariate analysis was conducted with referral as the dependent variable and covariates of sex, years in practice, practice setting, hospital admitting privileges, cancer patients seen in the past month, frequency of providing PC, self-rated knowledge score, and perceived accessibility of ROs. All covariates significant at P < .10 were selected for multivariate analysis. Predictors of actual knowledge scores were similarly identified. Significance level was considered P ≤ .05, and all statistical tests were 2-tailed.
RESULTS
Of the returned surveys, 66 were considered ineligible for analysis. These included respondents not actively practising or on maternity leave; respondents without cancer patients in their practices; or practitioners who had moved and provided no forwarding contact information. A total of 412 completed surveys were received, for an overall response rate of 31.8% (412 of 1294).
A total of 85.4% of respondents were FPs, 65.3% were men, and 44.9% practised in rural settings (Table 1). On average, physicians had 20 years of experience (range 1 to 60 years). The average year respondents completed training was 1984 (range 1940 to 2004). The average number of years in practice was 20 years (range 1 to 60 years). Of respondents, 97.6% (400 of 410) had seen cancer patients in the past month, 81.8% (332 of 406) sometimes or often provided PC, and 71.0% (282 of 397) had referred patients for PRT in the past year.
Table 1.
Respondent characteristics
| CHARACTERISTIC | N (%) |
|---|---|
| Sex (N = 412) | |
| • Male | 269 (65.3) |
| • Female | 137 (33.3) |
| • NS | 6 (1.5) |
| Practice setting (N = 412) | |
| • Rural | 185 (44.9) |
| • Urban | 180 (43.7) |
| • Mixed | 41 (10.0) |
| • NS | 6 (1.5) |
| Type of practice (N = 412) | |
| • Family physician | 352 (85.4) |
| • Medical oncology | 15 (3.6) |
| • Palliative care | 13 (3.2) |
| • Other or NS | 32 (7.7) |
| Hospital admitting privileges (N = 412) | |
| • Yes | 305 (74.0) |
| • No | 101 (24.5) |
| • NS | 6 (1.5) |
| Cancer patients seen in the past month (N = 412) | |
| • None | 10 (2.4) |
| • 1 to 5 | 213 (51.7) |
| • 6 to 10 | 95 (23.1) |
| • More than 10 | 92 (22.3) |
| • NS | 2 (0.5) |
| Providing care for patients with advanced cancer (N = 412) | |
| • Never | 3 (0.7) |
| • Rarely | 61 (14.8) |
| • Sometimes | 160 (38.8) |
| • Often | 184 (44.7) |
| • NS | 4 (1.0) |
| Involved in palliative care (N = 412) | |
| • Never | 8 (1.9) |
| • Rarely | 66 (16.0) |
| • Sometimes | 165 (40.0) |
| • Often | 167 (40.5) |
| • NS | 6 (1.5) |
| Referred patients for PRT in the past year (N = 412) | |
| • Yes | 282 (68.4) |
| • No | 115 (27.9) |
| • Not my referral centre or NS | 15 (3.6) |
| No. of patients referred in the past year (N = 282) | |
| • 1 to 5 | 192 (68.1) |
| • 6 to 10 | 49 (17.4) |
| • 11 to 20 | 22 (7.8) |
| • More than 20 | 19 (6.7) |
NS—not specified, PRT—palliative radiotherapy.
A total of 64.6% (266 of 412) had contacted an RO for advice in the past, most commonly regarding the management plan of a mutual patient (Table 2). Satisfaction was rated out of 10, with a mean (SD) score of 7.5 (2.0) for wait time for consultation, 7.7 (1.8) for wait time of PRT delivery, and 8.3 (1.4) for advice obtained. Perceived satisfaction of their patients’ PRT experience was 7.8 (1.7).
Table 2.
Satisfaction with service provided: Satisfaction was rated out of 10.
| CHARACTERISTICS | VALUES |
|---|---|
| Satisfaction with wait time for consultation (N = 412) | |
| • Mean | 7.5 |
| • SD | 2.0 |
| • Median | 8.0 |
| • Range | 1 to 10 |
| • NS or NA, n (%) | 117 (28.4) |
| Satisfaction with wait time for PRT delivery (N = 412) | |
| • Mean | 7.7 |
| • SD | 1.8 |
| • Median | 8.0 |
| • Range | 2 to 10 |
| • NS or NA, n (%) | 118 (28.6) |
| Satisfaction with communication back (N = 412) | |
| • Mean | 7.4 |
| • SD | 2.2 |
| • Median | 8.0 |
| • Range | 0 to 10 |
| • NS or NA, n (%) | 117 (28.4) |
| Perceived satisfaction of your patients (N = 412) | |
| • Mean | 7.8 |
| • SD | 1.7 |
| • Median | 8.0 |
| • Range | 1 to 10 |
| • NS or NA, n (%) | 121 (29.4) |
| Obtained advice from a radiation oncologist (N = 412), n (%) | |
| • Yes | 266 (64.6) |
| • No | 141 (34.2) |
| • NS or NA | 5 (1.2) |
| Ease of contacting a radiation oncologist (N = 412), n (%) | |
| • Very easy | 56 (13.6) |
| • Somewhat easy | 131 (31.8) |
| • Neither easy nor difficult | 47 (11.4) |
| • Somewhat difficult | 26 (6.3) |
| • Very difficult | 4 (1.0) |
| • NS or NA | 148 (36.0) |
| Type of advice obtained (N = 687), n (%) | |
| • Discussed current management plan | 184 (26.8) |
| • Determined suitability of referral | 151 (22.0) |
| • Made an urgent referral | 94 (13.7) |
| • Discussed side effects of treatment | 91 (13.2) |
| • Discussed admission or transfer of care | 83 (12.1) |
| • Discussed end-of-life care | 47 (6.8) |
| • Made a routine referral | 31 (4.5) |
| • Other | 6 (0.9) |
| Satisfaction with advice obtained (N = 412) | |
| • Mean | 8.3 |
| • SD | 1.4 |
| • Median | 8.0 |
| • Range | 2 to 10 |
| • NS or NA | 160 (38.9) |
NA—not applicable, NS—not specified, PRT—palliative radiotherapy.
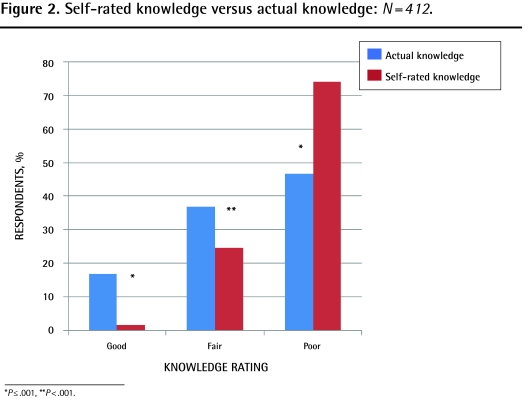
Actual knowledge of PRT was classified as poor for 46.6% (192 of 412) of respondents, fair for 36.7% (151 of 412), and good for 16.7% (69 of 412). The mean score was 5.6 of 11. The minimum life span usually required for PRT (1 month) was correctly identified by 15.3% of respondents. The correct efficacy of PRT was identified by 76.7% of respondents for brain metastases, 73.8% for bone metastases, 72.4% for airway obstruction, 68.5% for dysphagia, 56.6% for superior vena cava obstruction, 54.9% for SCC, and 47.8% for bleeding. A total of 41.0% of respondents correctly identified febrile neutropenia, 29.6% lymphedema, and 24.8% hypercalcemia as scenarios for which PRT is not effective.
Self-rated knowledge of PRT was classified as poor for 74.0% of respondents (305 of 412), fair for 24.5% (101 of 412), and good for 1.5% (6 of 412). The mean score was 3.9 of 12. The proportion of respondents who believed they had little or some knowledge of the mechanism of PRT was 69.9%; logistics was 84.6%; indications was 72.1%; and potential side effects was 68.2%.
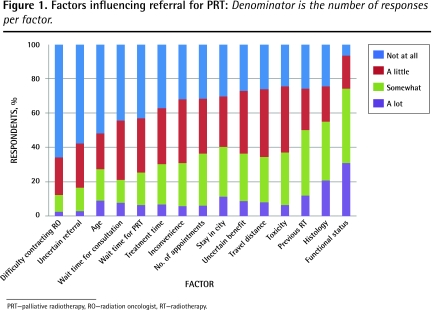
Factors influencing referral for PRT most often were functional status (93.1%; 349 of 375), histology (75.4%; 285 of 378), and concern about toxicity (75.4%; 281 of 373) (Figure 1). Logistic concerns, such as need for out-of-town patients to stay in the city, travel distance, or number of appointments required, were each taken into account “a lot” by 11% of respondents or less. Just over half of respondents considered wait times important.
Figure 1.
Factors influencing referral for PRT: Denominator is the number of responses per factor.
PRT—palliative radiotherapy, RO—radiation oncologist, RT—radiotherapy.
Physicians who had referred patients for PRT in the past year had been in practice on average 3 years longer. The average number of years in practice was 20.9 years for those who referred (273 of 282), with a range of 1 to 60 years; the average for respondents who did not refer (113 of 115) was 18.3 years, with a range of 1 to 50 years (P = .012). They had seen significantly more cancer patients per month (P < .001), had provided PC more frequently (P < .001), had higher self-rated PRT knowledge (P < .001), and had significantly higher actual knowledge (P < .001) (Table 3).
Table 3.
Characteristics of clinicians who referred and did not refer patients for PRT in the past year
| CHARACTERISTIC | REFERRED (N = 282), N (%) | DID NOT REFER (N = 115), N (%) | P VALUE |
|---|---|---|---|
| Practice setting* | .543 | ||
| • Rural | 127 (45.7) | 50 (44.2) | |
| • Urban | 120 (43.2) | 54 (47.8) | |
| • Mixed | 31 (11.2) | 9 (8.0) | |
| Accessibility of radiation oncologist† | .390 | ||
| • Somewhat or very easy | 154 (70.0) | 30 (76.9) | |
| • Neutral | 28 (12.7) | 2 (5.1) | |
| • Somewhat or very difficult | 38 (17.3) | 7 (17.9) | |
| Cancer patients seen in the past month‡ | < .001 | ||
| • None | 1 (0.4) | 9 (7.9) | |
| • 1 to 5 | 119 (42.3) | 85 (74.6) | |
| • 6 to 10 | 77 (27.4) | 16 (14.0) | |
| • More than 10 | 84 (29.9) | 4 (3.5) | |
| Provided palliative care§ | < .001 | ||
| • Never | 2 (0.7) | 6 (5.3) | |
| • Rarely | 22 (7.9) | 43 (38.1) | |
| • Sometimes | 113 (40.6) | 46 (40.7) | |
| • Often | 141 (50.7) | 18 (15.9) | |
| Self-rated knowledge score¶ | < .001 | ||
| • Poor | 189 (67.0) | 105 (91.3) | |
| • Fair | 87 (30.9) | 10 (8.7) | |
| • Good | 6 (2.1) | 0 (0.0) | |
| Actual knowledge score# | < .001 | ||
| • Poor | 116 (41.1) | 68 (59.1) | |
| • Fair | 103 (36.5) | 42 (36.5) | |
| • Good | 63 (22.3) | 5 (4.3) |
PRT—palliative radiotherapy.
N = 278 for clinicians who referred; N = 113 for clinicians who did not refer.
N = 220 for clinicians who referred; N = 39 for clinicians who did not refer.
N = 281 for clinicians who referred; N = 114 for clinicians who did not refer.
N = 278 for clinicians who referred; N = 113 for clinicians who did not refer.
N = 282 for clinicians who referred; N = 115 for clinicians who did not refer.
N = 282 for clinicians who referred; N = 115 for clinicians who did not refer.
Factors on univariate analysis predicting for referral were admitting privileges, sex (with men referring more often), years in practice, cancer patients seen per month, provision of PC, and self-rated knowledge. (As self-rated and actual knowledge were significantly correlated [P ≤ .001], only the former was included in the model.) On multivariate analysis, physicians with admitting privileges (P = .002) who provided PC (P = .004), saw more cancer patients (P = .002), rated their PRT knowledge as good or fair (P = .01), and were in practice longer (P = .004) were significantly more likely to have referred patients for PRT (Table 4). Sex was not independently predictive.
Table 4.
Predictors of referral for PRt in the past year: Statistically significant P values are boldface.
| PREDICTOR | ODDS RATIO FOR REFERRAL | 95% CI | P VALUE |
|---|---|---|---|
| Admitting privileges* | 2.65 | 1.44 to 4.87 | .002 |
| Years in practice (continuous) | 1.04 | 1.01 to 1.07 | .004 |
| Provided palliative care* | |||
| • Rarely | 1.44 | 0.26 to 8.02 | .68 |
| • Sometimes | 5.76 | 1.08 to 30.6 | .04 |
| • Often | 10.9 | 1.96 to 60.1 | .006 |
| Self-rated knowledge (good or fair)† | 2.74 | 1.28 to 5.89 | .01 |
| Cancer patients seen per month‡ | |||
| • More than 10 | 5.78 | 1.93 to 17.3 | .002 |
| Sex§ | 1.16 | 0.66 to 2.06 | .61 |
PRT—palliative radiotherapy.
Reference category: no, never, or none.
Reference category: poor.
Reference category: 0–10 patients per month.
Reference category: female.
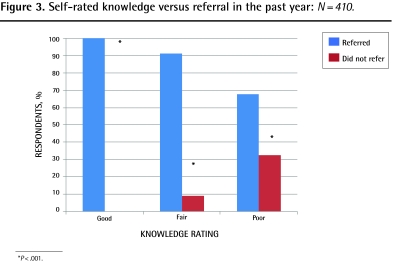
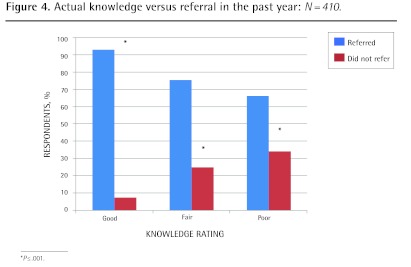
Significantly more respondents scored in the good (P ≤ .001) and fair (P < .001) categories for actual knowledge compared with self-rated knowledge, and more respondents rated themselves as having poor knowledge when their actual knowledge was fair or good (P ≤ .001) (Figure 2). Both self-rated and actual knowledge correlated with having referred patients for PRT (P ≤ .001) (Figures 3 and 4). Independent predictors of actual knowledge score were increasing frequency of provision of PC, and good or fair self-rated knowledge (Table 5).
Figure 2.
Self-rated knowledge versus actual knowledge: N=412.
*P ≤.001, **P<.001.
Figure 3.
Self-rated knowledge versus referral in the past year: N = 410.
*P < .001.
Figure 4.
Actual knowledge versus referral in the past year: N = 410.
*P ≤.001.
Table 5.
Predictors of actual knowledge score
| PREDICTOR | PARAMETER ESTIMATE (β) | 95% CI | P VALUE |
|---|---|---|---|
| Provided palliative care* | |||
| • Rarely | 1.54 | −0.35 to 3.43 | .11 |
| • Sometimes | 1.98 | 0.14 to 3.81 | .035 |
| • Often | 2.83 | 0.99 to 4.67 | .003 |
| Self-rated knowledge (good or fair)† | −2.06 | −2.65 to −1.46 | < .001 |
Reference category: never.
Reference category: poor.
DISCUSSION
As patients with advanced malignancy exhaust active treatment options and are discharged from follow-up at cancer centres, health care providers other than oncologists increasingly take over care. It is reasonable to expect these practitioners to have a basic knowledge of PRT indications and logistics.14,17 Unfortunately, a potential reason for inadequately managed cancer symptoms is inadequate knowledge.18 Two recently published studies used similar questionnaires to ours to determine PRT knowledge of referring practitioners in their catchment area.14,17
A 2006 Ontario survey was the first to demonstrate a direct relationship between level of knowledge and referral patterns.14 In that study, 97% of FP respondents had seen cancer patients within the past month, 80% provided PC, 54.1% referred patients for PRT, and 52.9% had contacted ROs for advice. Higher proportions of practitioners had fair or good PRT knowledge compared with the present study. The authors concluded that Ontario FPs were more likely to refer for PRT if they had higher actual knowledge; had been successful in contacting an RO; had more cancer patients in their practice; provided PC; and worked in a rural setting.14
A population-based survey in the Netherlands concentrated on PRT delivered in the last 3 months of life.17 The questionnaire was sent to all FPs working within the catchment area of 2 large radiotherapy departments in a region of 2.6 million inhabitants. Response rate was 45.2% (498 of 1100). Ninety-six percent of responding FPs considered themselves the most important care providers for terminally ill cancer patients. The most important factors affecting referral for PRT were general condition, presumed discomfort, and patient wishes. Almost 40% assessed their knowledge of PRT as modest. The authors concluded that respondent knowledge might have led to fewer referrals for PRT. The main difference between the patient population of Vulto et al and that of the practitioners in the present study is potential travel distance: 95% of surveyed physicians and 96% of their patients lived within 60 minutes of a radiotherapy centre.17 In comparison, in a review of 71 patients seen at the CCI for PRT, the average round-trip travel distance was 212 km.19
In a previous survey in our catchment area focusing on access to PRT for bone metastases, at least 50% of respondents believed the following were barriers to referral: delay in consultation with an RO; delay in PRT start; poor patient performance status; difficulty in arranging accommodation or transportation; and travel distance.5 On univariate analysis, significant predictors for referral from rural FPs were distance and difficulty with transportation or accommodation. Eighty-seven percent of respondents were not comfortable with their knowledge of PRT; the number of patients with bone metastases seen in the past year was the factor most independently associated with comfort level (P = .001).5
This lack of confidence with PRT indications was echoed in a 2008 study, in which 77% of 137 FP, FP resident, and nurse participants at an oncology-themed educational event were already involved in the care of cancer patients.15 Family practitioners in general were not very confident regarding the benefits of PRT, with 56% believing that they knew very little. Bone metastases and SCC were recognized as indications for PRT by approximately 90% of FPs, but more than 50% incorrectly identified hypercalcemia and febrile neutropenia as indications. Family physician knowledge of PRT was significantly higher than that of nurses or residents (P < .05).15
Limitations
The main limitation of any survey study is that of selection bias due to nonresponse. Results might not be entirely representative or generalizable, as it is not possible to reliably estimate whether answers accurately reflect the practice of all FPs, MOs, and PC specialists working in our catchment area or others. While our response rate compares favourably with other physician surveys, a third mailing might have slightly increased the response rate. Owing to anonymity, claims of past referral for PRT could not be independently verified. Evaluation of PRT-related knowledge was not exhaustive and did not use a validated instrument, although the questionnaire was based on that of a previously published study.14 It would be difficult to definitively conclude that all factors potentially related to referral patterns were assessed, and others (such as certification with the College of Family Physicians of Canada, location of training, location of practice, and amount of PRT-related training) might have been predictive. Although efforts were made to construct an exhaustive sampling frame, owing to the nature of remote northern practice, it is possible that some locum or refracting physicians were not included. Finally, owing to the small number of non-FP responding clinicians, analyses could not be subdivided by discipline.
Conclusion
Referring practitioners have less than optimal knowledge about PRT and its indications, which might lead to underreferral of appropriate patients for treatment. However, respondents are better informed than they believe themselves to be. Resident training in PC must include PRT, as it represents an effective modality for symptom control. As a result of the findings of this study, further efforts have been dedicated to providing PRT-related continuing medical education for practitioners and trainees in our region.
Acknowledgments
Presented in part at the 51st American Society for Radiation Oncology (ASTRO) Annual Meeting, November 1 to 5, 2009, in Chicago, IL. The authors wish to acknowledge all participating physicians.
EDITOR’S KEY POINTS
Palliative radiotherapy (PRT) is generally underused. Relying on nononcologists to refer symptomatic patients for PRT presumes a familiarity with its efficacy. Effectiveness of radiotherapy for certain clinical scenarios, such as palliation of brain and bone metastases, is well recognized. However, the benefit of radiotherapy for emergent indications such as spinal cord compression is not as well known.
Respondents’ self-rated PRT knowledge was significantly poorer than their actual level of knowledge (P ≤ .001); both correlated with having referred patients for PRT in the past year.
Wait times for PRT did not significantly influence clinicians’ decision to refer for treatment.
POINTS DE REPÈRE DU RÉDACTEUR
La radiothérapie palliative (RTP) est généralement sous-utilisée. Si l’on se fie à des non oncologistes pour orienter des patients symptomatiques en RTP, c’est probablement parce qu’on est conscient de son efficacité. L’efficacité de la radiothérapie pour certaines conditions cliniques, telles que le soulagement des métastases cérébrales et osseuses, est bien reconnue. Toutefois, les avantages de la radiothérapie pour certaines indications nouvelles comme la compression de la moelle épinière ne sont pas aussi bien connus.
L’évaluation qu’ont faite les répondants de leur propre connaissance de la RTP était significativement plus mauvaise que leur niveau de connaissance véritable (P ≤ ,001); ces deux indices étaient corrélés avec le fait d’avoir dirigé des patients en RTP durant l’année précédente.
Le temps d’attente pour la RTP n’avait pas influencé la décision des médecins de demander ce traitement.
Footnotes
This article has been peer reviewed.
Cet article a fait l’objet d’une révision par des pairs.
Contributors
All authors made substantial contributions to the conception, design, analysis, and interpretation of the submission, drafted the article and its revisions, and gave final approval of the version submitted.
Competing interests
None declared
References
- 1.Hoegler D. Radiotherapy for palliation of symptoms in incurable cancer. Curr Probl Cancer. 1997;21(3):129–83. doi: 10.1016/s0147-0272(97)80004-9. [DOI] [PubMed] [Google Scholar]
- 2.McWhinney IR, Hoddinott SN, Bass MJ, Gay K, Shearer R. Role of the family physician in the care of cancer patients. Can Fam Physician. 1990;36:2183–6. [PMC free article] [PubMed] [Google Scholar]
- 3.Wakefield MA, Beilby J, Ashby MA. General practitioners and palliative care. Palliat Med. 1993;7(2):117–26. doi: 10.1177/026921639300700205. [DOI] [PubMed] [Google Scholar]
- 4.Dworkind M, Shvartzman P, Adler PS, Franco ED. Urban family physicians and the care of cancer patients. Can Fam Physician. 1994;40:47–50. [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes EA, Hanson J, Neumann CM, Nekolaichuk CL, Bruera E. Communication between primary care physicians and radiation oncologists regarding patients with cancer treated with palliative radiotherapy. J Clin Oncol. 2000;18(15):2902–7. doi: 10.1200/JCO.2000.18.15.2902. [DOI] [PubMed] [Google Scholar]
- 6.Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol. 2007;25(11):1423–36. doi: 10.1200/JCO.2006.09.5281. [DOI] [PubMed] [Google Scholar]
- 7.Fairchild A, Harris K, Barnes E, Wong R, Lutz S, Bezjak A, et al. Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol. 2008;26(24):4001–11. doi: 10.1200/JCO.2007.15.3312. [DOI] [PubMed] [Google Scholar]
- 8.Loblaw DA, Laperriere NJ. Emergency treatment of malignant extra-dural spinal cord compression: an evidence-based guideline. J Clin Oncol. 1998;16(4):1613–24. doi: 10.1200/JCO.1998.16.4.1613. [DOI] [PubMed] [Google Scholar]
- 9.Pereira J, Phan T. Management of bleeding in patients with advanced cancer. Oncologist. 2004;9(5):561–70. doi: 10.1634/theoncologist.9-5-561. [DOI] [PubMed] [Google Scholar]
- 10.Rowell NP, Gleeson FV. Steroids, radiotherapy, chemotherapy and stents for superior vena caval obstruction in carcinoma of the bronchus. Cochrane Database Syst Rev. 2001;(4):CD001316. doi: 10.1002/14651858.CD001316. [DOI] [PubMed] [Google Scholar]
- 11.Tsao MN, Lloyd NS, Wong RK, Rakovitch E, Chow E, Laperierre N. Radiotherapeutic management of brain metastases: a systematic review and meta-analysis. Cancer Treat Rev. 2005;31(4):256–73. doi: 10.1016/j.ctrv.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Samant R, Gooi AC. Radiotherapy basics for family physicians. Potent tool for symptom relief. Can Fam Physician. 2005;51:1496–501. [PMC free article] [PubMed] [Google Scholar]
- 13.Huang J, Zhou S, Groome P, Tyldesley S, Zhang-Solomans J, MacKillop WJ. Factors affecting the use of palliative radiotherapy in Ontario. J Clin Oncol. 2001;19(1):137–44. doi: 10.1200/JCO.2001.19.1.137. [DOI] [PubMed] [Google Scholar]
- 14.Samant RS, Fitzgibbon E, Meng J, Graham ID. Family physicians’ perspectives regarding palliative radiotherapy. Radiother Oncol. 2006;78(1):101–6. doi: 10.1016/j.radonc.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Berrang T, Samant R. Palliative radiotherapy knowledge among community family physicians and nurses. J Cancer Educ. 2008;23(3):156–60. doi: 10.1080/08858190802039136. [DOI] [PubMed] [Google Scholar]
- 16.Dillman DA. Mail and telephone surveys: the total design method. New York, NY: John Wiley and Sons; 1978. [Google Scholar]
- 17.Vulto A, van Bommel M, Poortmans P, Lybeert M, Louwman M, Baart R, et al. General practitioners and referral for palliative radiotherapy: a population-based survey. Radiother Oncol. 2009;91(2):267–70. doi: 10.1016/j.radonc.2009.01.009. Epub 2009 Mar 25. [DOI] [PubMed] [Google Scholar]
- 18.Elliott TE, Murray DM, Elliott BA, Braun B, Oken MM, Johnson KM, et al. Physician knowledge and attitudes about cancer pain management: a survey from the Minnesota cancer pain project. J Pain Symptom Manage. 1995;10(7):494–504. doi: 10.1016/0885-3924(95)00100-d. [DOI] [PubMed] [Google Scholar]
- 19.Fairchild A, Pituskin E, Rose B, Ghosh S, Dutka J, Driga A, et al. The rapid access palliative radiotherapy program: blueprint for initiation of a one-stop multidisciplinary bone metastases clinic. Support Care Cancer. 2009;17(2):163–70. doi: 10.1007/s00520-008-0468-3. Epub 2008 Jun 20. [DOI] [PubMed] [Google Scholar]