Abstract
Two click chemistry-derived focused libraries based on the benz[d]isothiazol-3(2H)-one scaffold were synthesized and screened against Dengue virus and West Nile virus NS2B-NS3 proteases. Several compounds (4l, 7j-n) displayed noteworthy inhibitory activity toward Dengue virus NS2B-NS3 protease in the absence and presence of added detergent. These compounds could potentially serve as a launching pad for a hit-to-lead optimization campaign.
Introduction
The genus Flavivirus in the family Flaviviridae comprises an array of major viral pathogens, including West Nile virus (WNV), Dengue virus (DENV), Hepatitis C virus (HCV), Yellow Fever virus (YFV), and Japanese encephalitis virus (JEV).1 DENV is a mosquito-borne virus that has four serotypes: DENV-1, DENV-2, DENV-3, and DENV-4. Worldwide, DENV is the cause of 50-100 million infections annually, with approximately 500,000 cases progressing to dengue hemorrhagic fever/dengue shock syndrome, resulting in ∼25,000 deaths.2-3 There are no vaccines or small molecule therapeutics for the treatment of DENV infection; consequently, there is currently an urgent and unmet need for the discovery and development of therapeutic agents for DENV infection.
DENV is a small, enveloped virus with a single-stranded, positive sense 11-kb RNA genome, which encodes a polyprotein precursor. Co- and post-translational cleavage of the polyprotein produces three structural proteins (C, prM, and E) and seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) by the concerted action of host proteases (furin, signalase) and the two-component trypsin-like protease NS2B-NS3 (NS2B-NS3pro).4-5 Processing of the polyprotein by NS2B-NS3pro is essential for viral replication; consequently, NS2B-NS3pro has emerged as an attractive target for the discovery and development of therapeutics for DENV infection.6-7 NS3 is a multifunctional protein that comprises a protease (NS3pro), an RNA helicase, a nucleoside triphosphatase, and 5′- RNA triphosphatase activities.7 NS3pro is a serine endoprotease with a His51-Asp75-Ser135 catalytic triad and a strongly-preferred substrate specificity for a -X-K-R-R-G/S- sequence corresponding to the subsites -S4-S3-S2-S1-S1′-.8 Cleavage is at the P1-P1′ scissile bond (R-G/S). The hydrophilic core of the NS2B protein cofactor is required for optimal catalytic efficiency.9-10 X-ray crystal structures of NS2B-NS3pro of WNV and DENV-2 and DENV-1 have been reported.11-14 Inhibitors of NS2B-NS3 protease containing a highly charged peptidyl recognition element with a dibasic motif at P1-P2 coupled to an array of electrophilic warhead (aldehydes, trifluoromethyl ketones, and boronic acids)15-17 or non-peptidyl α-ketoamides,18 and others19-20 have been described.
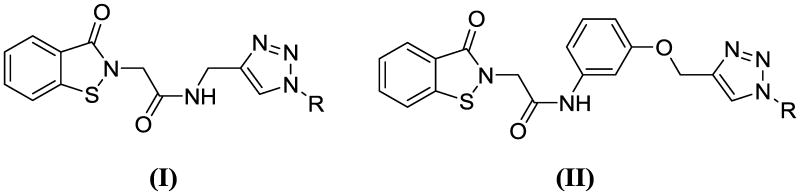
We describe herein the results of synthetic and biochemical studies related to the inhibition of DENV NS2B-NS3 protease by click chemistry-derived benz[d]isothiazol-3(2H)-one derivatives (I-II) (Figure 1).
Figure 1.
General structures of DENV2 NS2B-NS3pro inhibitors (I-II).
Chemistry
The synthesis of compounds 4a-p and 7a-p is summarized in Scheme 1. Thus, starting acid 121 was activated with 1,1′-carbonyldiimidazole (CDI) and then coupled to propargylamine to yield intermediate 2. Click chemistry methodology was employed to generate the desired triazole derivatives 4a-p by coupling compound 2 to an array of structurally diverse azides 3a-p. The latter were readily synthesized from the corresponding halides and sodium azide in dimethyl sulfoxide.22 Intermediate 1 was also coupled to aniline derivative 523 to yield compound 6, which was subsequently converted to compounds 7a-p via reaction with a series of azides 3a-p under click chemistry conditions. Compounds 4p and 7p were obtained by hydrolyzing compounds 4o and 7o, respectively.
Scheme 1.
Reagents and reaction conditions: i) CDI/THF/reflux then propargylamine or compound 5; ii) NaN3/DMSO; iii) NaOH/CH3OH, removal of CH3OH, then propargyl bromide/CH3CN; iv) sodium ascorbate/CuSO4/BuOH/H2O; v) TFA.
Biochemistry
The expression and purification of DENV NS2B-NS3pro and WNV NS2B-NS3pro have been previously described.24-25 Enzyme assays and inhibition studies were carried out as previously described26-29 and the results are summarized in Figures 2 and 3.
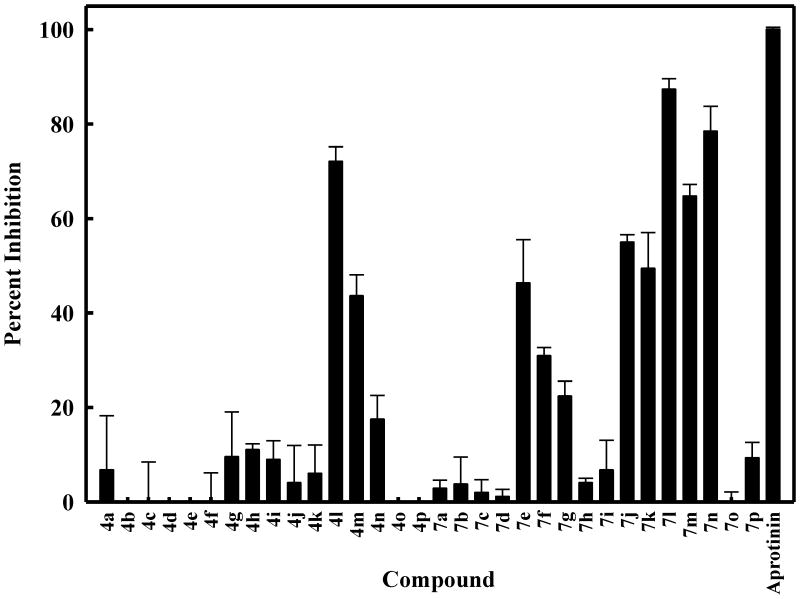
Figure 2.
Inhibition of DENV2 NS2B-NS3pro by derivatives of compounds (I-II). The concentrations of the tested compounds and DENV2 NS2B-NS3 protease were 25 μM and 25 nM, respectively. The buffer used contained 200 mM Tris hydrochloride, 6 mM NaCl, and 30% glycerol, pH 9.5. The percent values were calculated from the relative fluorescence units obtained in the presence and absence of tested compound. All assays were performed in triplicate and the average values are shown. The assays were performed as described in the Biochemistry methods section.
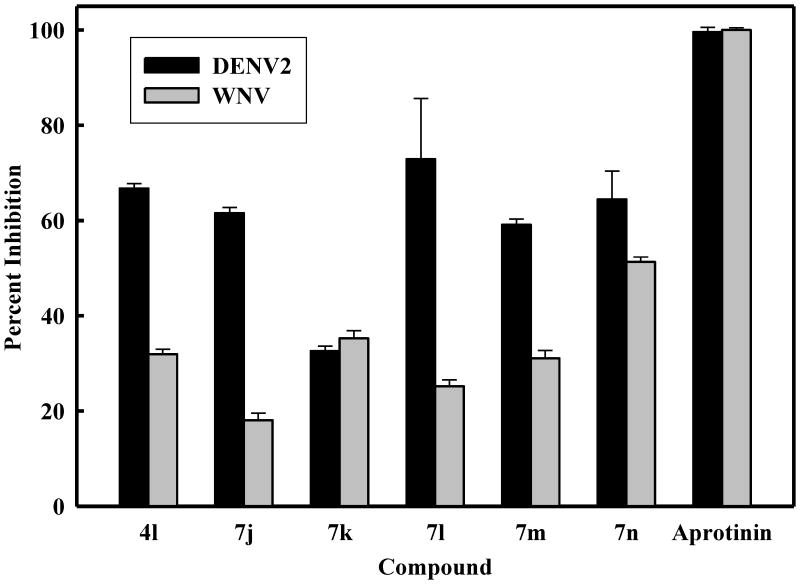
Figure 3.
Inhibition of DENV2 and WNV NS2B-NS3pro by selected derivatives of (I-II) at 25 μM. The concentrations of DENV2 and WNV NS2B-NS3 proteases were 25 nM and 28 nM, respectively. The buffer used contained 200 mM Tris hydrochloride, 6 mM NaCl, and 30% glycerol and 0.1% CHAPS, pH 9.5. The percent values were calculated from the relative fluorescence units obtained in the presence and absence of tested compound. All assays were performed in triplicate and the average values are shown. The assays were performed as described in the Biochemistry methods section.
Results and Discussion
Diseases caused by Dengue and related flaviviruses constitute a world-wide health problem for which there are currently no effective vaccines or small-molecule therapeutics. Consequently, there has been an increasing interest in the discovery and development of agents for DENV infection.19
As part of a program related to the discovery of DENV NS2B-NS3pro inhibitors, screening of a representative subset of compounds from a pharmacologically-rich in-house library of compounds lead to the identification of a benz[d]isothiazol-3(2H)-one derivative that exhibited inhibitory activity against DENV NS2B-NS3pro. This finding provided the impetus for the synthesis of two focused libraries based on general structures (I) and (II) (Figure 1). The high synthetic tractability of (I) and (II) made possible the facile synthesis of the desired compounds (Scheme 1). Considerations associated with the design strategy employed in the construction of the libraries included using click chemistry to generate an electron-rich ring with hydrogen acceptor capabilities and enhancing binding by probing the nature of R (Scheme 1, compounds 4a-p). The active site of DENV NS2B-NS3pro is rather shallow; consequently, we envisaged tethering the benz[d]isothiazol-3(2H)-one ring to the triazole ring via a m-aminophenol linker to provide additional favorable binding interactions with the enzyme (Scheme 1, compounds 7a-p).
The results related to the interaction of the synthesized compounds with DENV NS2B NS3pro are shown in Figure 2. With the exception of compounds 4l and 4m, the rest of the compounds based on structure (I) were inactive. However, several derivatives based on structure (II) showed noteworthy inhibitory activity (compounds 7e and 7j-n, Figure 2). The IC50 values of compounds 4l and 7j-7n were subsequently determined (Figure 4) and are listed in Table 2. The kinetics of the interaction of a representative inhibitor 7n against DENV2 NS2B-NS3pro was investigated in greater detail by determining Km and Vmax (Figures 5 and 6, Table 2), as well as the Ki (determined to be 4.77±0.05 μM). Because at micromolar concentrations many small molecules self– associate and the resulting colloid-like aggregates can lead to non-specific inhibition,30-31 the synthesized compounds were re-screened in the presence of 0.1% CHAPS to confirm the activity of the compounds and to eliminate the possibility that the compounds acted as promiscuous inhibitors.32-33 Indeed, the inhibitory activity of all active compounds remained essentially the same in the presence of detergent. It is evident from the results shown in Table 1 that the presence of a (phenoxy)phenyl moiety enhances inhibitory activity, however, the gain in potency is accompanied by a significant increase in molecular weight and hydrophobicity which are likely to impact adversely ADMET and drug-like characteristics.34-36
Figure 4.
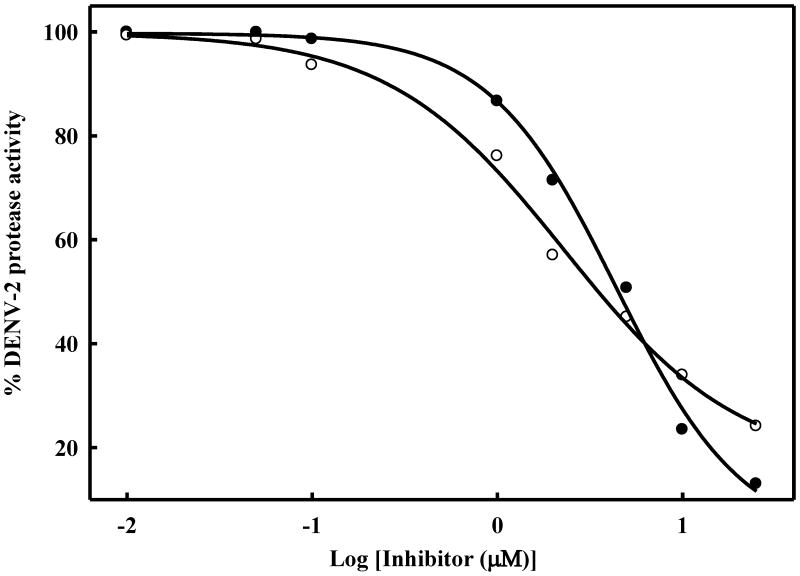
Determination of IC50 values of selected benz[d]isothiazol-3(2H)-one derivatives for inhibition of DENV2 NS2B-NS3 protease. The inhibitors were incubated wih DENV2 NS2B-NS3 protease (50 nM) in buffer (200 mM TrisHCl, 6 mM NaCl and 30% glycerol, pH 9.5) for 15 min. Bz-Nle-Lys-Arg-Arg-AMC (5.0 μM) was added to the mixture in a final volume of 100 μL. The fluorescence intensity was measured at 460 nm with excitation at 380 nm and converted to the percentage of protease activity in the absence and the presence of inhibitors. The inhibitors analyzed were as follows: (●) 7l; (○) 7n. The solid line is the theoretical fitting curve based on the Sigmoidal Equation. All the spectra were recorded at 37 °C and shown after subtraction of the buffer spectrum. The apparent IC50 for compounds 7l and 7n were 4.45 ± 0.06 and 3.48 ± 0.05, respectively.
Table 2. Kinetics of 7n against DENV2 NS2B-NS3pro.
| Inhibitor [7n] μM |
Km μM |
Vmax μM / min |
|---|---|---|
| 0 | 12.8732 ± 0.4064 | 0.0999 ± 0.0012 |
| 1 | 17.5003 ± 1.1541 | 0.1006 ± 0.0029 |
| 2 | 19.5461 ± 0.8851 | 0.0978 ± 0.0020 |
| 5 | 27.0101 ± 0.5086 | 0.0994 ± 0.0010 |
Figure 5.
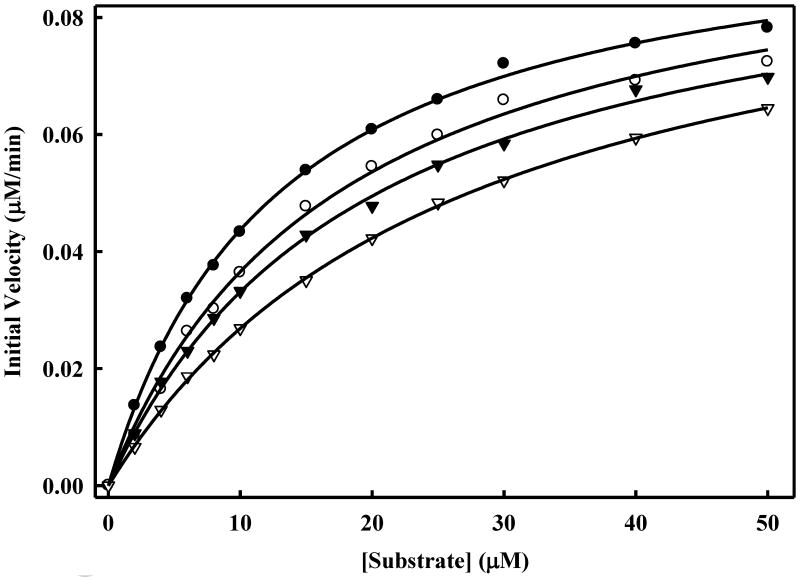
DENV2 NS2B-NS3pro activity in the absence and presence of inhibitor 7n. The in vitro protease assays were performed as described in the Experimental Section. The initial reaction rates of the tetra-peptide substrate cleavage catalyzed by 0.025 μM DENV2 NS2B-NS3 protease in the absence (●)-(●) and the presence of 1.0 μM (○)-(○), 2.0 μM (▾-▾) and 5.0 μM ((∇)-(∇) were determined by varying concentrations of tetra-peptide substrate (0, 2, 4, 6, 8, 10, 15, 20, 25, 30, 40 and 50 μM range). The reactions were initiated by the addition of DENV2 NS2B-NS3pro and the fluorescence intensity at 460 nm was monitored with an excitation at 380 nm. Reactions were less than 5% completion in all cases to maintain valid steady-state measurements. The calculated Kmapp and Vmax values are shown in Table 2.
Figure 6.
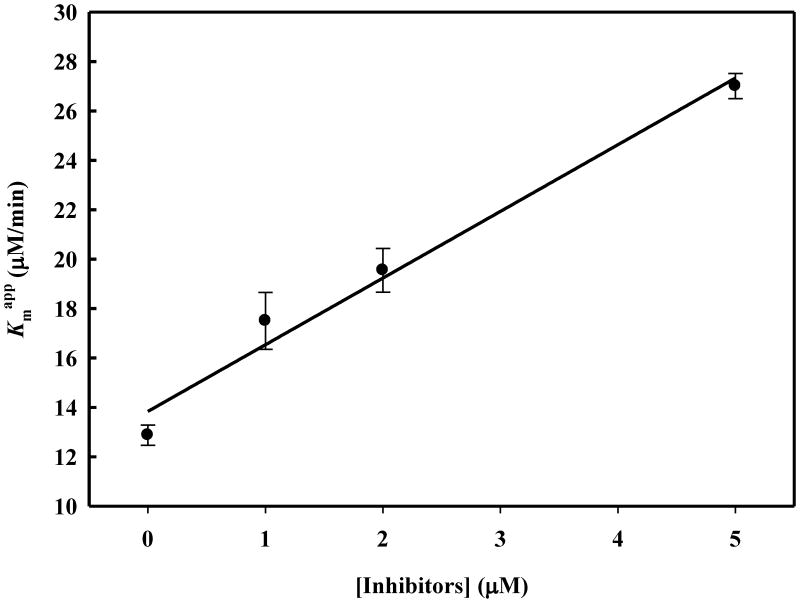
Plot of Km values versus concentration of compound 7n. Km values with no inhibitor and at different concentrations of inhibitor were calculated and plotted versus different concentrations of inhibitor. From these data, the Slope = 2.6988 ± 0.2712 μM and the Intercept = 12.8732 ± 0.7427 μM were obtained and the Ki value calculated from the equation Intercept / Slope is 4.77 ± 0.05 μM.
Table 1. IC50 values against DENV-2 proteasea.
| Compound | IC50 (μM) |
|---|---|
| 4l | 4.87 ± 0.07 |
| 7j | 6.22 ± 0.09 |
| 7k | 13.36 ± 0.21 |
| 7l | 4.45 ± 0.06 |
| 7m | 4.59 ± 0.07 |
| 7n | 3.48 ± 0.05 |
The IC50 values were determined as described under Experimental section.
The closely related West Nile virus (WNV), a member of the Flavivirus genus of the Flaviviridae family, is an emergent viral pathogen, for which there are no effective vaccines or antiviral agents.19,37-38 Consequently, the synthesized compounds were also screened against WNV NS2B-NS3pro27 and the results are summarized in Figure 3. Compounds 4l and 7j-n displayed weak inhibitory activity toward the enzyme.
Inspection of the structures of the six most active derivatives of compound (II) toward DENV NS2B-NS3pro suggests that they have in common an R group (keto, amide or ester carbonyl) capable of engaging in hydrogen bonding, as well as hydrophobic or π-π interactions. A plausible mode of binding of energy-minimized inhibitor 7j to the active site of DENV2 NS2B-NS3pro (homology model derived by Wichapong et al. from the corresponding WNV homolog13) is shown in Figure 7. This bound conformation, derived from molecular docking simulations, predicts that the ligand will occupy space adjacent to catalytic triad residues Ser135 and His51, with the core benz[d]isothiazol-3(2H)-one engaging in lipophilic interactions with the His51 and Trp50 side chains. The ligand triazole also accepts H-bonds from the backbone amide proton of Gly153 and the hydroxyl proton of Tyr161, donates a H-bond to the hydroxyl oxygen of Ser83, and engages in favorable lipophilic interactions with Pro132, Tyr150 and Tyr161. The terminal phenyl group on the ligand appears to be somewhat sterically limited by its presence in a relatively small lipophilic pocket, which might limit the prospects for lead optimization via aryl substituents, however the torsional flexibility availed from an aliphatic carbon immediately adjacent to the triazole (as per compounds 7k,l,m,n) may permit the ligand to bending downwards to engage with other lipophiles in the more spacious hydrophobic pocket marked by the nearby Tyr161.
Figure 7.
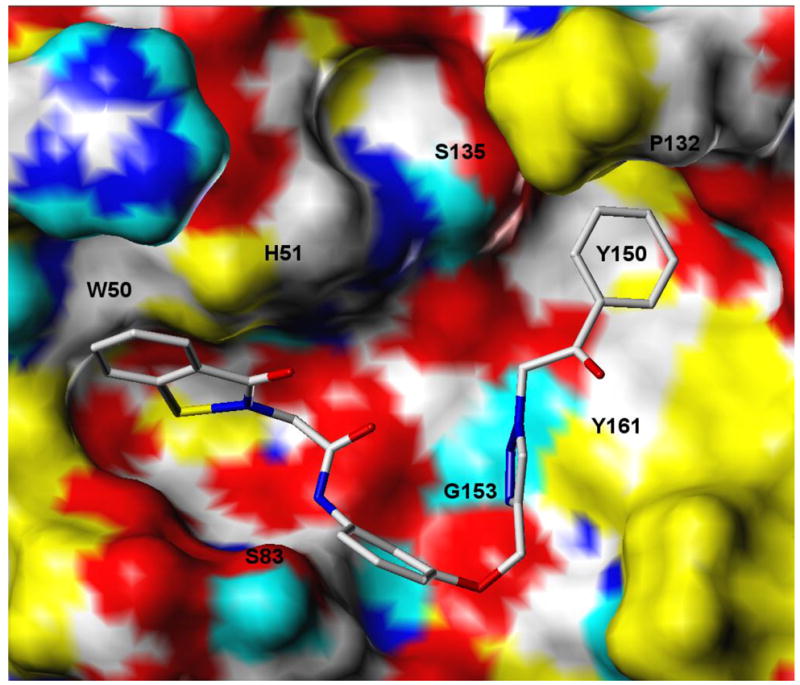
Conformation for inhibitor 7j bound to the catalytic site of DENV2 NS2B-NS3 protease, as predicted from molecular docking simulations. The receptor surface is colored as follows: red = polar O, blue = polar N, cyan = polar (donatable) H, white = weakly polar aliphatic or aryl CH groups, yellow = nonpolar lipophiles. The ligand is rendered in stick form according to standard CPK coloring.
In conclusion, the synthesis and in vitro evaluation of two focused libraries of compounds have resulted in the identification of several inhibitors of DENV2 NS2B-NS3pro. The novelty of the structures and high synthetic tractability provide a reasonable basis for a hit-to-lead optimization campaign.
Experimental Section
General
The 1H spectra were recorded on a Varian XL-300 or XL-400 NMR spectrometer. Melting points were determined on a Mel-Temp apparatus and are uncorrected. High resolution mass spectra (HRMS) were performed at the University of Kansas Mass Spectrometry Lab. Reagents and solvents were purchased from various chemical suppliers (Aldrich, Acros Organics, TCI America, and Bachem). Silica gel (230-450 mesh) used for flash chromatography was purchased from Sorbent Technologies (Atlanta, GA). Thin layer chromatography was performed using Analtech silica gel plates. The TLC plates for the final compounds were eluted using two different solvent systems and were visualized using iodine chamber and/or UV light. Each individual compound was identified as a single spot on TLC plate (purity greater than 95%). DENV2 NS2B-NS3 protease (or WNV NS2B-NS3pro) substrate Bz-Nle-Lys-Arg-Arg-AMC was purchased from Bachem, Torrance, CA. or custom synthesized by NeoBioScience, Cambridge, MA.
2-(3-Oxobenzo[d]isothiazol-2(3H)-yl)-N-(prop-2-ynyl)acetamide (2)
To a solution of compound 1 (10.45 g; 50 mmol) in dry THF (100 mL) was added portionwise a suspension of 1,1′-carbonyldiimidazole (8.10 g; 50 mmol) in THF (25 mL). The resulting solution was stirred at room temperature for 20 min and refluxed for 10 min. A solution of propargylamine (2.75 g; 50 mmol) in THF (25 mL) was added and the solution was allowed to stir at room temperature overnight. A precipitate formed which was collected by vacuum filtration, leaving compound 2 as a white solid (5.71 g; 46% yield), mp 156-158 °C. 1H NMR (CDCl3): δ 2.20 (t, J = 4.2 Hz, 1H), 4.05 (dd, J = 7.9, 7.9 Hz, 2H), 4.50 (s, 2H), 6.62 (s, 1H), 7.45 (t, J = 11.1 Hz, 1H), 7.60 (d, J = 4.3 Hz, 1H), 7.68 (t, J = 11.1 Hz, 1H), 8.06 (d, J = 4.3 Hz, 1H).
Azides 3a-j were synthesized as described below using standard literature procedures22
Benzyl azide (3a)
To a solution of sodium azide (4.32 g; 66 mmol) in dry dimethyl sulfoxide (120 mL) was added benzyl chloride (7.62 g; 60 mmol), and the reaction mixture was stirred at room temperature overnight. Water (80 mL) was carefully added to the reaction mixture and the aqueous layer was extracted with diethyl ether (2 × 150 mL). The combined organic extracts were washed with water (50 mL) and the organic layer was dried over anhydrous sodium sulfate. The drying agent was filtered off and the filtrate was concentrated, leaving compound 3a as a yellow oil (6.31 g; 79% yield). 1H NMR (CDCl3): δ 4.38 (s, 2H), 7.27-7.44 (m, 5H).
p-Fluorobenzyl azide (3b)
Yellow oil (99% yield). 1H NMR (CDCl3): δ 4.31 (s, 2H), 7.00-7.12(m, 2H), 7.24-7.35 (m, 2H).
m-Fluorobenzyl azide (3c)
Yellow oil (90% yield). 1H NMR (CDCl3): δ 4.38 (s, 2H), 6.98-7.13 (m, 3H), 7.33-7.40 (m, 1H).
p-Methoxybenzyl azide (3d)
Colorless oil (98% yield). 1H NMR (CDCl3): δ 3.80 (s, 3H), 4.23 (s, 2H), 6.90 (d, J = 6.9 Hz, 2H), 7.25 (d, J = 6.9 Hz, 2H).
Methyl 3-(azidomethyl)benzoate (3e)
Colorless oil (71% yield). 1H NMR (CDCl3): δ 3.98 (s, 3H), 4.41 (s, 2H), 7.42-7.59 (m, 3H), 8.00-8.15 (m, 1H).
(Azidomethyl)(phenyl)sulfane (3f)
Colorless oil (98% yield). 1H NMR (CDCl3): δ 4.58 (s, 2H), 7.25-7.39 (m, 3H), 7.50 (d, J = 8.3 Hz, 2H).
(3-Azidopropyl)benzene (3g)
Yellow oil (79% yield). 1H NMR (CDCl3): δ 1.85-2.00 (m, 2H), 2.72 (t, J = 25.0 Hz, 2H), 3.28 (t, J = 25.0 Hz, 2H), 7.18-7.40 (m, 5H).
(2-Azidoethyl)benzene (3h)
Yellow oil (41% yield). 1H NMR (CDCl3): δ 2.90 (t, J = 8.3 Hz, 2H), 3.50 (t, J = 8.3 Hz, 2H), 7.20-7.38 (m, 5H).
(2-Azidoethoxy)benzene (3i)
Yellow oil (69% yield). 1H NMR (CDCl3): δ 3.89 (t, J = 20.3 Hz, 2H), 4.29 (t, J = 20.3 Hz, 2H), 6.90-7.03 (m, 3H), 7.25-7.39 (m, 2H).
2-Azido-1-phenylethanone (3j)
Reddish oil (85% yield). 1H NMR (CDCl3): δ 4.59 (s, 2H), 7.43-7.70 (m, 3H), 7.95 (d, J = 8.3 Hz, 2H).
Representative synthesis of compound 3k-o
2-Azido-N-phenylacetamide (3k)
To a solution of aniline (9.3 mL; 100 mmol) in acetone (20 mL) kept in an ice bath was added 2-chloroacetyl chloride (4.1 mL; 50 mmol) dropwise with stirring. After the addition the ice bath was removed and the reaction mixture was stirred at room temperature for 4 h. Aqueous hydrochloric acid (10% v/v; 40 mL) was added to the reaction mixture, whereupon a white solid formed. The white precipitate was collected by vacuum filtration and washed with 5% hydrochloric acid (3 × 40 mL) and water (2 × 40 mL). The solid was allowed to air dry overnight (7.8 g; 92% yield), mp 122-124 °C. The dry solid (7.6 g; 45 mmol) was added to a solution of sodium azide (3.24 g; 49.5 mmol) in dry dimethyl sulfoxide (100 mL) and the reaction mixture was stirred at room temperature overnight. Water (50 mL) was added to the reaction mixture and the aqueous layer was extracted with diethyl ether (3 × 100 mL). The combined extracts were washed with water (50 mL) and dried by anhydrous sodium sulfate. The drying agent was filtered off and the filtrate concentrated, leaving compound 3k as a white solid (8.7 g; 98% yield), mp 57-59 °C. 1H NMR (CDCl3): δ 4.18 (s, 2H), 7.18 (t, J = 6.9 Hz, 1H), 7.35 (t, J = 8.6 Hz, 2H), 7.57 (d, J = 5.2 Hz, 2H), 8.00 (s, 1H).
2-Azido-N-(4-phenoxyphenyl)acetamide (3l)
Reddish oil (80% total yield). 1H NMR (CDCl3): δ 4.18 (s, 2H), 7.00 (m, 4H), 7.09 (t, J = 6.7 Hz, 1H), 7.35 (t, J = 6.7 Hz, 2H), 7.50 (d, J = 4.9 Hz, 2H), 8.03 (s, 1H).
2-Azido-N-(3-phenoxyphenyl)acetamide (3m)
Reddish oil (56% total yield). 1H NMR (CDCl3): δ 4.17 (s, 2H), 6.80 (m, 1H), 7.02 (d, J = 6.9 Hz, 2H), 7.15 (t, J = 8.6 Hz, 1H), 7.24-7.40 (m, 5H), 8.00 (s, 1H).
2-Azido-N-(2-phenoxyphenyl)acetamide (3n)
Brown oil (93% total yield). 1H NMR (CDCl3): δ 4.18 (s, 2H), 6.90 (d, J = 5.5 Hz, 1H), 7.00-7.20 (m, 6H), 7.39 (t, J = 9.1 Hz, 2H), 8.62 (s, 1H).
tert-Butyl 2-azidoacetate (3o)
Colorless oil (99% total yield). 1H NMR (CDCl3): δ 1.50 (s, 9H), 3.79 (s, 2H).
Representative synthesis of compounds 4a-p
N-((1-Benzyl-1H-1,2,3-triazol-4-yl)methyl)-2-(3-oxobenzo[d]isothiazol-2(3H)-yl)acetamide (4a)
To a solution of compound 2 (0.25 g; 1.0 mmol) and compound 3a (0.33 g; 2.5 mmol) in tert-butyl alcohol and water (1:1) (15 mL) were added sodium ascorbate (0.04 g; 0.2 mmol) and copper (II) sulfate pentahydrate (0.005 g; 0.02 mmol) and the reacting mixture was stirred at room temperature overnight. The disappearance of compound 2 was monitored by TLC. Water (25 mL) was added and the reaction mixture was stirred for 5 min. The precipitate was collected by vacuum filtration and washed with water (25 mL) and diethyl ether (30 mL), leaving compound 4a as a white solid (0.30 g; 79% yield), mp 193-195 °C. 1H NMR (CDCl3): 4.50 (s, 2H), 4.51 (s, 2H), 5.48 (s, 2H), 6.82 (s, 1H), 7.32-7.44 (m, 6H), 7.59 (d, J = 5.1 Hz, 1H), 7.64 (t, J = 6.9 Hz, 1H), 8.02 (s, 1H). HRMS (ESI): Calculated for C19H17N5O2S [M+Na]+ 402.1001; found 402.0992.
N-((1-(4-Fluorobenzyl)-1H-1,2,3-triazol-4-yl)methyl)-2-(3-oxobenzo[d]isothiazol-2(3H)-yl)acetamide (4b)
White solid (95% yield), mp 187-189 °C. 1H NMR (CDCl3): δ 4.49 (s, 2H), 4.51 (d, J = 5.2 Hz, 2H), 5.44 (s, 2H), 6.80 (s, 1H), 7.05 (t, J = 6.9 Hz, 2H), 7.26 (m, 1H), 7.43 (m, 2H), 7.59 (d, J = 5.2 Hz, 1H), 7.68 (d, J = 5.2 Hz, 1H), 8.02 (d, J = 5.2 Hz, 1H). HRMS (ESI): Calculated for C19H16FN5O2S [M+Na]+ 420.0906; found 402.0903.
N-((1-(3-Fluorobenzyl)-1H-1,2,3-triazol-4-yl)methyl)-2-(3-oxobenzo[d]isothiazol-2(3H)-yl)acetamide (4c)
White solid (78% yield), mp 191-193 °C. 1H NMR (CDCl3): δ 4.49 (s, 2H), 4.51 (d, J = 5.2 Hz, 2H), 5.44 (s, 2H), 6.80 (s, 1H), 6.98 (d, J = 6.9 Hz, 1H), 7.05 (d, J = 6.9 Hz, 1H), 7.36 (m, 1H), 7.43 (m, 2H), 7.59 (d, J = 5.2 Hz, 1H), 7.68(d, J = 5.2 Hz, 1H), 8.02 (d, J = 5.2 Hz, 1H). HRMS (ESI): Calculated for C19H16FN5O2S [M+H]+ 398.1087; found 398.1068.
N-((1-(4-Methoxybenzyl)-1H-1,2,3-triazol-4-yl)methyl)-2-(3-oxobenzo[d]isothiazol-2(3H)-yl)acetamide (4d)
White solid (92% yield), mp 188-190 °C. 1H NMR (CDCl3): δ 3.81 (s, 3H), 4.49 (s, 4H), 5.40 (s, 2H), 6.89 (d, J = 8.6 Hz, 2H), 7.22 (d, J = 8.6 Hz, 2H), 7.41 (m, 2H), 7.58 (d, J = 6.9 Hz, 1H), 7.63 (d, J = 6.9 Hz, 1H), 8.02 (d, J = 6.9 Hz, 1H). HRMS (ESI): Calculated for C20H19N5O3S [M+H]+ 410.1287; found 410.1297.
Methyl 4-((4-((2-(3-oxobenzo[d]isothiazol-2(3H)-yl)acetamido)methyl)-1H-1,2,3-triazol-1-yl)methyl)benzoate (4e)
White solid (10% yield), mp 138-140 °C. 1H NMR (CDCl3): δ 3.96 (s, 3H), 4.45 (s, 2H), 4.47 (d, J = 5.2 Hz, 2H), 5.55 (s, 2H), 6.80 (s, 1H), 7.40-7.49 (m, 8H), 8.01 (d, J = 5.2 Hz, 1H). HRMS (ESI): Calculated for C21H19N5O4S [M+Na]+ 460.1055; found 460.1044.
2-(3-Oxobenzo[d]isothiazol-2(3H)-yl)-N-((1-(phenylthiomethyl)-1H-1,2,3-triazol-4-yl)methyl)acetamide (4f)
White solid (49% yield), mp 176-178 °C. 1H NMR (CDCl3): δ 4.49 (s, 2H), 4.51 (s, 2H), 5.58 (s, 2H), 6.79 (s, 1H), 7.31(s, 3H), 7.41 (t, J = 3.4 Hz, 2H), 7.58 (d, J = 3.4 Hz, 1H), 7.65 (t, J = 3.4 Hz, 2H), 8.03 (d, J = 3.4 Hz, 1H). HRMS (ESI): Calculated for C19H17N5O2S2 [M+H]+ 412.0902; found 412.0909.
2-(3-Oxobenzo[d]isothiazol-2(3H)-yl)-N-((1-(3-phenylpropyl)-1H-1,2,3-triazol-4-yl)methyl)acetamide (4g)
Brown solid (75% yield), mp 143-145 °C. 1H NMR (CDCl3): δ 2.19-2.25 (m, 2H), 2.65 (t, J = 12.1 Hz, 2H), 4.34 (t, J = 8.6 Hz, 2H), 4.51 (s, 2H), 4.53 (d, 2H), 6.80 (s, 1H), 7.18 (d, J = 5.2 Hz, 2H), 7.20-7.35 (m, 2H), 7.42 (t, J = 8.6 Hz, 1H), 7.49-7.60 (m, 2H), 7.63 (t, J = 8.6 Hz, 1H), 8.01 (s, 1H). HRMS (ESI): Calculated for C21H21N5O2S [M+H]+ 408.1494; found 408.1475.
2-(3-Oxobenzo[d]isothiazol-2(3H)-yl)-N-((1-phenethyl-1H-1,2,3-triazol-4-yl)methyl)acetamide (4h)
Grey solid (78% yield), mp 173-175 °C. 1H NMR (CDCl3): δ 3.20 (t, J = 8.6 Hz, 2H), 4.46-4.59 (m, 6H), 6.81 (s, 1H), 7.10 (d, J = 6.9 Hz, 2H), 7.22-7.35 (m, 3H), 7.43 (t, J = 8.6 Hz, 2H), 7.59 (d, J = 5.2 Hz, 1H), 7.66 (t, J = 8.6 Hz, 2H), 8.02 (d, J = 5.2 Hz, 1H). HRMS (ESI): Calculated for C20H19N5O2S [M+H]+ 394.1338; found 394.1333.
2-(3-Oxobenzo[d]isothiazol-2(3H)-yl)-N-((1-(2-phenoxyethyl)-1H-1,2,3-triazol-4-yl)methyl)acetamide (4i)
Gray solid (82% yield), mp 168-170 °C. 1H NMR (CDCl3): δ 4.33 (t, J = 6.9 Hz, 2H), 4.51 (s, 2H), 4.53 (d, J = 5.2 Hz, 2H), 4.75 (t, J = 6.9 Hz, 2H), 6.80 (s, 1H), 6.88 (d, J = 6.9 Hz, 2H), 7.00 (t, J = 8.6 Hz, 1H), 7.25-7.32 (m, 1H), 7.42 (t, J = 8.6 Hz, 2H), 7.53 (d, J = 6.9 Hz, 1H), 7.63 (t, J = 8.6 Hz, 2H), 7.75 (s, 1H), 8.02 (d, J = 5.2 Hz, 1H). HRMS (ESI): Calculated for C20H19N5O3S [M+Na]+ 432.1106; found 410.1120.
N-((1-(2-Oxo-2-phenylethyl)-1H-1,2,3-triazol-4-yl)methyl)-2-(3-oxobenzo[d]isothiazol-2(3H)-yl)acetamide (4j)
Brown solid (90% yield), mp 180-182 °C. 1H NMR (DMSO): δ 4.40 (d, J = 3.7 Hz, 2H), 4.50 (s, 2H), 6.18 (s, 2H), 7.42 (t, J = 9.1 Hz, 1H), 7.60-7.79 (m, 5H), 7.85-8.10 (m, 4H), 8.80 (t, J = 9.1 Hz, 1H). HRMS (ESI): Calculated for C20H17N5O3S [M+H]+ 408.1130; found 408.1150.
N-((1-(2-Oxo-2-(phenylamino)ethyl)-1H-1,2,3-triazol-4-yl)methyl)-2-(3-oxobenzo[d]isothiazol-2(3H)-yl)acetamide (4k)
White solid (74% yield), mp 176-178 °C. 1H NMR (DMSO): δ 4.40 (d, J = 4.2 Hz, 2H), 4.51 (s, 2H), 5.38 (s, 2H), 7.10 (t, J = 8.3 Hz, 1H), 7.39 (t, J = 8.3 Hz, 2H), 7.45 (t, J = 8.3 Hz, 1H), 7.60 (d, J = 6.5 Hz, 1H), 7.70 (t, J = 8.3 Hz, 1H), 7.91 (d, J = 6.5 Hz, 1H), 8.00 (d, J = 6.5 Hz, 2H), 8.82 (s, 1H), 10.51 (s, 1H). HRMS (ESI): Calculated for C20H18N6O3S [M+Na]+ 445.1059; found 445.1068.
N-((1-(2-Oxo-2-(4-phenoxyphenylamino)ethyl)-1H-1,2,3-triazol-4-yl)methyl)-2-(3-oxobenzo[d]isothiazol-2(3H)-yl)acetamide (4l)
Gray solid (65% yield), mp 240-242 °C. 1H NMR (DMSO): δ 4.39 (d, J = 4.3 Hz, 2H), 4.50 (s, 2H), 5.30 (s, 2H), 6.95-7.05 (m, 3H), 7.10 (t, J = 6.5 Hz, 2H), 7.38-7.45 (m, 3H), 7.60 (d, J = 6.5 Hz, 1H), 7.69 (t, J = 6.5 Hz, 2H), 7.90 (d, J = 4.3 Hz, 1H), 8.00 (d, J = 4.3 Hz, 2H), 8.78 (t, J = 6.5 Hz, 1H), 10.51 (s, 1H). HRMS (ESI): Calculated for C26H22N6O4S [M+Na]+ 537.1321; found 537.1338.
N-((1-(2-Oxo-2-(3-phenoxyphenylamino)ethyl)-1H-1,2,3-triazol-4-yl)methyl)-2-(3-oxobenzo[d]isothiazol-2(3H)-yl)acetamide (4m)
Gray solid (66% yield), mp 220-222 °C. 1H NMR (DMSO): δ 4.39 (d, J = 4.3 Hz, 2H), 4.50 (s, 2H), 5.31 (s, 2H), 6.78 (d, J = 4.3 Hz, 2H), 7.03 (d, J = 4.3 Hz, 2H), 7.39 (t, J = 6.5 Hz, 2H), 7.29-7.48 (m, 4H), 7.70 (t, J = 6.5 Hz, 1H), 7.89 (d, J = 4.3 Hz, 1H), 7.98 (d, J = 4.3 Hz, 2H), 8.79 (t, J = 6.5 Hz, 1H), 10.56 (s, 1H). HRMS (ESI): Calculated for C26H22N6O4S [M+Na]+ 537.1321; found 537.1355.
N-((1-(2-Oxo-2-(2-phenoxyphenylamino)ethyl)-1H-1,2,3-triazol-4-yl)methyl)-2-(3-oxobenzo[d]isothiazol-2(3H)-yl)acetamide (4n)
Gray solid (77% yield), mp 213-215 °C. 1H NMR (DMSO): δ 4.38 (d, J = 4.3 Hz, 2H), 4.50 (s, 2H), 5.39 (s, 2H), 6.87 (m, 1H), 7.02-7.21 (m, 6H), 7.40-7.46 (m, 3H), 7.70 (t, J = 6.9 Hz, 1H), 7.85-8.05 (m, 3H), 8.79 (t, J = 5.2 Hz, 1H), 10.09 (s, 1H). HRMS (ESI): Calculated for C26H22N6O4S [M+Na]+ 537.1321; found 537.1339.
tert-Butyl 2-(4-((2-(3-oxobenzo[d]isothiazol-2(3H)-yl)acetamido)methyl)-1H-1,2,3-triazol-1-yl)acetate (4o)
White solid (80% yield), mp 177-179 °C. 1H NMR (CDCl3): δ 4.52 (s, 2H), 4.59 (d, J = 3.4 Hz, 2H), 5.01 (s, 2H), 6.79 (s, 1H), 7.43 (t, J = 6.8 Hz, 1H), 7.56-7.70 (m, 3H), 8.04 (d, J = 5.2 Hz, 1H). HRMS (ESI): Calculated for C18H21N5O4S [M+H]+ 404.1393; found 404.1391.
2-(4-((2-(3-Oxobenzo[d]isothiazol-2(3H)-yl)acetamido)methyl)-1H-1,2,3-triazol-1-yl)acetic acid (4p)
A solution of compound 4o (0.21 g; 0.5 mmol) in TFA (15 mL) was allowed to stir at RT for 5 h while monitoring the disappearance of the starting material by TLC. TFA was evaporated off and the residue was treated with diethyl ether (75 mL) and stirred for 30 min at RT. A white solid formed which was collected by suction filtration (0.13 g; 75% yield), mp 230-232 °C. 1H NMR (DMSO): δ 4.38 (d, J = 5.6 Hz, 2H), 4.50 (s, 2H), 5.25 (s, 2H), 7.43 (t, J = 5.4 Hz, 1H), 7.71 (t, J = 5.4 Hz, 1H), 7.83-8.00 (m, 3H), 8.79 (t, J = 5.4 Hz, 1H), 13.38 (s, 1H). HRMS (ESI): Calculated for C14H14N5O4S [M+Na]+ 348.0767; found 348.0779.
3-(Prop-2-ynyloxy)aniline (5)
Sodium hydroxide (4.30 g; 107.5 mmol) and 3-aminophenol (11.0 g; 100.9 mmol) were dissolved in methanol (200 mL) at gentle heating. The volatiles were evaporated off and the residual water was removed by four successive co-distillations with absolute ethanol (80 mL). The residue was dissolved in dry acetonitrile (200 mL) and propargyl bromide (14.24 g; 119.7 mmol) was added in three portions over 1 h period. The reaction mixture was stirred overnight, concentrated, and partitioned between 0.1 M sodium hydroxide (200 mL) and diethyl ether (200 mL). The layers were separated and the aqueous layer was extracted with diethyl ether (2 × 200 mL). The combined organic extracts were washed with brine (100 mL) and dried over anhydrous sodium sulfate. Evaporation of the solvent left a brown oily residue which was dissolved in methanol (160 mL) and treated with 4M hydrochloric acid in dioxane (54 mL; 215.3 mmol). The volatiles were removed and the resulting brownish solid was suspended in boiling ethyl acetate (140 mL). Cooling the suspension to room temperature yielded a white crystalline solid which was collected by suction filtration and dried. The solid was dissolved in water (50 mL) and the pH was adjusted to ∼11 using 10% aqueous sodium hydroxide (35 mL). The aqueous layer was extracted with ethyl acetate (2 × 100 mL), dried over sodium sulfate, filtered, and concentrated, leaving a yellow oil (10.3 g; 70% yield). 1H NMR (CDCl3): δ 2.50 (t, J = 3.1 Hz, 1H), 3.65 (br s, 2H), 4.63 (d, J = 3.1 Hz, 2H), 6.29-6.40 (m, 3H), 7.05 (t, J = 7.8 Hz, 1H).
2-(3-Oxobenzo[d]isothiazol-2(3H)-yl)-N-(3-(prop-2-ynyloxy)phenyl)acetamide (6)
To a solution of compound 1 (6.27g; 30 mmol) in dry THF (60 mL) was added portion wise a suspension of carbonyldiimidazole (4.86 g; 30 mmol) in THF (15 mL) and the mixture was stirred at RT for 20 min and then refluxed for 10 min. A solution of compound 5 (4.41 g; 30 mmol) in THF (15 mL) was added and the reaction mixture was stirred at RT overnight. A white precipitate formed which was collected by suction filtration (4.46 g; 44% yield), mp 173-175°C. 1H NMR (CDCl3): δ 2.40-2.60 (m, 1H), 4.60 (s, 2H), 4.65 (s, 2H), 6.30-6.42 (m, 1H), 6.75 (d, J = 5.5 Hz, 1H), 7.00-7.30 (m, 2H), 7.45 (t, J = 7.3 Hz, 2H), 7.60 (d, J = 3.6 Hz, 1H), 7.70 (d, J = 3.6 Hz, 1H), 8.10 (d, J = 3.6 Hz, 1H), 8.63 (s, 1H).
Representative synthesis of compounds 7a-p
N-(3-((1-Benzyl-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-2-(3-oxobenzo[d]isothiazol-2(3H)-yl)acetamide (7a)
A solution of compound 6 (0.34 g; 1.0 mmol) and compound 3a (0.33 g; 2.5 mmol) in tert-butyl alcohol and water (1:1) (20 mL was treated with sodium ascorbate (0.20 g; 1.0 mmol) and copper (II) sulfate pentahydrate (0.02 g; 0.10 mmol) and the reacting mixture was stirred at room temperature overnight. TLC analysis showed the presence of unreacted compound 6. An extra portion of compound 3a (0.33 g; 2.5 mmol) was added and the reaction mixture was stirred at 40°C overnight. Water (35 mL) was added and the resulting mixture was stirred for 5 min while kept in an ice bath. A precipitate formed which was collected by vacuum filtration and washed with water (25 mL) and diethyl ether (100 mL), leaving compound 7a as a gray solid (0.33 g; 70% yield), mp 185-187 °C. 1H NMR (CDCl3): δ 4.60 (s, 2H), 5.18 (s, 2H), 5.50 (s, 2H), 6.70 (d, J = 5.5 Hz, 1H), 7.01 (d, J = 5.5 Hz, 1H), 7.15-7.40 (m, 5H), 7.41-7.72 (m, 6H), 8.09 (d, J = 5.5 Hz, 1H), 8.75 (s, 1H). HRMS (ESI): Calculated for C25H21N5O3S [M+Na]+ 494.1263; found 494.1258.
N-(3-((1-(4-Fluorobenzyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-2-(3-oxobenzo[d]isothiazol-2(3H)-yl)acetamide (7b)
Gray solid (94% yield), mp 185-187 °C. 1H NMR (CDCl3): δ 4.60 (s, 2H), 5.18 (s, 2H), 5.49 (s, 2H), 6.71 (d, J = 6.3 Hz, 1H), 6.98-7.10 (m, 3H), 7.18-7.37 (m, 4H), 7.44 (t, J = 5. 6 Hz, 1H), 7.55-7.74 (m, 4H), 8.10 (d, J = 4.3 Hz, 1H), 8.63 (s, 1H). HRMS (ESI): Calculated for C25H20FN5O3S [M+Na]+ 512.1169; found 512.1190.
N-(3-((1-(3-Fluorobenzyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-2-(3-oxobenzo[d]isothiazol-2(3H)-yl)acetamide (7c)
Gray solid (93% yield), mp 198-200 °C. 1H NMR (CDCl3): δ 4.60 (s, 2H), 5.20 (s, 2H), 5.52 (s, 2H), 6.72 (d, J = 5.5 Hz, 1H), 6.95-7.10 (m, 4H), 7.19-7.40 (m, 3H), 7.45 (t, J = 7.4 Hz, 1H), 7.60 (d, J = 5.5 Hz, 2H), 7.69 (t, J = 7.4 Hz, 1H), 8.10 (d, J = 5.5 Hz, 1H), 8.62 (s, 1H). HRMS (ESI): Calculated for C25H20FN5O3S [M+H]+ 490.1349; found 490.1345.
N-(3-((1-(4-Methoxybenzyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-2-(3-oxobenzo[d]isothiazol-2(3H)-yl)acetamide (7d)
White solid (68% yield), mp 163-165 °C. 1H NMR (CDCl3): δ 3.80 (s, 3H), 4.59 (s, 2H), 5.17 (s, 2H), 5.45 (s, 2H), 6.70 (d, J = 5.4 Hz, 1H), 6.92 (d, J = 5.4 Hz, 2H), 7.00 (d, J = 3.6 Hz, 1H), 7.18-7.35 (m, 4H), 7.45-7.55 (m, 2H), 7.60 (d, J = 3.6 Hz, 1H), 7.68 (d, J = 3.6 Hz, 1H), 8.10 (d, J = 5.4 Hz, 1H), 8.60 (s, 1H). HRMS (ESI): Calculated for C26H23N5O4S [M+H]+ 502.1549; found 502.1526.
Methyl 4-((4-((3-(2-(3-oxobenzo[d]isothiazol-2(3H)-yl)acetamido)phenoxy)methyl)-1H-1,2,3-triazol-1-yl)methyl)benzoate (7e)
White solid (15% yield), mp 98-100 °C. 1H NMR (CDCl3): δ 3.95 (s, 3H), 4.60 (s, 2H), 5.19 (s, 2H), 5.59 (s, 2H), 6.70 (d, J = 5.6 Hz, 1H), 7.00 (d, J = 5.6 Hz, 1H), 7.10-7.40 (m, 3H), 7.40-7.72 (m, 5H), 8.00-8.20 (m, 3H), 8.61 (s, 1H). HRMS (ESI): Calculated for C27H23N5O5S [M+Na]+ 552.1318; found 552.1302.
2-(3-Oxobenzo[d]isothiazol-2(3H)-yl)-N-(3-((1-(phenylthiomethyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)acetamide (7f)
White solid (60% yield), mp 158-160 °C. 1H NMR (CDCl3): δ 4.60 (s, 2H), 5.18 (s, 2H), 5.60 (s, 2H), 6.71 (d, J = 5.6 Hz, 1H), 7.01 (d, J = 5.6 Hz, 1H), 7.20-7.39 (m, 5H), 7.48 (t, J = 7.7 Hz, 1H), 7.60-7.75 (m, 4H), 8.11 (d, J = 3.7 Hz, 1H), 8.62 (s, 2H). HRMS (ESI): Calculated for C25H21N5O3S2 [M+Na]+ 526.0984; found 526.0969.
2-(3-Oxobenzo[d]isothiazol-2(3H)-yl)-N-(3-((1-(3-phenylpropyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)acetamide (7g)
White solid (40% yield), mp 169-171 °C. 1H NMR (CDCl3): δ 2.20-2.31(m, 2H), 2.63 (t, J = 8.9 Hz, 2H), 4.35 (t, J = 7.1 Hz, 2H), 4.59 (s, 2H), 5.20 (s, 2H), 6.75 (d, J = 3.6 Hz, 1H), 7.00 (d, J = 3.6 Hz, 1H), 7.14-7.40 (m, 7H), 7.45 (t, J = 5.4 Hz, 1H), 7.58-7.73 (m, 3H), 8.07 (d, J = 3.6 Hz, 1H), 8.60 (s, 1H). HRMS (ESI): Calculated for C27H25N5O3S [M+H]+ 500.1756; found 500.1748.
2-(3-Oxobenzo[d]isothiazol-2(3H)-yl)-N-(3-((1-phenethyl-1H-1,2,3-triazol-4-yl)methoxy)phenyl)acetamide (7h)
White solid (52% yield), mp 175-177 °C. 1H NMR (CDCl3): δ 3.20 (t, J = 8.9 Hz, 2H), 4.59 (t, J = 8.9 Hz, 2H), 4.60 (s, 2H), 5.16 (s, 2H), 6.69 (d, J = 5.4 Hz, 2H), 7.00 (d, J = 5.4 Hz, 1H), 7.03-7.40 (m ,8H), 7.45 (t, J = 7.1 Hz, 1H), 7.60 (d, J = 3.6 Hz, 1H), 7.65 (d, J = 3.6 Hz, 1H), 8.09 (d, J = 3.6 Hz, 1H), 8.62 (s, 1H). HRMS (ESI): Calculated for C26H23N5O3S [M+H]+ 486.1600; found 486.1597.
2-(3-Oxobenzo[d]isothiazol-2(3H)-yl)-N-(3-((1-(2-phenoxyethyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)acetamide (7i)
Gray solid (96% yield), mp 163-165 °C. 1H NMR (DMSO-d6): δ 4.39 (t, J = 4.4 Hz, 2H), 4.63 (s, 2H), 4.79 (t, J = 4.4 Hz, 2H), 5.10 (s, 2H), 6.78 (d, J = 4.3 Hz, 1H), 6.89-7.00 (m, 3H), 7.12-7.38 (m, 5H), 7.42 (t, J = 6.5 Hz, 1H), 7.70 (t, J = 6.5 Hz, 1H), 7.90 (d, J = 4.3 Hz, 1H), 8.00 (d, J = 4.3 Hz, 1H), 8.29 (s, 1H), 10.28 (s, 1H). HRMS (ESI): Calculated for C26H23N5O4S [M+H]+ 502.1549; found 502.1547.
N-(3-((1-(2-Oxo-2-phenylethyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-2-(3-oxobenzo[d]isothiazol-2(3H)-yl)acetamide (7j)
Gray solid (76% yield), mp 165-167 °C. 1H NMR (CDCl3): δ 4.60 (s, 2H), 5.22 (s, 2H), 5.82 (s, 2H), 6.77 (d, J = 4.3 Hz, 1H), 7.02 (d, J = 4.3 Hz, 1H), 7.15-7.35 (m, 3H), 7.40-7.70 (m, 6H), 7.80 (s, 1H), 8.00 (d, J = 4.3 Hz, 1H), 8.09 (d, J = 4.3 Hz, 1H), 8.60 (s, 1H). HRMS (ESI): Calculated for C26H21N5O4S [M+H]+ 500.1393; found 500.1377.
N-(3-((1-(2-Oxo-2-(phenylamino)ethyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-2-(3-oxobenzo[d]isothiazol-2(3H)-yl)acetamide (7k)
White solid (74% yield), mp 205-207°C. 1H NMR (DMSO): δ 4.66 (s, 2H), 5.12 (s, 2H), 5.38 (s, 2H), 6.80 (d, J = 4.2 Hz, 1H), 7.02-7.39 (m, 7H), 7.41 (t, J = 6.5 Hz, 1H), 7.59 (d, J = 4.2 Hz, 2H), 7.69 (t, J = 6.5 Hz, 1H), 7.90 (d, J = 4.2 Hz, 1h), 8.00 (d, J = 4.2 Hz, 1H), 8.22 (s, 1H), 10.38 (s, 1H). HRMS (ESI): Calculated for C26H22N6O4S [M+H]+ 515.1502; found 515.1505.
N-(3-((1-(2-Oxo-2-(4-phenoxyphenylamino)ethyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-2-(3-oxobenzo[d]isothiazol-2(3H)-yl)acetamide (7l)
Brown solid (90% yield), mp 198-200 °C. 1H NMR (DMSO): δ 4.66 (s, 2H), 5.13 (s, 2H), 5.36 (s, 2H), 6.80 (d, J = 4.3 Hz, 1H), 6.92-7.03 (m, 2H), 7.08-7.30 (m, 4H), 7.32-7.49 (m, 5H), 7.60 (d, J = 6.3 Hz, 1H), 7.70 (t, J = 6.5 Hz, 1H), 7.91 (d, J = 4.3 Hz, 1H), 8.00 (d, J = 4.3 Hz, 1H), 8.24 (s, 1H), 10.39 (s, 1H). HRMS (ESI): Calculated for C32H26N6O5S [M+Na]+ 629.1583; found 629.1583.
N-(3-((1-(2-Oxo-2-(3-phenoxyphenylamino)ethyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-2-(3-oxobenzo[d]isothiazol-2(3H)-yl)acetamide (7m)
Gray solid (22% yield), mp 230-232 °C. 1H NMR (DMSO): δ 4.65 (s, 2H), 5.11 (s, 2H), 5.30 (s, 2H), 6.78 (t, J = 10.6 Hz, 2H), 7.02 (d, J = 6.7 Hz, 1H), 7.17 (d, J = 6.7 Hz, 1H), 7.20-7.44 (m, 9H), 7.70 (t, J = 8.5 Hz, 1H), 7.91 (d, J = 4.3 Hz, 1H), 8.00 (d, J = 4.3 Hz, 1H), 8.20 (s, 1H), 10.33 (s, 1H), 10.52 (s, 1H). HRMS (ESI): Calculated for C32H26N6O5S [M+Na]+ 629.1583; found 629.1586.
N-(3-((1-(2-Oxo-2-(2-phenoxyphenylamino)ethyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-2-(3-oxobenzo[d]isothiazol-2(3H)-yl)acetamide (7n)
White solid (94% yield), mp 185-187 °C. 1H NMR (CDCl3): δ 4.59 (s, 2H), 5.13 (s, 2H), 5.19 (s, 2H), 6.70 (d, J = 5.4 Hz, 1H), 6.80-6.95 (m, 3H), 6.95-7.38 (m, 7H), 7.45 (t, J = 5.4 Hz, 1H), 7.59-7.75 (m, 3H), 8.10 (m, 2H), 8.32 (d, J = 5.4 Hz, 1H), 8.61 (s, 1H). HRMS (ESI): Calculated for C32H26N6O5S [M+Na]+ 629.1583; found 629.1573.
tert-Butyl 2-(4-((3-(2-(3-oxobenzo[d]isothiazol-2(3H)-yl)acetamido)phenoxy)methyl)-1H-1,2,3-triazol-1-yl)acetate (7o)
White solid (82% yield), mp 188-190 °C. 1H NMR (CDCl3): δ 1.46 (s, 9H), 4.60 (s, 2H), 5.03 (s, 2H), 5.20 (s, 2H), 6.75 (d, J = 5.4 Hz, 1H), 7.00 (d, J = 5.4 Hz, 1H), 7.18-7.38 (m, 3H), 7.44 (t, J = 7.3 Hz, 1H), 7.58-7.75 (m, 2H), 8.10 (d, J = 5.4 Hz, 1H), 8.60 (s, 1H). HRMS (ESI): Calculated for C24H25N5O5S [M+H]+ 496.1655; found 496.1641.
2-(4-((3-(2-(3-Oxobenzo[d]isothiazol-2(3H)-yl)acetamido)phenoxy)methyl)-1H-1,2,3-triazol-1-yl)acetic acid (7p)
Compound 7o (0.25 g; 0.5 mmol) was treated with TFA (15 mL) and the solution was stirred at RT for 5 h. TFA was evaporated off and the residue was treated with diethyl ether (100 mL) and stirred at RT for 30 min. The solid was collected by suction filtration, leaving a white powder (0.11 g; 50% yield), mp 115-117 °C. 1H NMR (DMSO): δ 4.65 (s, 2H), 5.11 (s, 2H), 5.30 (s, 2H), 6.79 (d, J = 5.6 Hz, 1H), 7.15-7.28 (m, 2H), 7.35 (s, 1H), 7.44 (t, J = 5.6 Hz, 1H), 7.76 (t, J = 5.6 Hz, 1H), 7.90 (d, J = 4.2 Hz, 1H), 8.00 (d, J = 4.2 Hz, 1H), 8.20 (s, 1H), 10.39 (s, 1H). HRMS (ESI): Calculated for C20H17N5O5S [M+H]+ 440.1029; found 440.1024.
DENV2 NS2B-NS3pro expression and purification
The construction of the pQE30-NS2BH(QR)NS3pro expression plasmid has been described previously.25 The protease was expressed from E. coli strain Top 10 F′ (Invitrogen) transformed by pQE30-NS2BH(QR)-NS3pro plasmid and purified as described.26 DENV2 NS2B-NS3pro contains the hydrophilic NS2B cofactor peptide (NS2BH) linked to the N-terminal NS3 protease domain via Q-R (P2 and P1 residues at the NS2B-NS3 junction site).
WNV NS2B-NS3pro expression and purification
The expression and purification of the WNV NS2BH-NS3pro containing the 5 amino acid spacer between the NS2B and NS3pro domains was previously described.25
In vitro DENV2 and WNV NS2B-NS3pro assays and inhibition studies
The compounds were dissolved in dimethylsulfoxide (DMSO) to make 50 mM stock solutions. The compounds were screened at 25 M in 1% v/v DMSO in the final reaction mixture. Protease assays were performed in triplicates in Greiner Black 96 well plates. Each assay consisted of the reaction mixture of 100 L containing 200 mM TrisHCl buffer, pH 9.5, 30% glycerol, 25 nM DENV2 NS2B-NS3 protease (or 28 nM WNV NS2BH-NS3pro) and the compound. The enzyme and the compound were pre-incubated at room temperature prior to addition of the substrate (5 M), Bz-Nle-Lys-Arg-Arg-AMC. The time course of the reaction at 37°C was followed at every 90 sec intervals for up to 30 min in a monochrometer-based spectrofluorometer (Molecular Devices, Sunnyvale, CA) at excitation and emission wavelengths of 380 and 460 nm, respectively. The percent inhibition for each compound at 25 M was first determined. For determining IC50 values, the range of 10 nM, 50 nM, 0.1, 1, 2, 5, 10, and 25 M concentrations of selected compounds were used. IC50 values were calculated using the SigmaPlot software.
Molecular Modeling
Molecular docking calculations were carried out via the Surflex program36 requesting 5 distinct randomized starting conformations for the ligand, and 100 final docked poses to select from. For these calculations, ligands were sketched and optimized (according to default molecular mechanics constraints and force fields) via the SYBYL 8.1 program (Tripos Associates, St. Louis, MO, 2009), and the receptor was modeled based on the homology model generated for DENV NS3/NS2Bpro13 from the corresponding WNV homolog (the protein protonation state used herein presumed anionic aspartate and glutamate groups, and cationic arginines and lysines).
Acknowledgments
The generous financial support of this work by the National Institutes of Health (AI082068) is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lindenbach BD, Thiel HJ, Rice RC. In: Fields Virology. 5th. Knipe DM, Howley PM, editors. one. Lippincott, Williams, and Wilkins; Philadelphia: 2007. pp. 1101–1152. [Google Scholar]
- 2.Stevens AJ, Gahan ME, Mahalingam S, Keller PA. J Med Chem. 2009;52:7911. doi: 10.1021/jm900652e. [DOI] [PubMed] [Google Scholar]
- 3.Noble CG, Chen YL, Dong H, Gu F, Lim SP, Schul W, Wang QY, Shi PY. Antiviral Res. 2010;85:450. doi: 10.1016/j.antiviral.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Natarajan S. Gen Mol Biol. 2010;33:214. doi: 10.1590/S1415-47572010000200002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yusof R, Clum S, Wetzel M, Murthy HM, Padmanabhan R. J Biol Chem. 2000;275:9963. doi: 10.1074/jbc.275.14.9963. [DOI] [PubMed] [Google Scholar]
- 6.Sampath A, Padmanabhan R. Antiviral Res. 2009;81:6. doi: 10.1016/j.antiviral.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lescar J, Luo D, Xu T, Sampath A, Lim SP, Canard B, Vasudevan SG. Antiviral Res. 2008;80:94. doi: 10.1016/j.antiviral.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Schechter I, Berger A. Biochem Biophys Res Comm. 1967;27:157. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- 9.Niyomrattanakit P, Winoyanuwattikun P, Chanprapaph S, Angsuthanasombat C, Panyim S, Katzenmeier G. J Virol. 2004;78:13708. doi: 10.1128/JVI.78.24.13708-13716.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wichapong K, Pianwanit S, Sippl W, Kokpol S. J Mol Recognit. 2010;23:283. doi: 10.1002/jmr.977. [DOI] [PubMed] [Google Scholar]
- 11.Schiering N, Kroemer M, Renatus M, Erbel P, D'Arcy A. Nat Struct Mol Biol. 2006;13:372. doi: 10.1038/nsmb1073. [DOI] [PubMed] [Google Scholar]
- 12.Chandramouli S, Joseph JS, Daudenarde S, Gatchalian J, Cornillez-Ty C, Kuhn P. J Virol. 2010;84:3059. doi: 10.1128/JVI.02044-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wichapong K, Pianwanit S, Sippl W, Kokpol S. J Mol Recognit. 2010;23:283. doi: 10.1002/jmr.977. [DOI] [PubMed] [Google Scholar]
- 14.Aleshin AE, Shiryaev SA, Strongin AY, Liddington RC. Protein Sci. 2007;16:795. doi: 10.1110/ps.072753207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yin Z, Patel SJ, Wang WL, Wang G, Chan WL, Rao KR, Alam J, Jeyaraj DA, Ngew X, Patel V, Beer D, Lim SP, Vasudevan SG, Keller TH. Bioorg Med Chem Lett. 2006;16:36. doi: 10.1016/j.bmcl.2005.09.062. [DOI] [PubMed] [Google Scholar]
- 16.Yin Z, Patel SJ, Wang WL, Chan WL, Ranga Rao KR, Ngew X, Patel V, Beer D, Knox JE, Ma NL, Ehrhardt C, Lim SP, Vasudevan SG, Keller TH. Bioorg Med Chem Lett. 2006;16:40. doi: 10.1016/j.bmcl.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 17.Knox JE, Ma NL, Yin Z, Patel SJ, Wang WL, Wang G, Chan WL, Ranga Rao KR, Wang G, Ngew X, Patel V, Beer D, Lim SP, Vasudevan SG, Keller TH. J Med Chem. 2006;49:6585. doi: 10.1021/jm0607606. [DOI] [PubMed] [Google Scholar]
- 18.Steuer C, Gege C, Fischl W, Heinonen KH, Bartenschlager R, Klein CD. Bioorg Med Chem. 2011;19:4067. doi: 10.1016/j.bmc.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 19.Cregar-Hernandez L, Jiao GS, Johnson AT, Lehrer AT, Wong TA, Margosiak SA. Antivir Chem Chemother. 2011;21:209. doi: 10.3851/IMP1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parkinson T, Pryde DC. Future Med Chem. 2010;2:1181. doi: 10.4155/fmc.10.195. [DOI] [PubMed] [Google Scholar]
- 21.Dou D, Alex D, Du B, Tiew KC, Aravapalli S, Mandadapu SR, Calderone R, Groutas WC. Bioorg Med Chem. 2011;19:5782. doi: 10.1016/j.bmc.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 22.Shen J, Woodward R, Kedenburg JP, Liu X, Chen M, Fang L, Sun D, Wang PG. J Med Chem. 2008;51:7417. doi: 10.1021/jm8005355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urankar D, Košmrlj J. J Comb Chem. 2008;10:981. doi: 10.1021/cc8001475. [DOI] [PubMed] [Google Scholar]
- 24.Yon C, Teramoto T, Mueller N, Phelan J, Ganesh VK, Murthy KH, Padmanabhan R. J Biol Chem. 2005;280:27412. doi: 10.1074/jbc.M501393200. [DOI] [PubMed] [Google Scholar]
- 25.Yusof RS, Clum M, Wetzel H, Murthy HM, Padmanabhan R. J Biol Chem. 2000;265:9963. doi: 10.1074/jbc.275.14.9963. [DOI] [PubMed] [Google Scholar]
- 26.Mueller NH, Yon C, Ganesh VK, Padmanabhan R. Int J Biochem Cell Biol. 2007;39:606. doi: 10.1016/j.biocel.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 27.Dou D, Viwanathan P, Li Y, He G, Alliston KR, Lushington GH, Brown-Clay JD, Padmanabhan R, Groutas WC. J Comb Chem. 2010;12:836. doi: 10.1021/cc100091h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Lim P, Beer D, Patel V, Wen D, Tumanut C, Tully DC, Williams JA, Jiricek J, Priestle JP, Harris JL, Vasudevan SG. J Biol Chem. 2005;280:28766. doi: 10.1074/jbc.M500588200. [DOI] [PubMed] [Google Scholar]
- 29.Mueller NH, Pattabiraman N, Ansarah-Sobrinho C, Viswanathan P, Pierson TC, Padmanabhan R. Antimicrob Agents Chemother. 2008;52:3385. doi: 10.1128/AAC.01508-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coan KED, Maltby DA, Burlingame AL, Shoichet BK. J Med Chem. 2009;52:2067. doi: 10.1021/jm801605r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGovern SL, Helfand BT, Feng B, Shoichet BK. J Med Chem. 2003;46:4265. doi: 10.1021/jm030266r. [DOI] [PubMed] [Google Scholar]
- 32.Ezgimen MD, Mueller NH, Teramoto T, Padmanabhan R. Bioorg Med Chem. 2009;17:3278. doi: 10.1016/j.bmc.2009.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng BY, Shoichet BK. Nat Protoc. 2006;1:550. doi: 10.1038/nprot.2006.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edwards MP, Price DA. Ann Rep Med Chem. 2010;45:381. [Google Scholar]
- 35.Leeson PD, Springthorpe B. Nat Rev Drug Disc. 2007;6:881. doi: 10.1038/nrd2445. [DOI] [PubMed] [Google Scholar]
- 36.Leeson PD, Empfield JR. Ann Rep Med Chem. 2010;45:393. [Google Scholar]
- 37.Chappell KJ, Stoermer MJ, Fairlie DP, Young PR. Curr Top Med Chem. 2008;15:2771. doi: 10.2174/092986708786242804. [DOI] [PubMed] [Google Scholar]
- 38.Stoermer MJ, Chappell KJ, Liebscher S, Jensen CM, Gan CH, Gupta PK, Xu WJ, Young PR, Fairlie DP. J Med Chem. 2008;51:5714. doi: 10.1021/jm800503y. [DOI] [PubMed] [Google Scholar]
- 39.Jain AN. J Med Chem. 2003;46:499. doi: 10.1021/jm020406h. [DOI] [PubMed] [Google Scholar]