Abstract
Topographical cues from the extracellular microenvironment can influence cellular activity including proliferation and differentiation. Information on the effects of material topography on tenogenic differentiation of human mesenchymal stem cells (human MSCs) is limited. A methodology using the principles of isoelectric focusing has previously been developed in our laboratory to synthesize electrochemically aligned collagen (ELAC) threads that mimics the packing density, alignment and strength of collagen dense connective tissues. In the current study, human MSCs were cultured on ELAC and randomly-oriented collagen threads and the effect of collagen orientation on cell morphology, proliferation and tenogenic differentiation was investigated. The results indicate that higher rates of proliferation were observed on randomly oriented collagen threads compared to ELAC threads. On the other hand, tendon specific markers such as scleraxis, tenomodulin, tenascin-C and collagen-III were significantly increased on ELAC threads compared to randomly oriented collagen threads. Additionally, osteocalcin, a specific marker of bone differentiation was suppressed on ELAC threads. Previous studies have reported that BMP-12 is a key growth factor to induce tenogenic differentiation of human MSCs. To evaluate the synergistic effect of BMP-12 and collagen orientation, human MSCs were cultured on ELAC threads in culture medium supplemented with and without BMP-12. The results revealed that BMP-12 did not have an additional effect on the tenogenic differentiation of human MSCs on ELAC threads. Together, these results suggest that ELAC induces tenogenic differentiation of human MSCs by presenting an aligned and dense collagen substrate, akin to the tendon itself. In conclusion, ELAC has a significant potential to be used as a tendon replacement and in the development of an osteotendinous construct towards the regeneration of bone-tendon interfaces.
Keywords: collagen, topography, mesenchymal stem cells, differentiation, tenogenic, tendon
1. Introduction
Tendon injuries do not heal completely due to low cellularity [1] and poor vascularization [2]. Current strategies to treat damaged tendons such as suturing, autografts or allografts are often associated with limitations like injury relapse [3], donor site morbidity [4] and risk of disease transmission, respectively. A potential solution suggested is the in vitro differentiation of mesenchymal stem cells (MSCs) on a mechanically competent biomaterial followed by transplantation to facilitate tendon repair [5, 6]. While the differentiation of MSCs to bone, cartilage and fat lineages have been well established, the lack of specific biomarkers has hampered researchers from fully exploring the ability of MSCs to undergo tenogenic differentiation. However, in the recent past, identification of tendon specific markers like scleraxis [7–9] and tenomodulin [10] has spurred significant interest in devising strategies to stimulate tenogenic differentiation of MSCs.
While there is no well-established differentiation media towards the tenocytic lineage, biological and mechanical stimuli have been employed to induce the differentiation of MSCs towards the tendon lineage. BMP-12, either transfected into the cell or supplemented in the culture medium, has been reported to induce the differentiation of MSCs to tenocytes [11–13]. Adipose derived stem cells treated with GDF-5 have been reported to express tendon specific markers [14, 15]. Connective tissue growth factor (CTGF) has been shown to induce fibroblastic differentiation of MSCs by increasing the expression of type I collagen and tenascin-C [16]. Cyclic stretching has also been reported to definitively stimulate the tenogenic differentiation of MSCs suggesting that a mechanoactive environment may be essential to promote such differentiation for tendon repair applications [17–19].
Although application of external stimuli to control cellular activity is widely implemented, it is more desirable to develop bioactive materials that can modulate cell behavior by itself without any external stimuli. Collagen based biomaterials are of high interest for tendon tissue engineering applications due to their biocompatibility [20–24], biodegradability and also because collagen is naturally present abundantly in tendons [25]. Previous studies have shown that material topography and matrix stiffness of collagen-based biomaterials positively regulate cell response thereby demonstrating promise for tendon repair [26, 27]. Oriented collagen I membranes seeded with human dermal fibroblasts and tenocytes have been reported to better support cell adhesion, alignment and proliferation signifying that material topography controls cellular activity [26]. However, the ability of these membranes to induce tenogenic differentiation when seeded with MSCs has not been investigated. Additionally, the mechanical properties of these membranes are weak and do not match the tissue level mechanical properties of native tendon. Bone marrow stromal cells seeded on collagen functionalized polyacrylamide substrates with gradient mechanical properties have been shown to preferentially undergo tenogenic differentiation within a narrow stiffness range [27]. While the study definitively demonstrates that matrix stiffness influences cell fate, the stiffness of the substrates employed (20–80 kPa) are several orders of magnitude lower than the stiffness of native tendon (500–1000 MPa).
For a biomaterial to be functional in vivo, it is desirable that its mechanical properties match those of native tendon to be able to withstand daily loads. We have previously developed a methodology using the principles of isoelectric focusing to process collagen molecules to obtain electrochemically aligned collagen (ELAC) threads that mimic native tendon in terms of alignment, density and mechanical properties [28, 29]. While the in vitro [30] response to the material in terms of cell migration has been investigated, the ability of the material to support tenogenic differentiation of MSCs is unknown. In the current study, we hypothesized that the aligned collagen topography of ELAC threads will stimulate the differentiation of human mesenchymal stem cells (human MSCs) towards the tendon lineage. To test this hypothesis, human MSCs were cultured on ELAC and randomly oriented collagen threads and the effect of collagen orientation on cell morphology, proliferation and tenogenic differentiation was investigated in the absence of bioinductive factors. Furthermore, human MSCs were cultured on ELAC threads in culture medium supplemented with and without BMP-12 to evaluate the synergistic effect of BMP-12 and collagen orientation on the tenogenic differentiation of human MSCs.
2. Materials and Methods
2.1 Synthesis of ELAC Threads
The protocol followed to synthesize ELAC threads was modified from Cheng et al [28]. Briefly, acid soluble monomeric collagen (Advanced Biomatrix, San Diego, CA) was diluted two-fold using Rnase/Dnase free water (Invitrogen, Carlsbad, CA) and the pH was adjusted to 8–10 using 1N NaOH. The collagen solution was then dialyzed against ultrapure water at 12 °C for 18 hours to remove salts. Dialyzed collagen was loaded between two stainless steel wires (electrodes) across which 20 VDC was applied for 6 hours. In the presence of electric current, a pH gradient develops between the electrodes and the collagen molecules align along the isoelectric point. Aligned collagen was then treated with 1X PBS for 6 hours at 37 °C to facilitate fibril formation [31]. Following PBS treatment, ELAC threads were recovered from the electrochemical cells, immersed in isopropyl alcohol overnight, washed once with Rnase/Dnase free water (Invitrogen, Carlsbad, CA) and dried for 1 hour. The ELAC threads were then crosslinked with 0.625% genipin (Wako Pure Chemical, Osaka, Japan) in 95% ethanol solution for 3 days. The resultant ELAC threads were 8 cm long and 50–100 µm in diameter.
2.2 Synthesis of Random Collagen Threads
Acid soluble monomeric collagen solution (8 parts) was mixed with 10x PBS (1 part) and the pH was adjusted to neutral using 0.1N NaOH (1 part). The collagen solution was poured onto a glass slide and gelled by incubating at 37 °C for 2 hours. The collagen gel formed was crosslinked with 0.625% genipin in 95% ethanol for 3 days. Thin strips of the crosslinked collagen gel were cut using a razor blade and used as random collagen threads.
2.3 Validation of the stemness of human MSCs
Human MSCs (P2) were purchased from Lonza (Walkersville, MD). Cells were passaged three times in MSC-GM growth medium (Lonza) and used at P5 for all the experiments. To validate the stemness of the human MSCs at P5, their multilineage differentiation capability was confirmed following the manufacturer’s instructions. Briefly, for osteogenic differentiation, human MSCs (P5) were seeded at a density of 3000 cells/cm2 and cultured in MSC-GM growth medium. At day 1, the growth media was replaced with osteogenic induction medium (Lonza) and the cells were cultured till day 14 with medium changes every 3 days. At day 14, cells were fixed with 10% formaldehyde and osteogenic differentiation was evaluated by Alizarin Red S staining for calcium deposits. Differentiated cells containing mineral deposits stain bright red by the alizarin red solution. For adipogenic differentiation, human MSCs (P5) were seeded at a density of 20,000 cells/cm2 and cultured up to confluence (3 days) in MSC-GM growth medium. At day 3, the growth media was replaced with adipogenic induction medium (Lonza) and the cells were cultured till day 14 with medium changes every 3 days. At day 14, cells were fixed with 10% formalin, incubated with 60% isopropanol, washed and stained with Oil Red O. After rinsing, the cells were counterstained with hematoxylin. Lipids stain red by Oil Red O and nuclei stain blue by hematoxylin. Cells cultured for 14 days in MSC-GM growth medium (no induction) subjected to similar staining protocols were used as controls.
2.4 Effect of Collagen Orientation on Cell Morphology, Adhesion and Proliferation
Random collagen and ELAC threads were cut into 1 cm long pieces, sterilized in 70% ethanol, washed with 1x PBS and placed into an ultralow attachment 24 well plate (Corning). Each well consisted of either two random collagen or six ELAC threads (n=6 wells/group). The thread numbers were set as such to match cell seeding surface area between groups. To evaluate the effect of collagen orientation on cell adhesion and proliferation, human MSCs (P5) were seeded onto wells containing the threads at a density of 10,000 cells/cm2. The growth medium composed of α-MEM supplemented with 10% MSC-Qualified FBS (Invitrogen) and 1% penicillin/streptomycin. Four hours after seeding the unattached cells were removed by replacing the growth medium and the adherent cells were cultured for 12 days. At periodic intervals, cell proliferation was quantified using alamar blue assay by following the manufacturer’s instructions. Briefly, 0.5 ml of alamar blue mix (culture medium + 10% alamar blue) was added to each well and incubated for 2 hours at 37 °C. Following this, 100 µl of alamar blue mix from each well was transferred to a 96 well plate in triplicate and the absorbance was recorded at 570 nm and 600 nm. Cell number was quantified by calculating the percentage reduction in alamar blue and comparing the values to a standard curve generated using known number of cells. Cell adhesion was determined as the ratio of the number of cells calculated using alamar blue assay four hours after seeding to the initial cell seeding density.
Cell morphology was visually examined by staining the cell actin filaments using AlexaFluor 488 Phalloidin at day 1 and day 14. Briefly, cells were fixed with 3% formaldehyde (with 0.1% TritonX-100) for 10 minutes and washed with 1x PBS. The actin filaments were stained by incubating the cells in AlexaFluor 488 Phalloidin at 37 °C for 20 minutes. Following this, the stain was washed with 1x PBS and high quality images of the actin stained cells were taken using an Olympus Microscope.
2.5 Effect of Collagen Orientation on Tenogenic Differentiation of Human MSCs
Random collagen and ELAC threads were cut into 2 cm long pieces, sterilized in 70% ethanol, washed with 1x PBS and placed into an ultralow attachment 6 well plate (Corning). Each well consisted of either two random collagen or five ELAC threads. The thread numbers were set as such to match cell seeding surface area between groups. A sample size of n=5 wells/group/time point was used. To evaluate the effect of collagen orientation on tenogenic differentiation of human MSCs, passage-5 human MSCs were seeded onto the wells containing the threads at a density of 15,000 cells/cm2. The culture medium composed of α-MEM supplemented with 10% MSC-Qualified FBS (Invitrogen) and 1% penicillin/streptomycin. Four hours after seeding the unattached cells were removed by replacing the growth medium and the adherent cells were cultured for 14 days. Culture medium was changed every 3 days. At periodic intervals (days 3, 7 and 14), total RNA was extracted by lysing the cells using TRIZOL reagent (Invitrogen) and following the manufacturer’s protocol. Briefly, to facilitate phase separation, chloroform was added to the trizol homogenized samples and the samples were centrifuged at 12000 g for 15 minutes at 4 °C. The RNA was collected from the top aqueous phase into a separate tube, precipitated by adding isopropanol and pelleted by centrifuging at 12000 g for 10 minutes at 4 °C. The RNA pellet was then washed with 75% ethanol, dried, resuspended in Rnase/Dnase free water (Invitrogen) and stored at −80 °C until use. Next, 1 µg of total RNA was used for reverse transcription to synthesize cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Taqman gene expression assays (Applied Biosystems) for tendon specific genes (scleraxis, tenomodulin), tendon related genes (tenascin C, collagen III) and bone specific genes (runx2, osteocalcin) were used to evaluate the expression of the genes by quantitative real time-PCR (Applied Biosystems 7500 Real Time PCR System). The relative fold change in target gene expression was quantified using the 2deltadeltaCt method by normalizing the target gene expression to β-actin and relative to the expression on the random collagen control.
2.6 Effect of Collagen Orientation on Cell Morphology and Alignment using Scanning Electron Microscopy
Cell morphology and alignment was examined using scanning electron microscopy. At days 2 and 14, ELAC and random collagen thread samples from the differentiation study were fixed in 2% glutaraldehyde (in PBS) for 1 hour. Following this, the threads were washed with PBS, dehydrated in a graded series of ethanol (10%, 30%, 50%, 70%, 90% and 100%) and subjected to critical point drying. The threads were then mounted onto a stage, sputter coated with gold and visualized under a scanning electron microscope (FEI Nova nanoSEM).
2.7 Effect of BMP-12 on the Tenogenic Differentiation of Human MSCs on ELAC Threads
ELAC threads were cut into 2 cm long pieces, sterilized in 70% ethanol, washed with 1x PBS and placed into an ultralow attachment 6 well plate (Corning). Each well consisted five ELAC threads and a sample size of n=5 wells/group/time point was used. To evaluate the effect of BMP-12 on the tenogenic differentiation of human MSCs on ELAC threads, passage-5 human MSCs were seeded onto the wells containing the threads at a density of 15000 cells/cm2 and subjected to two different treatments. In the first group, cells were cultured in growth medium composing of α-MEM supplemented with 10% MSC-Qualified FBS (Invitrogen) and 1% penicillin/streptomycin. Four hours after seeding the unattached cells were removed by replacing the growth medium and the cells were cultured for 14 days with medium change every three days. In the second group, cells were treated with BMP-12 as described by Lee J.Y. et al. At first, culture was initiated similar to the first group and the unattached cells were removed after four hours. After 12 hours, cells were starved by replacing the growth medium with media containing 1% MSC-FBS for 12 hours. Following this, cells were treated with 10 ng/ml BMP-12 in the same low-serum (1%) medium for an additional 12 hours. After BMP-12 treatment, the cells were cultured in regular growth medium up to day 14 with medium change every three days. At periodic intervals (days 3, 7 and 14), the total RNA was extracted, cDNA was synthesized and qPCR was performed to evaluate the expression of tendon specific and tendon related genes as described in section 2.6. The relative fold change in target gene expression was quantified using the 2deltadeltaCt method by normalizing the target gene expression to β-actin and relative to the expression on the ELAC (w/o BMP-12) control.
2.8 Statistical Analysis
The results for cell adhesion (n=6/group), cell proliferation (n=6/group) and cell differentiation (n=5/group) were analyzed using Mann-Whitney U test. Statistical significance was set at p < 0.05.
3. Results
3.1 Validation of the Stemness of Human MSCs
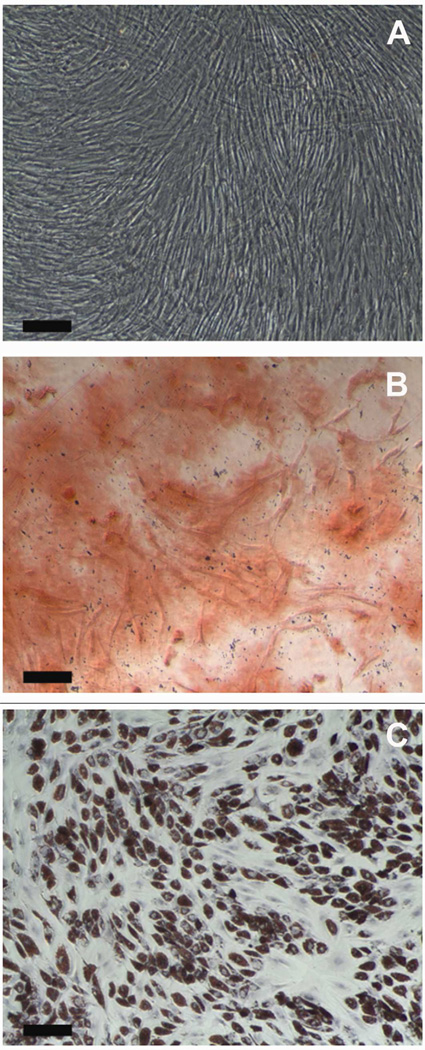
Alizarin red S staining confirmed the presence of calcium deposits at day 14 of the human MSC (P5) culture in osteogenic differentiation media (Fig. 1B). Human MSCs (P5) cultured in adipogenic differentiation media for 14 days showed the presence of lipid droplets as confirmed by Oil red O staining (Fig. 1C). Human MSCs (P5) cultured in standard growth medium (MSC-GM, Lonza) were negative for both Alizarin red S and Oil red O staining (Fig. 1A). These results confirm the stemness of the passage-5 human MSCs used for the proliferation and differentiation experiments in the current study.
Figure 1.
Validation of the Stemness of Human MSCs. (A) Day 14 – Control (No induction), (B) Day 14 - Alizarin red S staining after induction of osteogenic differentiation of human MSCs, (C) Day 14 – Oil red O staining after induction of adipogenic differentiation of human MSCs. Scale Bar: 200 µm.
3.2 Effect of Collagen Orientation on Adhesion and Proliferation of Human MSCs
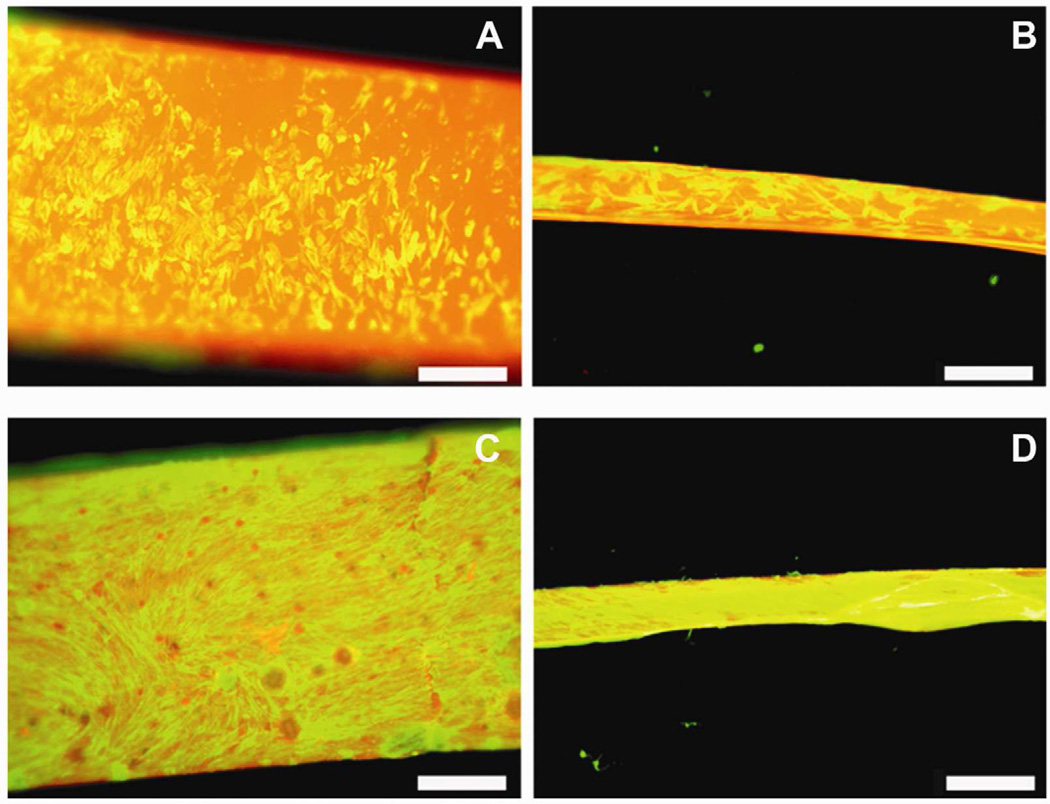
Visual examination of actin stained images revealed that the cells were uniformly seeded on both ELAC and random collagen threads at day 1 (Fig. 2A and 2B). By day 14, a confluent cell layer was observed suggesting significant cell proliferation on both thread types (Fig. 2C and 2D).
Figure 2.
Visual Examination of Cell Morphology and Distribution by Actin Staining. Actin stained images of Random thread at day 1 (A), ELAC thread at day 1 (B), Random thread at day 14 (C), ELAC thread at day 14 (D). Scale Bar: 0.5 mm.
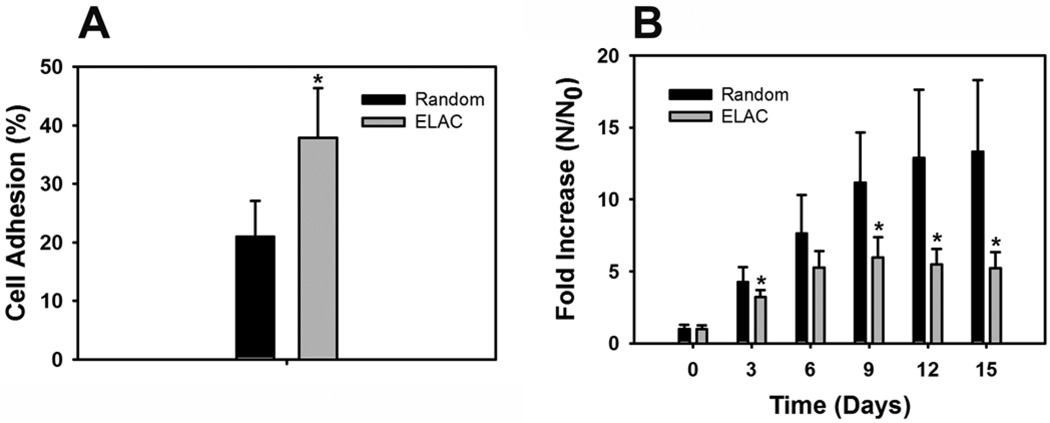
Alamar blue assay revealed that cell adhesion was two-fold higher on ELAC threads (40%) compared to random collagen threads (20%) (Fig. 3A). Cell proliferation results indicate that both ELAC and random collagen threads support the growth of human MSCs (Fig. 3B). More importantly, up to 15-fold increase in cell number was observed on random collagen threads compared to around 5-fold increase in cell number on ELAC threads. These results indicate that while higher cell adhesion was observed on ELAC threads, cell proliferation was significantly higher on random collagen threads compared to ELAC threads.
Figure 3.
Effect of Collagen Orientation on Cell Adhesion and Proliferation. (A) Cell Adhesion - Cell adhesion was two-fold higher on ELAC threads compared to random collagen threads. (B) Cell Proliferation – Higher rates of cell proliferation was observed on random collagen threads compared to ELAC threads. (* indicates statistical significance between ELAC and random collagen threads).
3.3 Effect of Collagen Orientation on Tenogenic Differentiation of Human MSCs
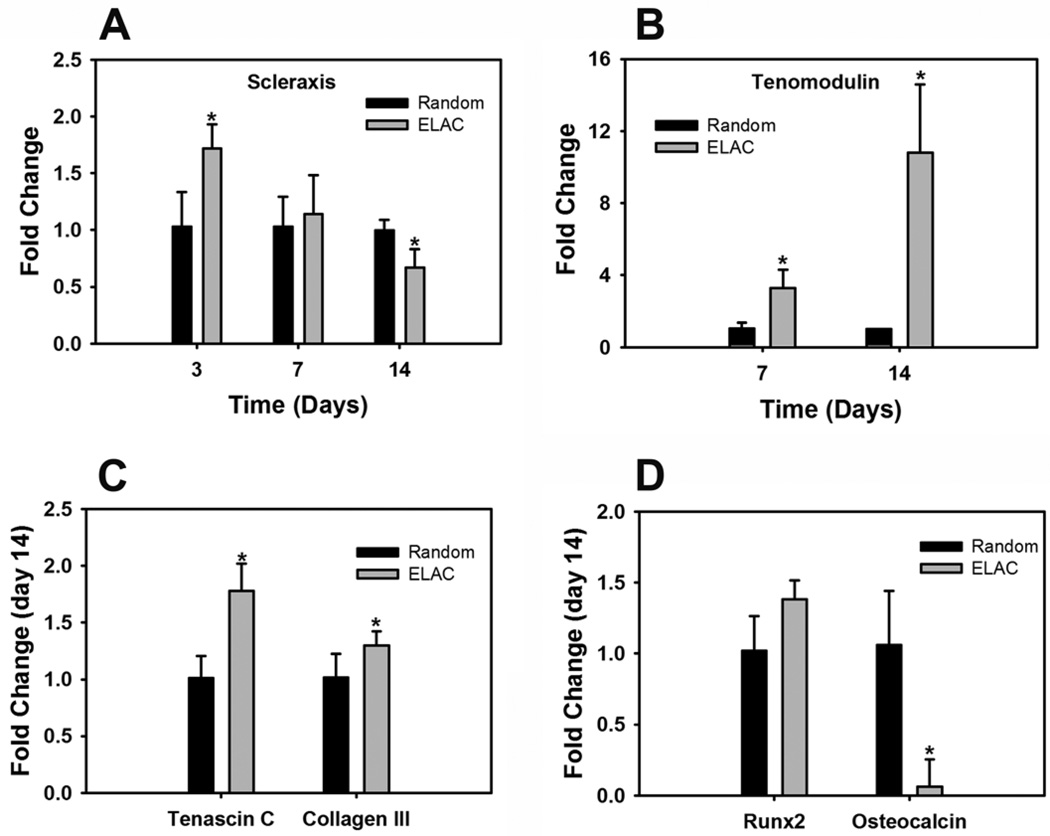
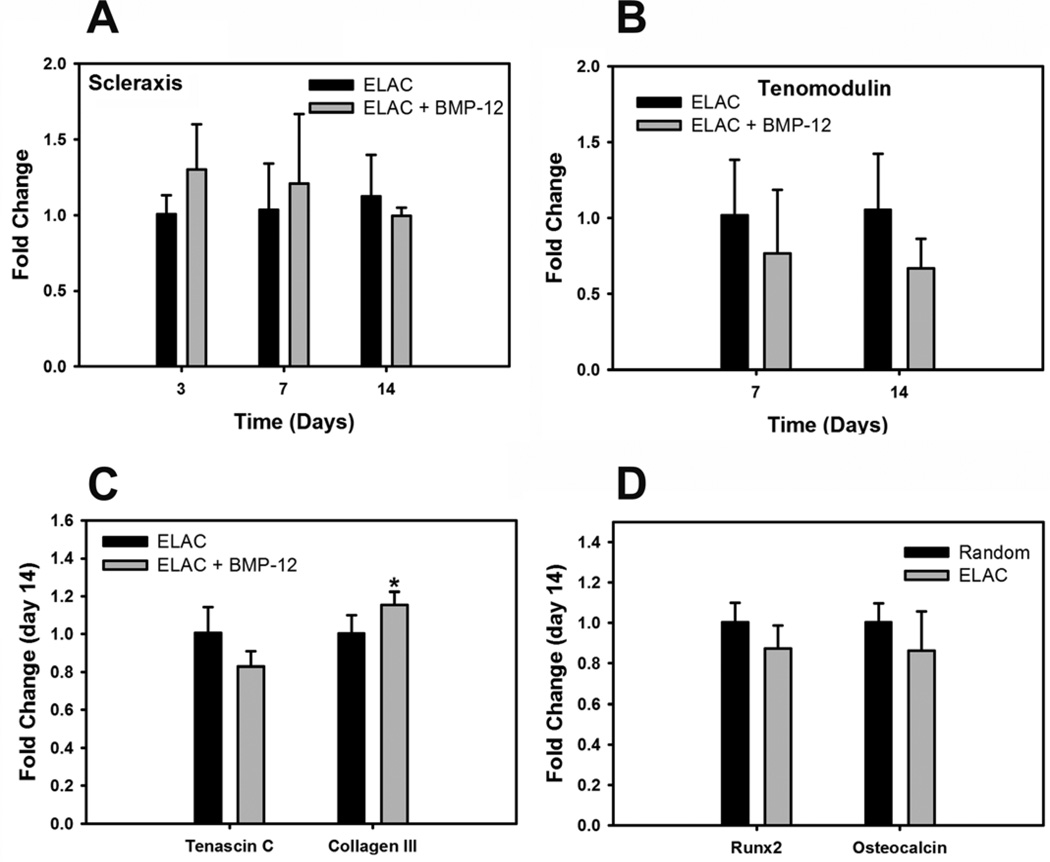
Scleraxis, an early marker for tendon differentiation, was significantly greater on ELAC threads compared to random collagen threads at day 3 (Fig. 4A). Tenomodulin, a mature marker for tendon differentiation, was 3-fold greater at day 7 and 10-fold greater at day 14 on ELAC threads compared to random collagen threads (Fig. 4B). Additionally, the expression of tendon related genes like tenascin-C and collagen-III was also greater on ELAC threads at day 14 (Fig. 4C). On the other hand, osteocalcin, a specific marker for bone differentiation, was significantly greater on random collagen threads compared to ELAC threads at day 14 (Fig. 4D). These results indicate that ELAC by a material itself promotes tenogenic differentiation and suppresses osteogenic differentiation of human MSCs.
Figure 4.
Effect of Collagen Orientation on Tenogenic Differentiation of Human MSCs. (A) Scleraxis, (B) Tenomodulin, (C) Tendon ECM Genes (Tenascin-C and Collagen-III), (D) Bone Differentiation Markers (Runx2 and Osteocalcin). Increase in the expression of tendon specific (scleraxis and tenomodulin) and tendon related (tenascin-D and collagen-III) genes indicates that the oriented collagen topography of ELAC threads stimulates the tenogenic differentiation of human MSCs. Data are normalized to β-actin and relative to the random collagen control. . (* indicates statistical significance between ELAC and random collagen threads).
3.4 Effect of Collagen Orientation on Cell Morphology using Scanning Electron Microscopy
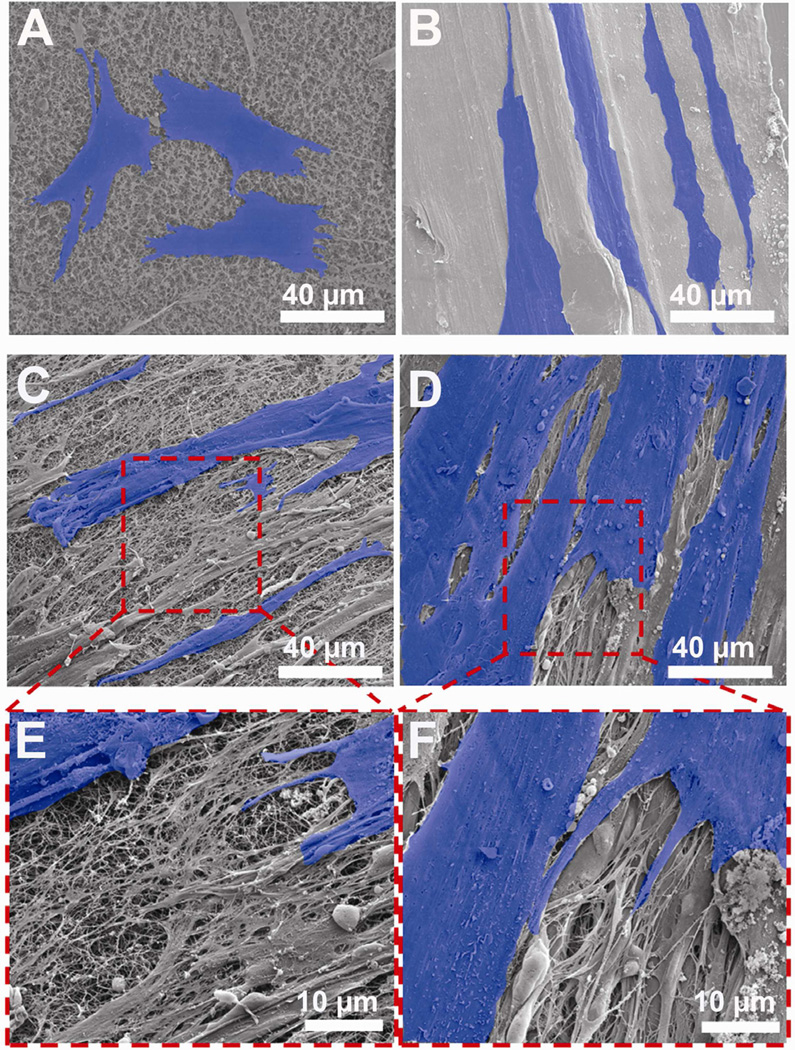
Scanning electron microscopy (SEM) was used to evaluate the differences in cell morphology in response to collagen orientation. High resolution SEM micrographs at day 2 showed the presence of well-defined filament-like cell processes originating from the cell body indicating that the cells adhere well on both random collagen and ELAC threads (Fig. 5A and 5B). While the cells on random collagen threads exhibited a more polygonal phenotype, cells on ELAC threads expressed an elongated and spindle shaped fibroblastic morphology. Additionally, cells on ELAC threads appeared to orient parallel to the aligned collagen topography. No such preferential cell orientation was observed on random collagen threads.
Figure 5.
Effect of Collagen Orientation on Cell Morphology, Alignment and ECM Deposition by Scanning Electron Microscopy. (A) Random – day 2, (B) ELAC – day 2, (C) Random – day 14, (D) ELAC – day 14, (E) Random – day 14 (High Mag), (F) ELAC – day 14 (High Mag). Cells have been highlighted in blue for clarity.
SEM analyses at day 14 showed that cells proliferated and formed a dense layer on both random collagen and ELAC threads (Fig. 5C and 5D). Unlike day 2, no clear difference in cell morphology between the two thread types was evident at day 14. However, preferential orientation of cells along the aligned collagen topography on ELAC was apparent even at day 14. Additionally, high magnification SEM images revealed deposition of a porous matrix-like material on both random collagen and ELAC threads (Fig. 5E and 5F). Together, these results suggest that human MSCs sense the topographical differences between ELAC and random collagen threads.
3.5 Evaluation of Synergistic Effect of BMP-12 and Collagen Orientation on Tenogenic Differentiation of Human MSCs
BMP-12 treatment showed an increasing trend (p=0.06) in the expression of scleraxis on ELAC threads at day 3 compared to the control (Fig. 6A). However, the expression of other tendon markers like tenomodulin, tenascin-C and collagen-III and osteogenic markers like Runx2 and Osteocalcin at day 14 was comparable with BMP-12 treatment compared to without BMP-12 (Fig. 6B, 6C and 6D). These results indicate that BMP-12 had no additional effect over collagen orientation on the tenogenic differentiation of human MSCs.
Figure 6.
Effect of BMP-12 on Tenogenic Differentiation of Human MSCs on ELAC Threads. . (A) Scleraxis, (B) Tenomodulin, (C) Tendon ECM Genes (Tenascin-C and Collagen-III), (D) Bone Differentiation Markers (Runx2 and Osteocalcin). The expression of tendon specific genes (scleraxis and tenomodulin) was comparable with or without BMP-12 treatment indicating that BMP-12 did not have an additive effect over collagen orientation on the tenogenic differentiation of human MSCs. Data are normalized to β-actin and relative to ELAC without BMP-12 control. (* indicates statistical significance between ELAC and random collagen threads).
4. Discussion
Tendon extracellular matrix (ECM) is mainly composed of highly aligned collagen type I fibers. Anisotropic collagen-based biomaterials present a natural environment to the cells populating it and hence may act as ideal replacements for damaged tendon [25]. ELAC is a pure collagen based biomaterial that mimics native tendon in terms of its density (>1000 mg/ml) and mechanical strength [28]. While previous studies have demonstrated that aligned collagen matrices better support cell adhesion [30], proliferation [26] and the multilineage differentiation potential of MSCs [32], this is the first study that thoroughly investigates and demonstrates the ability of a dense aligned collagen based biomaterial to specifically stimulate tenogenic differentiation of human MSCs. Evidence of such differentiation is key in developing a mechanically viable functional tissue engineered construct with significant potential to enhance tendon and ligament healing.
Cell proliferation results indicate that higher rate of cell expansion was observed on random collagen threads compared to ELAC threads (Fig. 3). A possible reason may be that twofold higher number of cells initially attach on ELAC threads thereby limiting the available surface area for proliferation. Cell proliferation reached a plateau at day 6 on ELAC compared to day 12 on the random collagen threads indicating that cells reached confluence faster on ELAC compared to random collagen threads. However, the rate of proliferation before confluence (day 3) was still lower on ELAC compared to random collagen threads. The lower cell proliferation may be indicative of active cell differentiation taking place on ELAC threads.
Scleraxis is a basic helix-loop-helix transcription factor reported to be a highly specific early marker for tendon differentiation [7–9]. The force transmitting tendons of scleraxis-null mutant mice have been reported to show severe defects suggesting that scleraxis is critical for tendon development [33]. Park et al. reported that growth differentiation factor-5 (GDF-5) treatment of rat adipose derived MSCs resulted in an early increase in scleraxis expression following which the expression of scleraxis decreased [15]. A similar outcome was evident in the current study in which an initial increase in the expression of scleraxis (day 3) on ELAC threads was followed by a subsequent decrease in its expression with the duration of the culture (Fig. 4A). An early increase in scleraxis expression may indicate onset of tenogenic differentiation and can be used as a useful criteria to screen potential tenoinductive factors in future studies.
Scleraxis has been reported to positively regulate the expression of tenomodulin [34]. The results of the current study are consistent with previous reports [15, 34] such that an initial upregulation (day 3) of scleraxis on the ELAC threads was followed by an increase in the expression of tenomodulin towards the end (day 7 and day 14) of the culture. Tenomodulin has been deemed necessary for tendon maturation [35] and is regarded as a specific marker for tenogenic differentiation [10, 34]. A 10-fold increase in the expression of tenomodulin on ELAC threads compared to random collagen threads at day 14 clearly demonstrates that human MSCs preferentially differentiate towards the tendon lineage on ELAC threads (Fig. 4B). Although not specific markers, the late increase in the expression of tendon-related ECM genes like tenascin-C and collagen III further substantiates that the oriented collagen topography of ELAC threads stimulates tenogenic differentiation of human MSCs.
MSC-collagen gel composites contracted onto sutures and implanted into patellar tendon defects resulted in ectopic bone formation in 28% of the MSC-collagen grafted tendons [36]. Excessive contraction of the collagen gel has been attributed as a possible reason for this outcome. Previous studies have confirmed that matrix elasticity directs stem cell differentiation [37]. While soft and compliant matrices support neuronal differentiation [38], stiffer matrices have been shown to promote osteogenic differentiation [37, 39]. To confirm the specificity of ELAC threads to induce tenogenic differentiation, the expression of bone-specific (Runx2 and Osteocalcin) genes were evaluated. The results showed that the expression of osteocalcin was significantly lower on ELAC threads compared to random collagen threads indicating that osteogenic differentiation is suppressed on ELAC threads. These results confirm the ability of ELAC to unambiguously induce tenogenic differentiation thus making it a promising candidate for tendon tissue engineering applications.
Scanning electron microscopy revealed that cells orient parallel to the aligned collagen on ELAC threads suggesting that the cells are able to sense the topography of the material (Fig. 5). These results are in agreement with a previous study that used tendon stem cells and reported that the cells orient along the aligned poly(L-lactic acid) fibrous scaffold [40]. Cell adhesion, shape and extracellular matrix interaction have been reported to regulate stem cell lineage commitment by modulating specific signaling pathways [41, 42]. While the cells on the random collagen threads express a more polygonal morphology, cells on the ELAC threads assume a spindle shaped fibroblastic morphology very early in culture (day 2). It is likely that the difference in the matrix stiffness between the ELAC and random collagen threads may have influenced the lineage commitment of stem cells by modulating the focal adhesion structure and cell cytoskeleton shape.
The remodeling phase of tendon healing (post inflammatory and proliferative phases) begins at six weeks and lasts up to one year to form scar-like tendon tissue [43]. We have recently reported the in vivo response to ELAC threads by implanting them into the rabbit patellar tendon [44]. The results showed that ELAC degrades gradually and completely in a time-frame of eight months when implanted in the tendon proper. A mild inflammation was observed around the ELAC threads for up to four months. To put in perspective, the level of inflammation was comparable to that observed around standard surgical sutures. The mild-inflammation was largely resolved and was remodeled by eight months. This in vivo study impacted ELAC as is, and the study did not involve seeding cells onto the ELAC threads prior to implantation. Future studies will focus on implanting cell seeded ELAC threads which may counter deleterious effects of inflammation and expedite de novo matrix synthesis. In the context of the current in vitro study, some evidence of porous matrix like deposition was observed by SEM (Fig. 5E and 5F). However, to definitively demonstrate cell secreted ECM synthesis, further studies involving longer term cultures (4–6 weeks) in culture medium supplemented with ascorbic acid is needed. Ascorbic acid is essential for catalyzing collagen crosslink formation and; thus, for retaining the collagen formed during the culture period on the substrate.
Although an initial increase in scleraxis expression is promising, it may be desirable to maintain the expression of scleraxis by employing alternative strategies like biological or mechanical stimuli in synergy with aligned collagen topography to further augment tendon differentiation. BMP-12 is believed to be a key cytokine for tenogenic differentiation of human MSCs. BMP-12 gene transfection has been reported to induce tenogenic differentiation of rhesus bone marrow MSCs [13]. Equine marrow MSCs have been shown to undergo tenogenic differentiation when treated with BMP-12 supplemented culture medium for 14 days [11]. More recently, Lee et al. have reported that a brief stimulation (12 hours) of rat MSCs with BMP-12 was sufficient to induce and maintain tenogenic differentiation both in vitro and in vivo [12]. The BMP-12 treatment strategy employed in the current study was adopted from Lee et al. [12]. Human MSCs cultured on ELAC threads were briefly exposed to BMP-12 for 12 hours and then cultured for 14 days in regular growth medium (w/o BMP-12). The results indicated that the expression of tendon-specific genes like scleraxis and tenomodulin did not increase with BMP-12 treatment of ELAC seeded cells suggesting that BMP-12 did not have an additional effect over collagen orientation on the tenogenic differentiation of human MSCs. It is likely that the effect of collagen orientation on tenogenic differentiation was more dominant and masked the ability of BMP-12 to induce such differentiation. It is possible that the protocol developed by Lee et al. may be optimal for the tenogenic differentiation of rat MSCs on random collagen sponge scaffolds, but not necessarily on human MSCs on oriented collagen matrices. However, since an increasing trend in the expression of scleraxis (p=0.06) after BMP-12 treatment was observed at day 3 of the current study, it is likely that the presence of BMP-12 throughout the culture duration may have augmented the tenogenic differentiation of human MSCs. Further studies are needed to determine the optimal BMP-12 delivery strategy for the tenogenic differentiation of MSCs on aligned collagen matrices. Mechanical stimulation has been previously reported to maintain scleraxis expression [17] and hence may be a promising alternative to enhance tendon differentiation.
5. Conclusion
The current study demonstrates that an anisotropically oriented dense collagen matrix promotes tenogenic differentiation of human MSCs, even in the absence of bioinductive cues. The results suggest that ELAC induces such differentiation topographically by providing a collagen substrate that is densely packed and uniformly aligned, akin to the tendon itself. Therefore, ELAC when seeded with MSCs and implanted into the tendon has significant potential to initiate repair by stimulating tenogenic differentiation and thereby promoting the formation of a functional tissue. ELAC can also be used in the development of osteotendinous constructs towards the regeneration of bone-tendon interfaces.
Acknowledgement
This study was funded by a grant from NIH (1R21AR056060).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Squier CA, Magnes C. Spatial relationships between fibroblasts during the growth of rat-tail tendon. Cell Tissue Res. 1983;234:17–29. doi: 10.1007/BF00217399. [DOI] [PubMed] [Google Scholar]
- 2.Fischer LP, Carret JP, Gonon GP, Sayfi Y. Arterial vascularization of the patellar ligament (ligamentum patellase) and of the Achilles tendon (tendo calcaneous) in man. Bull Assoc Anat (Nancy) 1976;60:323–334. [PubMed] [Google Scholar]
- 3.Krackow KA, Thomas SC, Jones LC. Ligament-tendon fixation: analysis of a new stitch and comparison with standard techniques. Orthopedics. 1988;11:909–917. doi: 10.3928/0147-7447-19880601-11. [DOI] [PubMed] [Google Scholar]
- 4.Kartus J, Movin T, Karlsson J. Donor-site morbidity and anterior knee problems after anterior cruciate ligament reconstruction using autografts. Arthroscopy. 2001;17:971–980. doi: 10.1053/jars.2001.28979. [DOI] [PubMed] [Google Scholar]
- 5.Butler DL, Juncosa-Melvin N, Boivin GP, Galloway MT, Shearn JT, Gooch C, et al. Functional tissue engineering for tendon repair: A multidisciplinary strategy using mesenchymal stem cells, bioscaffolds, and mechanical stimulation. J Orthop Res. 2008;26:1–9. doi: 10.1002/jor.20456. [DOI] [PubMed] [Google Scholar]
- 6.Lui PP, Rui YF, Ni M, Chan KM. Tenogenic differentiation of stem cells for tendon repair-what is the current evidence? J Tissue Eng Regen Med. 2011;5:e144–e163. doi: 10.1002/term.424. [DOI] [PubMed] [Google Scholar]
- 7.Brent AE, Schweitzer R, Tabin CJ. A somitic compartment of tendon progenitors. Cell. 2003;113:235–248. doi: 10.1016/s0092-8674(03)00268-x. [DOI] [PubMed] [Google Scholar]
- 8.Cserjesi P, Brown D, Ligon KL, Lyons GE, Copeland NG, Gilbert DJ, et al. Scleraxis: a basic helix-loop-helix protein that prefigures skeletal formation during mouse embryogenesis. Development. 1995;121:1099–1110. doi: 10.1242/dev.121.4.1099. [DOI] [PubMed] [Google Scholar]
- 9.Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, et al. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855–3866. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- 10.Jelinsky SA, Archambault J, Li L, Seeherman H. Tendon-selective genes identified from rat and human musculoskeletal tissues. J Orthop Res. 2010;28:289–297. doi: 10.1002/jor.20999. [DOI] [PubMed] [Google Scholar]
- 11.Violini S, Ramelli P, Pisani LF, Gorni C, Mariani P. Horse bone marrow mesenchymal stem cells express embryo stem cell markers and show the ability for tenogenic differentiation by in vitro exposure to BMP-12. BMC Cell Biol. 2009;10:29. doi: 10.1186/1471-2121-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JY, Zhou Z, Taub PJ, Ramcharan M, Li Y, Akinbiyi T, et al. BMP-12 treatment of adult mesenchymal stem cells in vitro augments tendon-like tissue formation and defect repair in vivo. PLoS One. 2011;6:e17531. doi: 10.1371/journal.pone.0017531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang QW, Chen ZL, Piao YJ. Mesenchymal stem cells differentiate into tenocytes by bone morphogenetic protein (BMP) 12 gene transfer. J Biosci Bioeng. 2005;100:418–422. doi: 10.1263/jbb.100.418. [DOI] [PubMed] [Google Scholar]
- 14.James R, Kumbar SG, Laurencin CT, Balian G, Chhabra AB. Tendon tissue engineering: adipose-derived stem cell and GDF-5 mediated regeneration using electrospun matrix systems. Biomed Mater. 2011;6:025011. doi: 10.1088/1748-6041/6/2/025011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park A, Hogan MV, Kesturu GS, James R, Balian G, Chhabra AB. Adipose-derived mesenchymal stem cells treated with growth differentiation factor-5 express tendon-specific markers. Tissue Eng Part A. 2010;16:2941–2951. doi: 10.1089/ten.tea.2009.0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee CH, Moioli EK, Mao JJ. Fibroblastic differentiation of human mesenchymal stem cells using connective tissue growth factor. Conf Proc IEEE Eng Med Biol Soc. 2006;1:775–778. doi: 10.1109/IEMBS.2006.259866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuo CK, Tuan RS. Mechanoactive tenogenic differentiation of human mesenchymal stem cells. Tissue Eng Part A. 2008;14:1615–1627. doi: 10.1089/ten.tea.2006.0415. [DOI] [PubMed] [Google Scholar]
- 18.Song G, Luo Q, Xu B, Ju Y. Mechanical stretch-induced changes in cell morphology and mRNA expression of tendon/ligament-associated genes in rat bone-marrow mesenchymal stem cells. Mol Cell Biomech. 2010;7:165–174. [PubMed] [Google Scholar]
- 19.Farng E, Urdaneta AR, Barba D, Esmende S, McAllister DR. The effects of GDF-5 and uniaxial strain on mesenchymal stem cells in 3-D culture. Clin Orthop Relat Res. 2008;466:1930–1937. doi: 10.1007/s11999-008-0300-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chvapil M, Speer DP, Holubec H, Chvapil T, King DH. Collagen fibers as a temporary scaffold for replacement of ACL in goats. J Biomed Mat Res. 1993;27:313–325. doi: 10.1002/jbm.820270305. [DOI] [PubMed] [Google Scholar]
- 21.Koob TJ, Willis TA, Hernandez DJ. Biocompatibility of NDGA-polymerized collagen fibers. I. Evaluation of cytotoxicity with tendon fibroblasts in vitro. J Biomed Mat Res. 2001;56:31–39. doi: 10.1002/1097-4636(200107)56:1<31::aid-jbm1065>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 22.Koob TJ, Willis TA, Qiu YS, Hernandez DJ. Biocompatibility of NDGA-polymerized collagen fibers. II. Attachment, proliferation, and migration of tendon fibroblasts in vitro. J Biomed Mat Res. 2001;56:40–48. doi: 10.1002/1097-4636(200107)56:1<40::aid-jbm1066>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 23.Law JK, Parsons JR, Silver FH, Weiss AB. An evaluation of purified reconstituted type 1 collagen fibers. J Biomed Mat Res. 1989;23:961–977. doi: 10.1002/jbm.820230902. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein JD, Tria AJ, Zawadsky JP YPK, Christiansen D, Silver FH. Development of a reconstituted collagen tendon prosthesis. A preliminary implantation study. J Bone Joint Surg Am. 1989;71:1183–1191. [PubMed] [Google Scholar]
- 25.Calve S, Dennis RG, Kosnik PE, 2nd, Baar K, Grosh K, Arruda EM. Engineering of functional tendon. Tissue Eng. 2004;10:755–761. doi: 10.1089/1076327041348464. [DOI] [PubMed] [Google Scholar]
- 26.Gigante A, Cesari E, Busilacchi A, Manzotti S, Kyriakidou K, Greco F, et al. Collagen I membranes for tendon repair: effect of collagen fiber orientation on cell behavior. J Orthop Res. 2009;27:826–832. doi: 10.1002/jor.20812. [DOI] [PubMed] [Google Scholar]
- 27.Sharma RI, Snedeker JG. Biochemical and biomechanical gradients for directed bone marrow stromal cell differentiation toward tendon and bone. Biomaterials. 2010;31:7695–7704. doi: 10.1016/j.biomaterials.2010.06.046. [DOI] [PubMed] [Google Scholar]
- 28.Cheng X, Gurkan UA, Dehen CJ, Tate MP, Hillhouse HW, Simpson GJ, et al. An electrochemical fabrication process for the assembly of anisotropically oriented collagen bundles. Biomaterials. 2008;29:3278–3288. doi: 10.1016/j.biomaterials.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 29.Kishore V, Paderi JE, Akkus A, Smith KM, Balachandran D, Beaudoin S, et al. Incorporation of a decorin biomimetic enhances the mechanical properties of electrochemically aligned collagen threads. Acta Biomater. 2011;7:2428–2436. doi: 10.1016/j.actbio.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gurkan UA, Cheng X, Kishore V, Uquillas JA, Akkus O. Comparison of morphology, orientation, and migration of tendon derived fibroblasts and bone marrow stromal cells on electrochemically aligned collagen constructs. J Biomed Mater Res A. 2010;94:1070–1079. doi: 10.1002/jbm.a.32783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uquillas JA, Kishore V, Akkus O. Effects of phosphate-buffered saline concentration and incubation time on the mechanical and structural properties of electrochemically aligned collagen threads. Biomed Mater. 2011;6:035008. doi: 10.1088/1748-6041/6/3/035008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lanfer B, Seib FP, Freudenberg U, Stamov D, Bley T, Bornhauser M, et al. The growth and differentiation of mesenchymal stem and progenitor cells cultured on aligned collagen matrices. Biomaterials. 2009;30:5950–5958. doi: 10.1016/j.biomaterials.2009.07.039. [DOI] [PubMed] [Google Scholar]
- 33.Murchison ND, Price BA, Conner DA, Keene DR, Olson EN, Tabin CJ, et al. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development. 2007;134:2697–2708. doi: 10.1242/dev.001933. [DOI] [PubMed] [Google Scholar]
- 34.Shukunami C, Takimoto A, Oro M, Hiraki Y. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev Biol. 2006;298:234–247. doi: 10.1016/j.ydbio.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 35.Docheva D, Hunziker EB, Fassler R, Brandau O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol Cell Biol. 2005;25:699–705. doi: 10.1128/MCB.25.2.699-705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Awad HA, Boivin GP, Dressler MR, Smith FN, Young RG, Butler DL. Repair of patellar tendon injuries using a cell-collagen composite. J Orthop Res. 2003;21:420–431. doi: 10.1016/S0736-0266(02)00163-8. [DOI] [PubMed] [Google Scholar]
- 37.Engler AJ, Sweeney HL, Discher DE, Schwarzbauer JE. Extracellular matrix elasticity directs stem cell differentiation. J Musculoskelet Neuronal Interact. 2007;7:335. [PubMed] [Google Scholar]
- 38.Flanagan LA, Ju YE, Marg B, Osterfield M, Janmey PA. Neurite branching on deformable substrates. Neuroreport. 2002;13:2411–2415. doi: 10.1097/01.wnr.0000048003.96487.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garcia AJ, Reyes CD. Bio-adhesive surfaces to promote osteoblast differentiation and bone formation. J Dent Res. 2005;84:407–413. doi: 10.1177/154405910508400502. [DOI] [PubMed] [Google Scholar]
- 40.Yin Z, Chen X, Chen JL, Shen WL, Hieu Nguyen TM, Gao L, et al. The regulation of tendon stem cell differentiation by the alignment of nanofibers. Biomaterials. 2010;31:2163–2175. doi: 10.1016/j.biomaterials.2009.11.083. [DOI] [PubMed] [Google Scholar]
- 41.Gao L, McBeath R, Chen CS. Stem cell shape regulates a chondrogenic versus myogenic fate through Rac1 and N-cadherin. Stem Cells. 2010;28:564–572. doi: 10.1002/stem.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 43.Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am. 2005;87:187–202. doi: 10.2106/JBJS.D.01850. [DOI] [PubMed] [Google Scholar]
- 44.Kishore V, Uquillas JA, Dubikovsky A, Alshehabat M, Snyder PW, Breur GJ, Akkus O. In vivo response to electrochemically aligned collagen bioscaffolds. J Biomed Mater Res B Appl Biomater. 2012 doi: 10.1002/jbm.b.31962. (in press). [DOI] [PubMed] [Google Scholar]