Abstract
Calorie restriction (CR) reduces the rate of cell proliferation in mitotic tissues. It has been suggested that this reduction in cell proliferation may mediate CR-induced increases in longevity. However, the mechanisms that lead to CR-induced reductions in cell proliferation rates remain unclear. To evaluate the CR-induced physiological adaptations that may mediate reductions in cell proliferation rates, we altered housing temperature and access to voluntary running wheels to determine the effects of food intake, energy expenditure, percent body fat, and body weight on proliferation rates of keratinocytes, liver cells, mammary epithelial cells, and splenic T-cells in C57BL/6 mice. We found that ∼20% CR led to a reduction in cell proliferation rates in all cell types. However, lower cell proliferation rates were not observed with reductions in 1) food intake and energy expenditure in female mice housed at 27°C, 2) percent body fat in female mice provided running wheels, or 3) body weight in male mice provided running wheels compared with ad libitum-fed controls. In contrast, reductions in insulin-like growth factor I were associated with decreased cell proliferation rates. Taken together, these data suggest that CR-induced reductions in food intake, energy expenditure, percent body fat, and body weight do not account for the reductions in global cell proliferation rates observed in CR. In addition, these data are consistent with the hypothesis that reduced cell proliferation rates could be useful as a biomarker of interventions that increase longevity.
Keywords: food intake, energy expenditure, percent body fat, body weight
calorie restriction (CR), defined as a reduction in caloric intake without malnutrition, increases lifespan, delays the onset of age-related diseases, and slows the functional decline of various organs (42). Although the mechanisms mediating these effects are unclear, one of the most rapid and robust effects of CR is a reduction in the rate of cell proliferation in mitotic tissues (25, 28, 38). The turnover rates of keratinocytes, liver cells, mammary epithelial cells (MECs), splenic T cells, and prostate cells are decreased by 30–50% following several weeks of CR, and the effects persist throughout the intervention period (25). It has been suggested that this reduction in cell proliferation could mediate many of the longevity and anti-cancer effects of CR by delaying replicative senescence and reducing the promotional phase of carcinogenesis (11, 42). Considering that the rate of cell proliferation is an integrated response that comprises many genetic and hormonal signals and appears to be mechanistically linked to aging, this cellular process represents a potential target for monitoring therapeutic interventions aimed at delaying the onset of age-related diseases. Accordingly, identifying upstream physiological inputs or hormonal signals that may mediate reduced cell proliferation rates in response to CR could provide insights into mechanisms of aging and age-related diseases.
Reductions in food intake (18, 43), energy expenditure (37), percent body fat (3, 5, 36), and body weight (20, 34, 35) have all been suggested to mediate the effects of CR. To date, however, it is still not clear which, if any, of these physiological adaptations are sufficient to increase longevity. In addition, the hormonal mediators translating these physiological adaptations to cellular responses have not been fully characterized. Progress in this field has been limited by the difficulty in dissociating the individual physiological effects of CR as well as the time and resources required for classical longevity studies in mice, which take up to 3 years to complete (19).
Previous studies have established that changes in ambient housing temperature and voluntary wheel running can alter food intake, energy expenditure, percent body fat, and body weight (22, 26). We hypothesized that the use of these interventions might allow for the assessment of alterations in these physiological adaptations on cell proliferation rates independent of classical CR. In addition, if cell proliferation rates can serve as a biomarker of interventions that increase lifespan, the application of this biomarker-based approach could provide an efficient method for investigating the effects of different factors on health and longevity. However, the effects of altering these physiological parameters through changes in ambient temperature or voluntary wheel running on cell proliferation have not previously been established.
Therefore, the goal of this study was to mimic physiological adaptations to CR through changes in ambient housing temperature and voluntary wheel running to determine the effects of food intake, energy expenditure, percent body fat, and body weight on “global” cell proliferation rates (keratinocyte, liver cell, MEC, and splenic T cell proliferation) in young C57BL/6 mice.
METHODS
Mice and diets.
Twelve-week-old C57BL/6 mice (Charles River Breeding, Wilmington, MA) were housed individually and maintained under temperature- and light-controlled conditions (12:12-h light-dark cycle, lights on at 0700 and off at 1900). For all experiments, mice were provided semipurified AIN-93M diet (Bio-Serv, Frenchtown, NJ) and free access to water. Mice on restricted diets were provided with food daily at ∼1200. Food intake was recorded for each individual mouse every other day. All protocols and procedures were approved by the University of California Berkeley Animal Use Committee.
Experiment 1 design.
After 1 wk of adaptation to their environments and AIN-93M diet, female C57BL/6 mice were randomly assigned to one of three groups (n = 3–5): ad libitum fed and housed at 22°C in a temperature-controlled room (AL/22), ad libitum fed and housed at 27°C in a temperature-controlled room (AL/27), or pair fed the same amount as the AL/27 group but housed at 22°C in a temperature-controlled room (PF/22) (each mouse in the PF/22 group was provided food equal to the average food intake of the AL/27 group from the previous day). The groups were designed to modulate two of the physiological adaptations to CR, reduced food intake and reduced energy expenditure, independent of CR itself. Mice were maintained on the feeding regimen for 4 wk and labeled with 2H2O for the final 3 wk.
Experiment 2 design.
After 1 wk of adaptation to their environments and AIN-93M diet, female C57BL/6 mice were assigned randomly to one of four groups (n = 15): ad libitum fed and sedentary (AL/SED), ad libitum fed and provided 24-h access to a voluntary running wheel (AL/EX), pair fed the same amount as the AL/SED group and provided 24-h access to a voluntary running wheel (PF/EX), or calorie restricted to body weight match the PF/EX and sedentary (CR/SED) groups. The groups were designed to modulate two of the physiological adaptations to CR, reduced percent body fat and reduced body weight, independent of CR itself. Mice were maintained on the feeding regimen for 5 wk and labeled with 2H2O for the final 3 wk.
Experiment 3 design.
The group design for experiment 3 was identical to that of experiment 2, except 12-wk-old male C57BL/6 mice instead of female mice were used. Male C57BL/6 mice were used in this experiment since they have been shown elsewhere to compensate incompletely to voluntary exercise through ad libitum food intake, and therefore, they weigh less than ad libitum-fed sedentary controls (23). The groups were designed to modulate one of the physiological adaptations to CR, reduced body weight, independent of CR itself. Mice were maintained on the feeding regimen for 5 wk and labeled with 2H2O for the final 3 wk.
Voluntary exercise.
AL/EX and PF/EX mice in experiments 2 and 3 were provided 24-h access to a 24-cm running wheel (mini-Mitter) attached to a digital counter. Revolutions were recorded daily.
Body weight measurement, 2H2O labeling, and blood and tissue collection.
The body weight of each mouse was measured one to three times/wk. Mice were labeled with an intraperitoneal injection of 100% 2H2O (0.35 ml/10 g body wt) 3 wk prior to the end of the study and were then provided 8% 2H2O as drinking water for the remainder of the study, as described previously (8). Upon completion of each experiment, mice were anesthetized under 3% isoflurane, and blood was collected via cardiac puncture, followed by cervical dislocation and tissue collection.
Keratinocyte isolation.
After euthanasia, the back of each mouse was shaved, followed by an application of Nair for complete hair removal (Carter Products, New York, NY). A small piece of the dorsal skin was dissected, washed with phosphate-buffered saline solution (PBS; Gibco, Grand Island, NY), cut into three small sections, and placed in 5 ml of PBS with 10 units of dispase II (Roche, Indianapolis, IN). Dorsal skins were incubated for 3.5 h, with shaking at 100 rpm at 37°C. The epidermis was then peeled from the dermis and collected for DNA isolation.
Liver cell isolation.
A section of the liver (∼30 mg) was dissected and homogenized, and total DNA from all liver cells was isolated.
MEC isolation.
MECs were isolated using a protocol adapted from Fata et al. (14). Briefly, the 4L and 4R inguinal mammary glands were removed, placed in Dulbecco's modified Eagle's medium (DMEM; Gibco, Grand Island, NY), and minced. The minced tissue was incubated in 20 ml of collagenase-trypsin solution [0.2% collagenase A (Worthington Biochemical, Lakewood, NJ), 0.2% trypsin, 5% fetal bovine serum in DMEM] for 20 min, with shaking at 100 rpm at 37°C. Following digestion, the tissue suspension was centrifuged at 1,500 rpm for 10 min, the collagenase-trypsin solution and upper fat layer were discarded, and the pellet was resuspended in 10 ml of DMEM. The suspension was pelleted via centrifugation at 1,500 rpm for 10 min, resuspended in 4 ml of DMEM containing 5 μl DNase (≥500 U/ml; Sigma, St. Louis, MO), shaken vigorously for 2 min, and then incubated at room temperature for 5 min. Six milliliters of DMEM was added to the suspension, which was then pelleted via centrifugation at 1,500 rpm for 10 min. The pellet was resuspended in 10 ml of DMEM, and the suspension was briefly centrifuged at 1,500 rpm and the supernatant discarded. The pellet was subjected to a total of three rounds of this brief differential centrifugation at 1,500 rpm. The resulting pellet containing the MECs was then collected for DNA isolation.
Splenic T cell isolation.
Upon dissection, the spleen was homogenized and passed through a 40-μM nylon cell strainer. T cells were isolated from the single cell suspension using mouse anti-CD90.2 microbeads and the MACS cell separation column, following the manufacturer's instructions (Miltenyi Biotec, Auburn, CA), pelleted, and then collected for DNA isolation.
Bone marrow cell isolation.
The femur was dissected for isolation of bone marrow cells. Bone marrow cells were flushed from the femur with 2 ml of PBS and were then pelleted and collected for DNA isolation.
DNA isolation.
DNA was extracted using DNeasy kits (Qiagen, Valencia, CA). Briefly, isolated keratinocytes, liver, MECs, T cells, and bone marrow cells were digested overnight at 37°C in proteinase K solution, followed by DNA isolation and elution into 200 μl of water.
DNA synthesis measurement.
Determination of 2H incorporation into purine deoxyribose (dR) of DNA was performed as described previously (8). Briefly, isolated DNA was hydrolyzed overnight at 37°C with nuclease S1 and potato acid phosphatase. Hydrolyzates were reacted with pentafluorobenzyl hydroxylamine and acetic acid and then acetylated with acetic anhydride and 1-methylimidazole. Dichloromethane extracts were dried, resuspended in ethyl acetate, and analyzed by gas chromatography-mass spectrometry on a DB-17 column with negative chemical ionization, using He as carrier and CH4 as reagent gas. The fractional molar isotope abundances at m/z 435 (M0 mass isotopomer) and 436 (M1) of the pentafluorobenzyl triacetyl derivative of purine dR were quantified using ChemStation software. Excess fractional M+1 enrichment (EM1) was calculated as
where sample and standard (std) refer to the analyzed sample and an unenriched pentafluorobenzyl triacetyl purine dR derivative standard, respectively. The fractional synthesis rate (f) of keratinocytes, liver, MECs and T cells was calculated by a comparison to bone marrow cells in the same animal, which represents an essentially fully turned over population of cells.
Body composition analysis.
Percent body fat was determined by chemical extraction (2). Briefly, mouse carcasses were weighed before and after freeze-drying to determine percent water. Dried carcasses were placed in a Soxhlet extraction apparatus and extracted in ether for 7 days and in acetone for 5 days. The carcasses were then removed and placed in a fume hood for 3 days to allow complete solvent evaporation and then weighed to determine percent body fat.
Serum analyte measurements.
On the day before the termination of each experiment, CR and PF mice were provided with food at ∼1200, as described in Mice and diets. All AL mice were given 24-h access to their food for the entirety of the experiments. On the termination day of each experiment, between 0900 and 1200, mice were anesthetized under 3% isoflurane, and blood was collected via cardiac puncture followed by cervical dislocation and tissue collection. Following centrifugation, serum was collected and stored at −20°C. Serum concentrations (ng/ml) of the following analytes were determined via ELISAs, following the manufacturer's instructions: insulin-like growth factor I (IGF-I; R & D Systems, Minneapolis, MN) in triplicate (experiment 2) or duplicate (experiment 3), insulin-like growth factor-binding protein-3 (IGFBP-3; R & D Systems) in duplicate, triiodothyronine (T3; Calbiotech, Spring Valley, CA) in singlet, and thyroxine (T4; Calbiotech) in singlet. The intra-assay coefficient of variation for the reported assays was 3.85% for IGF-I (experiment 2), 1.91% for IGF-I (experiment 3), and 3.81% for IGFBP-3.
Statistical analysis.
All results are presented as means ± SE. Repeated-measures ANOVA followed by Bonferroni post hoc test was used to analyze change in body weight over time. Following a significant result on repeated-measures ANOVA, single time point comparisons were made using one-way ANOVA followed by a Tukey post hoc test. For cell proliferation, differences between groups were analyzed by one-way ANOVA with Tukey post hoc test. For correlations between serum IGF-I and cell proliferation rates, linear regression analyses were performed and coefficient of determination values (r2) determined. Data were analyzed by Prism Graphpad software (version 5.0a).
RESULTS
Experiment 1: the effect of mimicking physiological adaptations to CR via changes in housing temperature on cell proliferation rates.
To determine the effects of reduced food intake and energy expenditure on cell proliferation rates independent of classical CR, female C57BL/6 mice were randomized into one of three groups: ad libitum fed and housed at the standard 22°C (AL/22), ad libitum fed and housed at 27°C (AL/27), or pair fed the same amount as the AL/27 group and housed at the standard 22°C (PF/22). The rationale for the group design in experiment 1 was based on two primary assumptions: 1) AL/27 mice are closer to thermoneutrality than AL/22 mice, and therefore, they will expend less energy to maintain body temperature; and 2) AL/27 mice will voluntarily decrease their food intake to maintain energy balance due to decreased energy expenditure, and therefore, they will have body weights comparable with AL/22 mice despite reduced food intake.
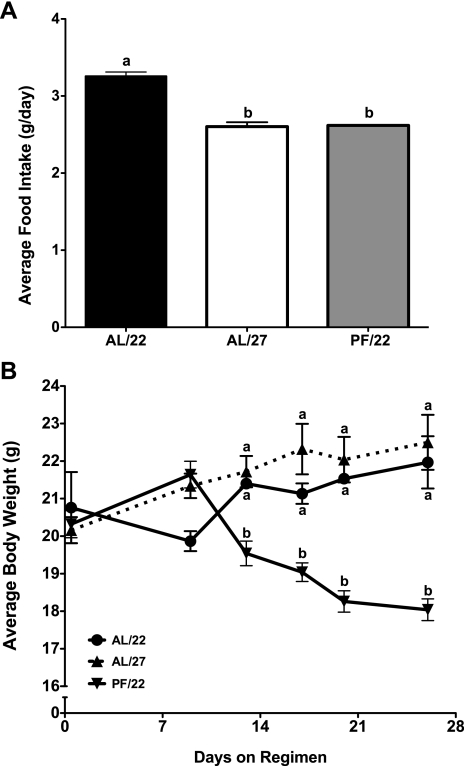
The average daily food intake of both the AL/27 and PF/22 mice was significantly lower than that of AL/22 mice (AL/22 3.3 ± 0.05, AL/27 2.6 ± 0.06, PF/22 2.6 ± 0 g/day; Fig. 1A). There was no difference in average body weight between AL/22 and AL/27 mice, whereas PF/22 mice weighed significantly less than both AL groups at the end of the experiment (AL/22 22.0 ± 0.7, AL/27 22.5 ± 0.7, PF/22 18.0 ± 0.3 g; Fig. 1B). The maintenance of body weight in the face of decreased food intake in AL/27 mice relative to AL/22 mice was presumably due to a decrease in energy expenditure.
Fig. 1.
Effect of housing temperature on food intake and body weight (experiment 1). Female mice were divided into 3 groups: ad libitum fed and housed at 22°C (AL/22), ad libitum fed and housed at 27°C (AL/27), and pair fed the same amount as the AL/27 group and housed at 22°C (PF/22). Food was provided once daily at 1200. A: food consumption was recorded every other day; data represent the mean food intakes over the 4-wk experiment. B: body weights were recorded once/wk; data represent the mean weekly body weights. All values are expressed as means ± SE; n = 3–5 for each group. One-way ANOVA with Tukey's post hoc test was used for all between-group analyses; means not sharing a common letter are significantly different (P < 0.05).
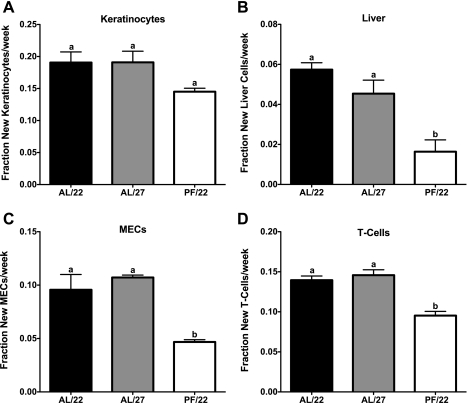
All mice remained on their regimens for a total of 4 wk, at which point they were euthanized and their tissues collected for cell proliferation analysis. Cell proliferation rates were significantly lower in all cell types analyzed in PF/22 mice compared with AL/22 mice (24, 71, 51, and 32% lower in keratinocytes, liver, MECs, and splenic T cells, respectively; Fig. 2, A–D). Interestingly, despite a significantly lower average daily food intake and energy expenditure compared with AL/22 mice, there were no differences in cell proliferation rates in any of the cell types analyzed in AL/27 mice compared with AL/22 mice (Fig. 2, A–D). These data indicate that reduced food intake and energy expenditure are not sufficient to account for the cell proliferation rate-lowering effects of CR.
Fig. 2.
Effect of housing temperature on cell proliferation rates (experiment 1). Cell proliferation rates in keratinocytes (A), liver (B), mammary epithelial cells (MECs; C), and splenic T cells (D). Values are expressed as means ± SE; n = 3–5 for each group. One-way ANOVA with Tukey's post hoc test was used for all between-group analyses; means not sharing a common letter are significantly different (P < 0.05).
Experiment 2: the effect of mimicking physiological adaptations to CR via voluntary wheel running on cell proliferation rates (female mice).
To determine the effects of reductions in percent body fat and body weight on cell proliferation rates independent of classical CR, female C57BL/6 mice were randomized into one of four groups: ad libitum fed and sedentary (AL/SED), ad libitum fed with free access to a voluntary running wheel (AL/EX), pair fed the same amount as the AL/SED group with free access to a voluntary running wheel (PF/EX), or calorie restricted to body weight match PF/EX mice and sedentary (CR/SED). The rationale for the group design in experiment 2 was based on three primary assumptions. 1) Female AL/EX mice, although they expend more energy than AL/SED mice, will voluntarily increase their food intake to compensate for an increase in energy expenditure, and therefore, they will have body weights comparable with AL/SED mice; 2) PF/EX mice will lose body weight compared with AL/SED; and 3) exercising mice in either dietary setting will have decreased percent body fat compared with AL/SED mice.
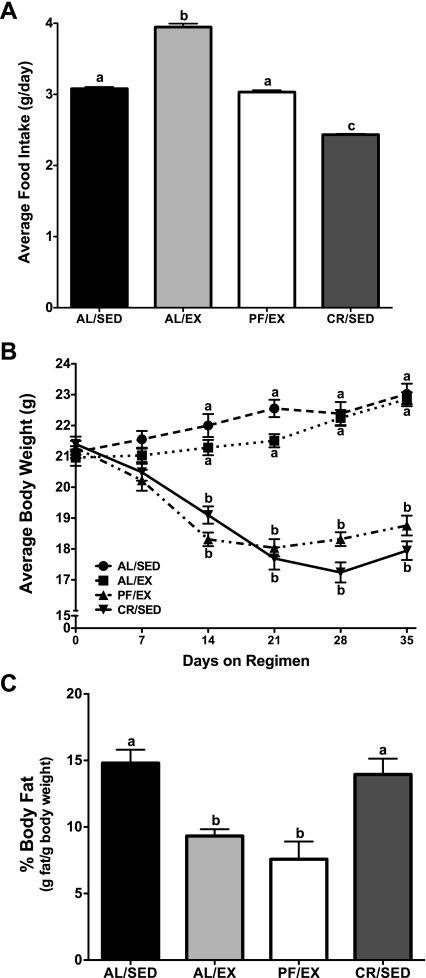
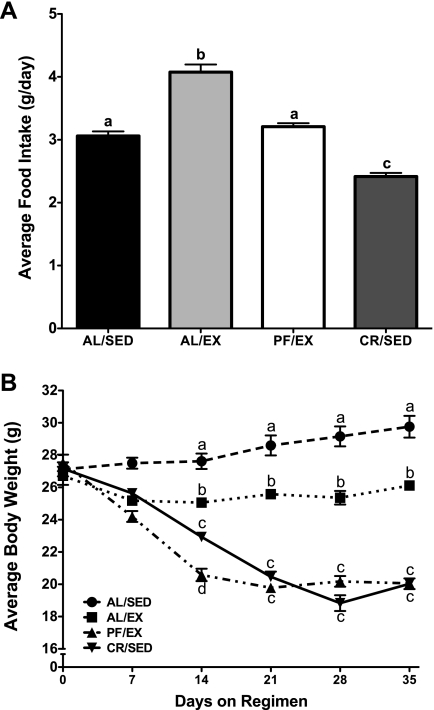
The average daily food intake of the AL/EX mice was significantly elevated compared with both AL/SED and PF/EX mice (AL/SED 3.1 ± 0.02, AL/EX 3.9 ± 0.05, PF/EX 3.0 ± 0.03, CR/SED 2.4 ± 0.01 g/day; Fig. 3A). There was no difference in average body weight between AL/SED and AL/EX mice at the end of the experiment. Similarly, there was no difference in average body weight between PF/EX and CR/SED mice, whereas mice in these two groups did have significantly lower body weights than both AL/SED and AL/EX mice (AL/SED 23.0 ± 0.3, AL/EX 22.9 ± 0.2, PF/EX 18.8 ± 0.3, CR/SED 18.0 ± 0.3 g; Fig. 3B). Body composition analyses confirmed that percent body fat was significantly reduced in AL/EX and PF/EX mice compared with AL/SED and CR/SED mice, which did not differ significantly from one another (AL/SED 14.8 ± 1.0, AL/EX 9.3 ± 0.5, PF/EX 7.6 ± 1.3, CR/SED 14.0 ± 1.2 g fat/g body wt; Fig. 3C).
Fig. 3.
Effect of voluntary wheel running on food intake, %body fat, and body weight in female mice (experiment 2). Female mice were divided into 4 groups: ad libitum fed and sedentary (AL/SED), ad libitum fed and provided free access to a running wheel (AL/EX), pair fed the same amount as AL/SED and provided free access to a running wheel (PF/EX), and calorie restricted to body weight match the PF/EX and sedentary groups (CR/SED). Food was provided once daily at 1200. A: food intake was recorded every other day; data represent the mean food intakes over the 5-wk experiment. B: body weights were recorded ∼3 times/wk; data represent the mean weekly body weights. C: following euthanization, %body fat was determined via chemical extraction from the carcass. Values are expressed as means ± SE; n = 15 for each group for food intake and body weight, and n = 5–14 for each group for %body fat. One-way ANOVA with Tukey's post hoc test was used for all between-group analyses; means not sharing a common letter are significantly different (P < 0.05).
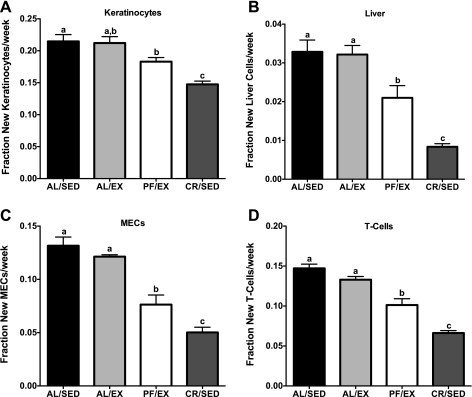
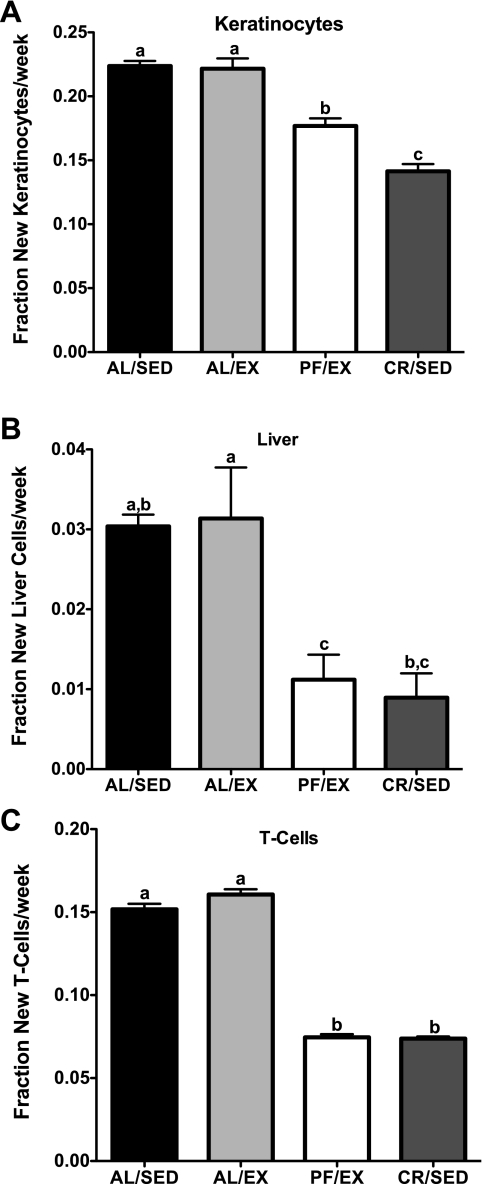
All mice remained on their regimens for a total of 5 wk, at which point they were euthanized and their tissues collected for cell proliferation analysis. Despite significantly lower percent body fat, there were no differences in cell proliferation rates in any of the cell types analyzed in AL/EX compared with AL/SED mice. Cell proliferation rates were significantly lower in all cell types analyzed in CR/SED mice compared with AL/SED mice (31, 75, 62, and 55% lower in keratinocytes, liver, MECs, and splenic T cells, respectively). Cell proliferation rates were also significantly lower in all cell types analyzed in PF/EX mice compared with AL/SED mice (15, 36, 42, and 31% lower in keratinocytes, liver, MECs, and splenic T cells, respectively). Interestingly, cell proliferation rates in CR/SED mice were significantly lower than in PF/EX mice, to which they were body weight matched (19, 60, 34, and 35% lower in keratinocytes, liver, MECs, and splenic T cells, respectively; Fig. 4, A–D).
Fig. 4.
Effect of voluntary wheel running on cell proliferation rates in female mice (experiment 2). Cell proliferation rates in keratinocytes (A), liver (B), MECs (C), and splenic T cells (D). Values are expressed as means ± SE; n = 10–15 for each group. One-way ANOVA with Tukey's post hoc test was used for all between-group analyses; means not sharing a common letter are significantly different (P < 0.05).
These data suggest that reductions in percent body fat are not sufficient to account for the cell proliferation rate-lowering effects of CR, whereas reductions in body weight may be associated with lower rates of cell proliferation. Interestingly, both groups of mice in which we observed an effect of reduced body weight on cell proliferation rates (PF/EX and CR/SED) were food restricted relative to what they would have eaten if fed ad libitum. As exemplified by the increased food intake of AL/EX mice, PF/EX mice would have consumed more food had they been given free access and not restricted to the food intake of AL/SED mice. These results suggest that the effects on cell proliferation rates could be due to a “perceived” reduction in energy intake rather than to reduced body weight per se. Accordingly, we designed experiment 3 with the intention of dissociating reduced body weight from CR.
Experiment 3: the effect of mimicking physiological adaptations to CR via voluntary wheel running on cell proliferation rates (male mice).
To determine the effect of reduced body weight on cell proliferation rates in the context of ad libitum food intake, male C57BL/6 mice were randomized into one of four groups: ad libitum fed and sedentary (AL/SED), ad libitum fed with free access to a voluntary running wheel (AL/EX), pair fed the same amount as the AL/SED group with free access to a voluntary running wheel (PF/EX), or calorie restricted to body weight match PF/EX and sedentary mice (CR/SED). The rationale for the group design in experiment 3 was based on the assumption that male AL/EX mice will voluntarily increase their food intake to counter their increased energy expenditure but will fail to fully compensate energetically, and therefore, they will have reduced body weight compared with AL/SED mice. Therefore, experiments 2 and 3 had identical designs but utilized differences in food intake and energy balance in response to exercise between female (experiment 2) and male (experiment 3) mice.
The average daily food intake of the AL/EX mice was significantly elevated compared with both AL/SED and PF/EX mice (AL/SED 3.1 ± 0.07, AL/EX 4.1 ± 0.1, PF/EX 3.2 ± 0.06, CR/SED 2.4 ± 0.01 g/day; Fig. 5A). However, AL/EX mice had significantly lower body weight compared with AL/SED mice at the end of the experiment (reduced by 12.4%; AL/SED 29.8 ± 0.7, AL/EX 26.1 ± 0.2, PF/EX 20.1 ± 0.2, CR/SED 20.0 ± 0.3 g; Fig. 5B). There was no difference in average body weight between PF/EX and CR/SED mice, whereas mice in these two groups did have significantly lower body weights than both AL/SED and AL/EX mice.
Fig. 5.
Effect of voluntary wheel running on food intake and body weight in male mice (experiment 3). Male mice were divided into 4 groups: AL/SED, AL/EX, PF/EX, and CR/SED. Food was provided once daily at 1200. A: food intake was recorded every other day. Data represent the mean food intakes over the 5-wk experiment. B: body weights were recorded ∼3 times/wk. Data represent the mean weekly body weights. Values are expressed as means ± SE; n = 3–5 for each group. One-way ANOVA with Tukey's post hoc test was used for all between-group analyses; means not sharing a common letter are significantly different (P < 0.05).
All mice remained on their regimens for a total of 5 wk, at which point they were euthanized and their tissues collected for cell proliferation analysis. Interestingly, despite a significant reduction in body weight compared with AL/SED, AL/EX male mice did not have significant differences in cell proliferation rates in any of the cell types analyzed. Cell proliferation rates were significantly lower in all cell types analyzed in CR/SED mice compared with AL/SED mice (37, 71, and 51% lower in keratinocytes, liver, and splenic T cells, respectively; Fig. 6, A–C).
Fig. 6.
Effect of voluntary wheel running on cell proliferation rates in male mice (experiment 3). Cell proliferation rates in keratinocytes (A), liver (B), and splenic T cells (C). Values are expressed as means ± SE; n = 3–5 for each group. One-way ANOVA with Tukey's post hoc test was used for all between-group analyses; means not sharing a common letter are significantly different (P < 0.05).
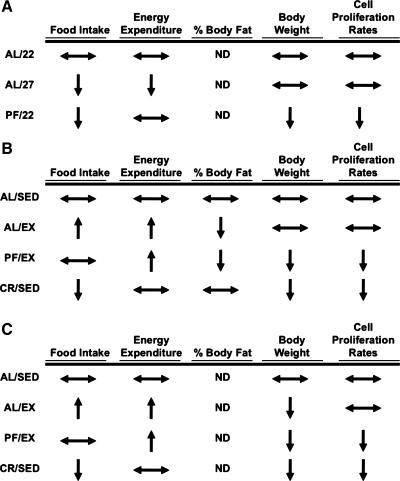
These data suggest that, in the context of ad libitum food intake, reduced body weight relative to sedentary controls is not sufficient to account for the cell proliferation rate-lowering effects of CR. Together, the results from experiments 1, 2, and 3 (Fig. 7, A–C) suggest that none of the classic adaptations to CR (reductions in food intake, energy expenditure, %body fat, and body weight) are sufficient to account for the reductions in “global” cell proliferation rates observed in CR mice.
Fig. 7.
Summary of the effects of the physiological adaptations to calorie restriction on cell proliferation rates (experiments 1, 2, and 3). Cell proliferation rates were measured under experimental conditions in which food intake and energy expenditure (experiment 1; A), %body fat (experiment 2; B), or body weight (C) were reduced, relative to ad libitum-fed controls, through changes in ambient housing temperature (experiment 1) or voluntary wheel running (experiments 2 and 3) independent of classic calorie restriction. Indicated changes in food intake, energy expenditure, %body fat, body weight, and cell proliferation rates are relative to AL/22 (experiment 1) or AL/SED (experiments 2 and 3) controls. ↔No change; ↑increased; ↓decreased. ND, not determined. Changes in energy expenditure were assumed based on changes in food intake and body weight.
Circulating factors and cell proliferation.
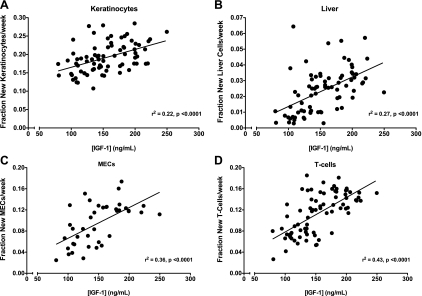
To identify circulating factors that may mediate global reductions in cell proliferation rates, we measured the concentrations of several circulating growth factors in mice from experiments 2 and 3. IGFBP-3, T3, and T4 serum levels were not different in any group in experiment 2 (Table 1). In contrast, serum IGF-I levels were significantly lower in CR/SED compared with both AL groups (Table 1). In addition, IGF-I serum levels correlated positively with cell proliferation rates in all cell types analyzed in all mice in experiments 2 and 3 (Fig. 8, A–D).
Table 1.
Circulating factors (experiment 2)
| Circulating Factor |
||||
|---|---|---|---|---|
| Group | IGF-I | IGFBP-3 | T3 | T4 |
| AL/SED | 163.9 ± 8.9ab | 489.2 ± 28.7a | 1.30 ± 0.07ab | 49.3 ± 3.4a |
| AL/EX | 182.3 ± 7.6a | 501.5 ± 48.6a | 1.44 ± 0.07a | 46.5 ± 2.3a |
| PF/EX | 142.1 ± 10.3bc | 416.5 ± 26.3a | 1.18 ± 0.1ab | 41.6 ± 2.2a |
| CR/SED | 123.1 ± 4.6c | 388.3 ± 20.5a | 1.14 ± 0.09b | 43.1 ± 2.5a |
Values are expressed as means ± SE (ng/ml); n = 15 (IGF-I), 15 (IGFBP-3), and 10 (T3 and T4) for each group. IGF-I, insulin-like growth factor I; IGFBP-3, IGF-binding protein-3; T3, triiodothyronine; T4, thyroxine; AL/SED, ad libitum fed and sedentary; AL/EX, ad libitum fed and provided 24-h access to a voluntary running wheel; PF/EX, pair fed the same amount as the AL/SED group and provided 24-h access to a voluntary running wheel; CR/SED, calorie restricted to body weight match the PF/EX and sedentary groups. Serum IGF-1, IGFBP-3, T3, and T4 levels were determined at the end of the 5-k experiment. One-way ANOVA with Tukey's post hoc test was used for all between-group analyses; means not sharing a common letter are significantly different (P < 0.05).
Fig. 8.
Correlation of serum insulin-like growth factor I (IGF-I) and cell proliferation (experiments 2 and 3). Serum IGF-I levels were determined at the end of the 5-wk experiments and correlated to keratinocyte (A), liver (B), MECs (experiment 2 only; C), and splenic T cell proliferation rates (D). Linear regression analyses were performed, and coefficient of determination values (r2) were determined; n = 40–80 mice/correlation.
DISCUSSION
The experiments presented here demonstrate that reductions in food intake, energy expenditure, percent body fat, or body weight cannot account for the decrease in cell proliferation rates in response to CR (results summarized in Fig. 7). The unifying feature associated with lower cell proliferation rates among all experiments was restriction of food intake below the level that the animals would have selected if given ad libitum access to food. Potential signals mediating these effects were explored, and reductions in serum IGF-I were associated with decreased cell proliferation rates. These data are also consistent with the hypothesis that reduced cell proliferation is a biomarker that correlates with interventions that increase longevity (11).
In all experiments, an ∼20% reduction in caloric intake led to reduced cell proliferation rates in keratinocytes, liver cells, MECs, and splenic T cells compared with ad libitum-fed controls (Figs. 2, 4, and 6). These data are consistent with previous work showing CR-induced reductions in cell proliferation rates in many tissues. Previous work using [3H]thymidine incorporation demonstrated decreased cell proliferation rates in the small intestine, colon, bladder, dermis, mammary tissue, and esophagus in response to 25% CR in Swiss Webster mice (28) and decreased cell proliferation of primary hepatocytes from calorie-restricted F344 rats (38). Work from our laboratory has confirmed these results in several tissues, based on 2H incorporation from heavy water into the deoxyribose moiety of purine deoxyribonucleosides in DNA, and extended the findings to include lower proliferation rates of keratinocytes and splenic T cells in CR mice (25, 41). Taken together with the present findings, it is clear that 20–40% CR leads to a robust decrease in cell proliferation rates across many tissues. However, until now, the physiological adaptations to CR that initiate this reduction in cell proliferation rate had not been systematically evaluated.
The overall goal of this work was to determine the effects of the physiological adaptations associated with CR on cell proliferation rates independent of classical CR. The first adaptation that we examined was food intake. In two separate experiments we found that decreased food intake (experiment 1) and increased food intake (experiment 2), although it maintained the same relative body weight as ad libitum-fed sedentary controls, had no effect on cell proliferation rates.
Although food intake has previously been suggested to mediate the longevity effects of CR (18, 29, 43), our data on cell proliferation rates are consistent with previous work on spontaneous tumor formation. Huffman et al. (26) demonstrated that prostate tumor development was reduced in CR mice housed at 23°C but not in ad libitum-fed mice housed at 28°C despite the fact that the latter group consumed the same amount of food as the CR group at 23°C. In conjunction with the present data, it appears that CR-induced reductions in cell proliferation rates and tumor promotion are not mediated by decreased food intake per se.
The second CR-associated physiological adaptation that we examined was whole body energy expenditure. In two separate experiments we demonstrated that neither decreases (experiment 1) nor increases (experiment 2) in energy expenditure had any effect on cell proliferation rates. It should be noted that energy expenditure was estimated by energy balance, not directly measured through oxygen consumption. In both experiments 1 and 2, there were significant changes in food intake, yet no change in body weight, implying that there were also changes in energy expenditure or food absorption. Neither increased housing temperature nor increased wheel running have been reported to affect food absorption; thus we believe that changes in energy expenditure are most likely to account for body weight maintenance in these settings of altered food intake.
Although reduced energy expenditure has been proposed to mediate the longevity effects of CR, our data on cell proliferation rates are consistent with the current views of energy expenditure and longevity. The “rate of living theory” proposed to explain CR-induced longevity (37) stated that CR animals have reduced metabolic rates and thus longer lifespan. More recent work has suggested that, whereas whole body energy expenditure does decrease in CR rodents (33), metabolic rate is actually increased in CR rodents when corrected for body weight (30, 32, 33). Our data here show that decreases in whole body energy expenditure cannot reproduce the cell proliferation rate-lowering effects of CR. Thus, it appears that decreased energy expenditure, either at the level of the whole body or corrected for lean body weight, does not mediate either the cell proliferation or longevity effects of CR.
The third CR-associated physiological adaptation that we examined was percent body fat. In experiment 2, a reduction in percent body fat had no effect on cell proliferation rates. These findings on cell proliferation rates are consistent with direct work on percent body fat and longevity. Ever since the first suggestion that decreased body fat could mediate the health benefits of CR (3), several studies have tested the role of percent body fat on longevity; however, none have shown a direct role. Bertrand et al. (4) showed no correlation between percent body fat and longevity in ad libitum-fed F344 rats and a positive correlation in those on CR. Testing the hypothesis more directly, Harrison et al. (18) studied lean and ob/ob mice that were either fed ad libitum or calorie restricted. They reported that ob/ob mice on CR lived longer than lean ad libitum-fed mice and as long as lean CR mice despite having a much higher percent body fat than either lean ad libitum-fed or CR mice, again dissociating percent body fat and longevity. Exercise and longevity studies have also shown a dissociation between percent body fat and longevity, where exercised rats have decreased percent body fat yet no increase in maximal lifespan (21). Accordingly, it appears that the effects of CR on cell proliferation rates and longevity are not mediated by reductions in percent body fat.
The final CR-associated physiological adaptation that we examined was body weight. From our first two experiments, it appeared that reduced body weight relative to ad libitum-fed sedentary controls was associated with lower cell proliferation rates. To determine the effect of reduced body weight relative to ad libitum-fed sedentary controls independent of food restriction, we used male mice that were provided free access to food and a running wheel. In experiment 3, AL/EX male mice weighed significantly less (12.4%) than AL/SED control mice (Fig. 5A), and yet there were no differences in cell proliferation rates between these two groups for any cell type analyzed (Fig. 6). We have found previously that 15% CR results in a 13% reduction in body weight and decreased cell proliferation rates relative to ad libitum-fed controls (unpublished data). Therefore, the lack of change in cell proliferation rates observed in AL/EX male mice cannot be attributed to their reduced body weight relative to AL/SED controls. Moreover, in experiment 2, CR/SED mice had lower cell proliferation rates than PF/EX mice in all cell types analyzed despite being matched for body weight (Figs. 3B and 4), suggesting that body weight could not fully account for the lower rates of cell proliferation observed in CR/SED mice. These data suggest that lower body weight, relative to ad libitum-fed sedentary controls, is not sufficient to account for the lower rates of cell proliferation in CR animals.
Our data on cell proliferation rates and body weight are consistent with previous work evaluating longevity in exercised rats. Since the original CR and longevity work by McCay et al. (34), there has been the notion that decreased body weight, relative to ad libitum-fed controls, mediates the longevity effects of CR. Consistent with this hypothesis, several studies have shown that exercise leads to decreased tumor formation and markers of cell proliferation in the context of restricted feeding (44, 45). However, studies investigating the effect of voluntary exercise have shown that whereas exercise dramatically reduces body weight in rats, it has no effect on maximal lifespan (21). Thus, it appears that reduced body weight per se is not sufficient to account for the lower rates of cell proliferation or increased longevity in CR animals. However, exercise in the setting of food restriction may lead to improved health.
In contrast to these physiological adaptations to CR, reductions in circulating IGF-I were associated with decreased cell proliferation rates. In experiment 2, circulating IGF-I levels were lower in both CR/SED and PF/EX mice compared with AL/SED controls (Table 1), and in experiments 2 and 3, IGF-I levels correlated strongly with cell proliferation rates across all mice in all cell types (Fig. 8). These data are consistent with many other studies reporting decreased IGF-I in CR mice (12, 15, 39). Interestingly, many of the genetic mouse models of increased longevity have disruptions in the GH-IGF-I axis with reduced IGF-I concentration or signaling (1, 9, 10, 24, 31). However, the mechanisms connecting IGF-I and longevity have not been established experimentally. One hypothesis is that reduced IGF-I signaling could increase longevity by reducing cell proliferation rates in younger animals, which would both preserve the replicative capacity of cells and inhibit tumor growth as animals age. In future studies, it will be informative to assess cell proliferation rates in models of altered longevity and to identify adaptations to CR that reduce IGF-I signaling.
If reductions in food intake, energy expenditure, percent body fat, and body weight are insufficient to account for the decreased cell proliferation rates and the increased longevity in CR animals, what might be the adaptations that signal or mediate these effects? A unifying feature of the interventions that reduced cell proliferation rates here was that food intake was restricted below levels that would have been selected by the animals if allowed ad libitum access to food. It is not clear how restriction below ad libitum intake could serve as a signal for cellular or biochemical events. However, one possibility is the striking alteration in the pattern of food intake that is observed as an adaptation to CR (6). In contrast to the relatively constant nibbling pattern of ad libitum-fed mice, CR mice consume their total daily allotment of food within several hours (7). We have shown that this pattern of food intake leads to a dramatic change in fatty acid metabolism, with a short, postprandial phase of fatty acid synthesis, followed by a prolonged period of fatty acid oxidation (7). It is possible that this diurnal pattern of repeated fasting may initiate signaling mechanisms that lead to lower rates of cell proliferation and increased longevity. Consistent with this hypothesis, mice on intermittent fasting regimens have reduced rates of cell proliferation and increased maximal lifespan (16, 40) despite no change in overall food intake, percent body fat, or body weight. In future studies, it will be important to investigate mechanisms by which repeated fasting and/or increased fatty acid oxidation might lead to decreased rates of cell proliferation, perhaps through regulation of the GH-IGF-I axis. In addition, it will be interesting to identify adaptations to CR that reduce free IGF-I, perhaps through changes in circulating IGFBP-1, a serum factor not assessed in this study.
Finally, these results are consistent with the hypothesis that reductions in cell proliferation rates could serve as a rapidly responsive biomarker of interventions that extend lifespan. Cell proliferation rates have been suggested to mediate the longevity effects of CR by preserving replicative capacity and reducing tumor promotion (11, 42). Cell proliferation rates are reduced rapidly and persistently in many tissues in response to CR (25, 28, 38) and alternate-day fasting (40), two dietary regimens that extend maximal lifespan. In contrast, several progeroid diseases are associated with increased rates of cell proliferation in early life (6, 13, 17, 27). In the present study, we show that the cell proliferation rate response is specific to the intervention of CR, which has been demonstrated elsewhere to extend maximal lifespan (42). Indeed, there were no changes in cell proliferation rates in response to increased housing temperature and voluntary wheel running, two interventions that mimic physiological adaptations to CR but cannot reproduce its health and longevity effects in isolation (21, 26). We also confirmed the rapid response of cell proliferation rates to CR and identified a correlation between cell proliferation rates and circulating IGF-I. Future work investigating the rates of cell proliferation in other models of longevity will be valuable in assessing its utility as a biomarker of aging.
GRANTS
This research was supported by National Institutes of Health Grant R01-AG-034297-ARRA.
DISCLOSURES
The authors have no conflicts of interest to report.
ACKNOWLEDGMENTS
We acknowledge the technical assistance of Mark Fitch, Simply Florcruz, Jerry Cai, Jon Banh, Ryan Yee, Alicia White, Marcy Dalidd, Lindsay Roberts, and Noreene Shibata.
REFERENCES
- 1. Bartke A, Brown-Borg H. Life extension in the dwarf mouse. Curr Top Dev Biol 63: 189–225, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Bell GE, Stern JS. Evaluation of body composition of young obese and lean Zucker rats. Growth 41: 63–80, 1977 [PubMed] [Google Scholar]
- 3. Berg BN, Simms HS. Nutrition and longevity in the rat. II. Longevity and onset of disease with different levels of food intake. J Nutr 71: 255–263, 1960 [PubMed] [Google Scholar]
- 4. Bertrand HA, Lynd FT, Masoro EJ, Yu BP. Changes in adipose mass and cellularity through the adult life of rats fed ad libitum or a life-prolonging restricted diet. J Gerontol 35: 827–835, 1980 [DOI] [PubMed] [Google Scholar]
- 5. Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science 299: 572–574, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Bridger JM, Kill IR. Aging of Hutchinson-Gilford progeria syndrome fibroblasts is characterised by hyperproliferation and increased apoptosis. Exp Gerontol 39: 717–724, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Bruss MD, Khambatta CF, Ruby MA, Aggarwal I, Hellerstein MK. Calorie restriction increases fatty acid synthesis and whole body fat oxidation rates. Am J Physiol Endocrinol Metab 298: E108–E116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Busch R, Neese RA, Awada M, Hayes GM, Hellerstein MK. Measurement of cell proliferation by heavy water labeling. Nat Protoc 2: 3045–3057, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology 141: 2608–2613, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology 144: 3799–3810, 2003 [DOI] [PubMed] [Google Scholar]
- 11. de Magalhaes JP, Faragher RG. Cell divisions and mammalian aging: integrative biology insights from genes that regulate longevity. Bioessays 30: 567–578, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Dunn SE, Kari FW, French J, Leininger JR, Travlos G, Wilson R, Barrett JC. Dietary restriction reduces insulin-like growth factor I levels, which modulates apoptosis, cell proliferation, and tumor progression in p53-deficient mice. Cancer Res 57: 4667–4672, 1997 [PubMed] [Google Scholar]
- 13. Faragher RG, Kill IR, Hunter JA, Pope FM, Tannock C, Shall S. The gene responsible for Werner syndrome may be a cell division “counting” gene. Proc Natl Acad Sci USA 90: 12030–12034, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fata JE, Mori H, Ewald AJ, Zhang H, Yao E, Werb Z, Bissell MJ. The MAPK(ERK-1,2) pathway integrates distinct and antagonistic signals from TGFalpha and FGF7 in morphogenesis of mouse mammary epithelium. Dev Biol 306: 193–207, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fontana L, Klein S. Aging, adiposity, and calorie restriction. JAMA 297: 986–994, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider N. Effects of intermittent feeding upon body weight and lifespan in inbred mice: interaction of genotype and age. Mech Ageing Dev 55: 69–87, 1990 [DOI] [PubMed] [Google Scholar]
- 17. Halaschek-Wiener J, Brooks-Wilson A. Progeria of stem cells: stem cell exhaustion in Hutchinson-Gilford progeria syndrome. J Gerontol A Biol Sci Med Sci 62: 3–8, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Harrison DE, Archer JR, Astle CM. Effects of food restriction on aging: separation of food intake and adiposity. Proc Natl Acad Sci USA 81: 1835–1838, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460: 392–395, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Holehan AM, Merry BJ. The control of puberty in the dietary restricted female rat. Mech Ageing Dev 32: 179–191, 1985 [DOI] [PubMed] [Google Scholar]
- 21. Holloszy JO. Exercise and food restriction in rats. J Nutr 122: 774–777, 1992 [DOI] [PubMed] [Google Scholar]
- 22. Holloszy JO. Exercise increases average longevity of female rats despite increased food intake and no growth retardation. J Gerontol 48: B97–B100, 1993 [DOI] [PubMed] [Google Scholar]
- 23. Holloszy JO, Smith EK, Vining M, Adams S. Effect of voluntary exercise on longevity of rats. J Appl Physiol 59: 826–831, 1985 [DOI] [PubMed] [Google Scholar]
- 24. Holzenberger M, Dupont J, Ducos B, Leneuve P, Géloën A, Even PC, Cervera P, Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature 421: 182–187, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Hsieh EA, Chai CM, Hellerstein MK. Effects of caloric restriction on cell proliferation in several tissues in mice: role of intermittent feeding. Am J Physiol Endocrinol Metab 288: E965–E972, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Huffman DM, Johnson MS, Watts A, Elgavish A, Eltoum IA, Nagy TR. Cancer progression in the transgenic adenocarcinoma of mouse prostate mouse is related to energy balance, body mass, and body composition, but not food intake. Cancer Res 67: 417–424, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Kill IR, Faragher RG, Lawrence K, Shall S. The expression of proliferation-dependent antigens during the lifespan of normal and progeroid human fibroblasts in culture. J Cell Sci 107: 571–579, 1994 [DOI] [PubMed] [Google Scholar]
- 28. Lok E, Nera EA, Iverson F, Scott F, So Y, Clayson DB. Dietary restriction, cell proliferation and carcinogenesis: a preliminary study. Cancer Lett 38: 249–255, 1988 [DOI] [PubMed] [Google Scholar]
- 29. Masoro EJ. Caloric Restriction: a Key to Understanding and Modulating Aging. Amsterdam: Elsevier, 2002 [Google Scholar]
- 30. Masoro EJ, Yu BP, Bertrand HA. Action of food restriction in delaying the aging process. Proc Natl Acad Sci USA 79: 4239–4241, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Masuda H, Chikuda H, Suga T, Kawaguchi H, Kuro-o M. Regulation of multiple ageing-like phenotypes by inducible klotho gene expression in klotho mutant mice. Mech Ageing Dev 126: 1274–1283, 2005 [DOI] [PubMed] [Google Scholar]
- 32. McCarter R, Masoro EJ, Yu BP. Does food restriction retard aging by reducing the metabolic rate? Am J Physiol Endocrinol Metab 248: E488–E490, 1985 [DOI] [PubMed] [Google Scholar]
- 33. McCarter RJ, Palmer J. Energy metabolism and aging: a lifelong study of Fischer 344 rats. Am J Physiol Endocrinol Metab 263: E448–E452, 1992 [DOI] [PubMed] [Google Scholar]
- 34. McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. 1935. Nutrition 5: 155–171; discussion 172, 1989 [PubMed] [Google Scholar]
- 35. Miller RA, Harper JM, Galecki A, Burke DT. Big mice die young: early life body weight predicts longevity in genetically heterogeneous mice. Aging Cell 1: 22–29, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 429: 771–776, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sacher G. Handbook of the Biology of Aging. New York: Van Nostrand Reinhold, 1977 [Google Scholar]
- 38. Shaddock JG, Chou MW, Casciano DA. Effects of age and caloric restriction on cell proliferation in hepatocyte cultures from control and hepatectomized Fischer 344 rats. Mutagenesis 11: 281–284, 1996 [DOI] [PubMed] [Google Scholar]
- 39. Sonntag WE, Lynch CD, Cefalu WT, Ingram RL, Bennett SA, Thornton PL, Khan AS. Pleiotropic effects of growth hormone and insulin-like growth factor (IGF)-1 on biological aging: inferences from moderate caloric-restricted animals. J Gerontol A Biol Sci Med Sci 54: B521–B538, 1999 [DOI] [PubMed] [Google Scholar]
- 40. Varady KA, Roohk DJ, Hellerstein MK. Dose effects of modified alternate-day fasting regimens on in vivo cell proliferation and plasma insulin-like growth factor-1 in mice. J Appl Physiol 103: 547–551, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Varady KA, Roohk DJ, McEvoy-Hein BK, Gaylinn BD, Thorner MO, Hellerstein MK. Modified alternate-day fasting regimens reduce cell proliferation rates to a similar extent as daily calorie restriction in mice. FASEB J 22: 2090–2096, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weindruch R. The Retardation of Aging and Disease by Dietary Restriction. Springfield, IL: C. C. Thomas, 1988 [Google Scholar]
- 43. Yu BP, Masoro EJ, McMahan CA. Nutritional influences on aging of Fischer 344 rats: I. Physical, metabolic, and longevity characteristics. J Gerontol 40: 657–670, 1985 [DOI] [PubMed] [Google Scholar]
- 44. Zhu Z, Jiang W, McGinley JN, Thompson HJ. Energetics and mammary carcinogenesis: effects of moderate-intensity running and energy intake on cellular processes and molecular mechanisms in rats. J Appl Physiol 106: 911–918, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhu Z, Jiang W, Sells JL, Neil ES, McGinley JN, Thompson HJ. Effect of nonmotorized wheel running on mammary carcinogenesis: circulating biomarkers, cellular processes, and molecular mechanisms in rats. Cancer Epidemiol Biomarkers Prev 17: 1920–1929, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]