Abstract
Impaired suppression of glucagon levels after oral glucose or meal ingestion is a hallmark of type 2 diabetes. Whether hyperglucagonemia after a β-cell loss results from a functional upregulation of glucagon secretion or an increase in α-cell mass is yet unclear. CD-1 mice were treated with streptozotocin (STZ) or saline. Pancreatic tissue was collected after 14, 21, and 28 days and examined for α- and β-cell mass and turnover. Intraperitoneal (ip) glucose tolerance tests were performed at day 28 as well as after 12 days of subcutaneous insulin treatment, and glucose, insulin, and glucagon levels were determined. STZ treatment led to fasting and post-challenge hyperglycemia (P < 0.001 vs. controls). Insulin levels increased after glucose injection in controls (P < 0.001) but were unchanged in STZ mice (P = 0.36). Intraperitoneal glucose elicited a 63.1 ± 4.1% glucagon suppression in control mice (P < 0.001), whereas the glucagon suppression was absent in STZ mice (P = 0.47). Insulin treatment failed to normalize glucagon levels. There was a significant inverse association between insulin and glucagon levels after ip glucose ingestion (r2 = 0.99). β-Cell mass was reduced by ∼75% in STZ mice compared with controls (P < 0.001), whereas α-cell mass remained unchanged (P > 0.05). α-Cell apoptosis (TUNEL) and replication (Ki67) were rather infrequently noticed, with no significant differences between the groups. These studies underline the importance of endogenous insulin for the glucose-induced suppression of glucagon secretion and suggest that the insufficient decline in glucagon levels after glucose administration in diabetes is primarily due to a functional loss of intraislet inhibition of α-cell function rather than an expansion of α-cell mass.
Keywords: type 2 diabetes, hyperglucagonemia, streptozotocin, intraislet insulin
both type 1 and type 2 diabetes are characterized by a reduction in β-cell mass leading to impaired insulin secretion (8, 16). In addition, an insufficient suppression of glucagon secretion after oral glucose or meal ingestion as well as fasting hyperglucagonemia have been described in patients with diabetes (18, 26). These elevated glucagon concentrations have been associated with an increased rate of hepatic gluconeogenesis and glycogen breakdown, thereby largely contributing to the hyperglycemia in patients with diabetes (9, 31).
The mechanisms and underlying causes for the elevations in glucagon levels in patients with diabetes are less well understood. In fact, some studies have suggested an independent α-cell defect to predispose the development of type 2 diabetes (2). However, the fact that normal glucose-tolerant individuals at a high genetic risk for type 2 diabetes, such as first-degree relatives, exhibit completely normal glucagon levels at fasting and after oral or intravenous glucose administration argues against this hypothesis (20). The alternative hypothesis is that the elevations of glucagon levels in diabetic patients develop as a consequence of other metabolic abnormalities in such individuals, such as impaired β-cell mass and function (13, 29). Consistent with the latter explanation, a rise in glucagon levels has been observed in different studies after an experimental reduction of β-cell mass, using either streptozotocin (STZ) or alloxan (11, 22). Futhermore, the direction of the blood flow within the islet is typically centripetal, entering the β-cell enriched core region before reaching the α-cells in the islet periphery (33), and different experimental studies have described an inhibitory effect of insulin on glucagon release (13). Collectively, these studies suggest that the increase in glucagon concentrations after a selective destruction of β-cells is driven by the loss of intraislet inhibition of α-cells.
However, although the inverse association between impaired β-cell mass and hyperglucagonemia seems to be well established, it is less clear whether this increase in glucagon levels is primarily due to an expansion of α-cell mass and turnover or rather due to a functional upregulation of glucagon release independent of any changes in α-cell number. In line with the former explanation, some studies have reported an expansion of α-cell mass in patients with type 2 diabetes (4, 38), but this has not been confirmed by all studies (16, 28). The later hypothesis of a functional upregulation of glucagon secretion would be supported by the observation of a close interaction of pulsatile insulin and glucagon secretion in Göttingen mini-pigs (22).
The present studies were therefore designed to address whether a selective loss of β-cells in mice results in a loss of glucose-induced glucagon suppression and, if so, whether this was due to an increase in α-cell mass and turnover or a purely functional up-regulation of glucagon release.
MATERIALS AND METHODS
Animal model.
CD-1 mice (6 wk of age, 20–25 g) were purchased from Charles River (Sulzfeld, Germany). Mice were treated by ip injections of either physiological saline (0.2 ml) or STZ (40 mg/kg body wt) dissolved in fresh citrate buffer (100 mM citrate, pH 4,5) on five consecutive days. Five control animals were killed by bleeding, without any injections, on day 0. Blood glucose determination (Clinical Analyzer; Nova Biomedical, Rödermark, Germany) was performed on blood samples taken from tail vein at day 0 before any injection and subsequently 14, 21, and 28 days after the first injection, respectively. On these days, groups of mice (as stated) were euthanized for morphological examination of the pancreas. Animal experiments were approved by the Landesamt für Verbraucherschutz Nordrhein-Westfalen (No. 50.8735.1 Nr. 105/5).
Pancreatic tissue processing.
After bleeding, the pancreases of mice were removed, sectioned, weighed, and analyzed in the head, body, and tail regions. Tissue fixation was performed with formalin (4%) overnight. Tissue sections were dehydrated (Sakura Tissue-TEK VIP, Staufen, Germany) and embedded in paraffin for subsequent analysis. Sections (5 μm) were cut from these paraffin blocks and immunohistochemically stained for insulin and glucagon. Briefly, the sections were deparaffinized using Xylol twice for 10 min followed by alcohol thrice for 5 min, and water was purified by reversed osmosis for another 5 min. The sections were permeabilized by heating in the microwave in antigen unmasking solution, pH 6, following cool down at room temperature and blocking in PBS containing 2% BSA for 1 h at room temperature. For insulin staining, the sections were incubated with the primary guinea pig antibody against insulin (1:400 Guinea Pig Anti-Swine, diluted in PBS with 2% BSA; Dako, Carpinteria, CA), and for glucagon staining with the primary mouse antibody against glucagon (1:200 mouse anti-glucagon monoclonal antibody, diluted in PBS with 2% BSA; Thermo Scientific, Rockford, IL) overnight at 4°C. Insulin and glucagon were detected by use of the streptavidin-alkaline phosphatase method (Dako) according to the manufacturer's protocol.
Replication was determined by immunohistochemistry for Ki67. First, tissue sections were deparaffinized, permeabilized, and blocked as stated and then incubated with the primary antibody against Ki67 (1:25 monoclonal rat anti-mouse Ki67 clone TEC-3, diluted in PBS with 2% BSA; Dako) over night at 4°C. Ki67 was detected by means of Cy3-conjugated secondary donkey anti-rat antibody (1:100, diluted in PBS with 2% BSA; Jackson ImmunoResearch Europe, Newmarket, UK).
Apoptosis was determined using the TUNEL method (In Situ Cell Death Detection Kit, TMR red; Roche, Mannheim, Germany) according to the manufacturer's protocol.
All tissue sections stained for Ki67 and TUNEL were simultaneously stained for glucagon. For these purposes, the tissue sections were incubated with the anti-glucagon antibody (monoclonal mouse, 1:200, diluted in PBS with 2% BSA; Thermo Scientific) for 30 min at 37°C. Glucagon was detected using Cy2-conjugated goat anti-mouse secondary antibody (1:100, diluted in PBS with 2% BSA; Jackson ImmunoResearch Europe). Finally, the nuclei were stained with DAPI (blue) for 30 min at room temperature. After two 5-min incubations with PBS, the sections were mounted with DakoFluorescence Mounting Medium.
Morphometric analysis.
For the determinations of pancreatic β-cell (BCM) and α-cell mass (ACM) at days 0, 14, 21, and 28, pancreatic sections each from the head, body, and tail regions stained for insulin or glucagon were imaged at ×100 magnification (×10 objective) using a Zeiss Axioplan microscope equipped with a motorized stage. A tile image of the entire tissue section was generated using the Mosaix tool of Zeiss Axiovision version 4.5 software. The fractional areas of the pancreas stained positive for insulin or glucagon were digitally quantified using a color-based threshold using Axiovision software as previously described (25). To minimize sampling bias, α-cell area was determined in three sections each from the pancreatic head, body, and tail. The mean values for fractional α- and β-cell area in the pancreatic head, body, and tail were used to compute ACM and BCM. Thus, the BCM and ACM of each pancreas were determined according to the following formulas: 1) BCM per pancreas (mg) = β-cell fractional area × pancreatic weight (mg); 2) ACM per pancreas (mg) = α-cell fractional area × pancreatic weight (mg).
To determine the frequencies of apoptosis and replication in α-cells, the total number of α-cells positive for TUNEL or Ki67 were determined in one pancreatic section from the head, body, and tail region and expressed in relation to the total number of α-cells. All islets present in the section were included in this analysis.
Intraperitoneal glucose tolerance test.
At day 28 and after 12 days of insulin treatment, intraperitoneal glucose tolerance tests (IPGTTs) were performed in fasting (12 h), unanesthetized mice. After baseline blood sampling (0 min), animals received an injection of glucose (2 g/kg body wt ip), with glucose, insulin, and glucagon concentrations measured at t = 30 min after glucose administration. Blood samples were taken from the tail vein, and plasma concentrations of glucose, insulin, and glucagon were determined.
Insulin treatment.
Exogenous insulin treatment was initiated 28 days after STZ administration over 12 consecutive days by subcutaneous implantation of the sustained-release insulin implants Linbit (Linshin Canada) under the mid-dorsal skin,according to the manufacturer's protocol. The insulin release rate was ∼0,05 U/day.
Assay procedures.
Glucose concentrations were determined by means of the glucose oxidase method with a clinical analyzer (Nova Biomedical, Rödermark, Germany). Insulin was determined using an ultrasensitive mouse ELISA (Mercodia, Uppsala, Sweden), according to the manufacturer's protocol. The determination of insulin was performed in duplicate with 5 μl of sample volume. The detection limit of the assay was ≤0.188 μg/l (32,712 pmol/l). Glucagon was measured using an ELISA (Alpco, Salem, OR) according to the manufacturer's protocol. The determination of glucagon was performed in duplicate with a 50-μl sample volume. The assay range was 50–10,000 pg/ml, the intra-assay coefficients of variation were 3.3–5.1%, and the interassay coefficients of variation were 7.3–18.9%.
Statistics.
Data are expressed as means ± SE, and groups of data were compared using an unpaired Student's t-test. The relationship between insulin and glucagon during an IPGTT was analyzed by nonlinear regression analysis using the software GraphPad Prism, version 3.0. A P value of <0.05 was considered to denote a significant difference.
RESULTS
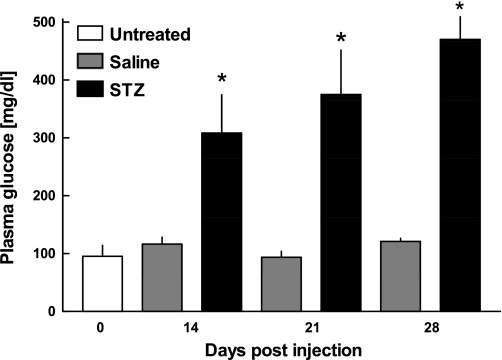
Repeated intraperitoneal administration of low-dose STZ led to the development of severe fasting hyperglycemia at t = 14, 21, and 28 days after treatment (P < 0.001), whereas the saline-treated control mice remained normoglycemic throughout the observation period (Fig. 1).
Fig. 1.
Plasma glucose levels of untreated mice (open bars; n = 5), saline-treated mice (gray bars; n = 6 at day 14, n = 6 at day 21, and n = 5 at day 28), and STZ-treated diabetic mice (filled bars; n = 6 at day 14, n = 4 at day 21, and n = 11 at day 28) at different time points after STZ or saline injection. Glucose values exceeding the upper detection limit (600 mg/dl) were considered equal to 600 mg/dl. Data are presented as means ± SE. *P < 0.001 vs. untreated mice.
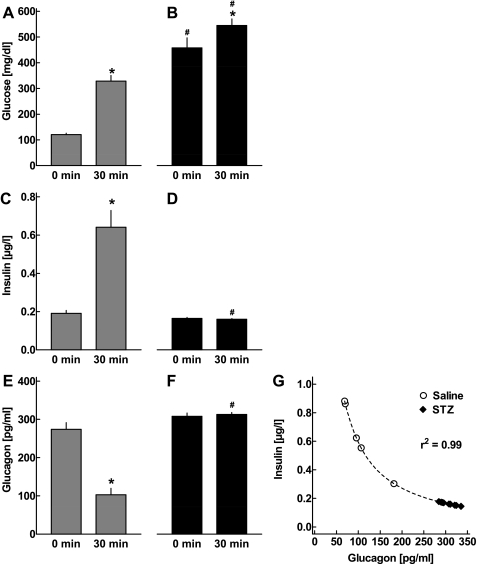
IPGTTs were performed 28 days after the first injection of STZ or saline. In control mice (n = 6), glucose concentrations increased significantly after the glucose challenge (Fig. 2A; P < 0.001), and this was accompanied by an ∼3.4-fold increase in insulin concentrations (Fig. 2C; P < 0.001). Glucagon concentrations were suppressed by 63.1 ± 4.1% at t = 30 min after the glucose challenge in the saline-treated animals (Fig. 2E; P < 0.001). Both fasting and post-challenge glucose concentrations were significantly higher in the STZ-diabetic mice (n = 13) compared with controls (Fig. 2B; P < 0.001). Insulin levels were almost unchanged by the glucose load in these animals (P = 0.36 vs. basal levels; Fig. 2D). Importantly, the glucose-induced suppression of glucagon was completely absent in the STZ-diabetic mice, with no detectable change in glucagon concentrations vs. fasting levels (plus 3.2 ± 2.0% vs. basal levels, P = 0.47). The glucagon levels measured 30 min after ip glucose administration in the STZ animals were significantly higher than in saline-treated controls (P < 0.001; Fig. 2F).
Fig. 2.
Plasma concentrations of glucose (A and B), insulin (C and D), and glucagon (E and F) in CD-1 mice 28 days after the first injection of saline (gray bars: A, C, E; n = 6) or STZ (filled bars: B, D, F; n = 13). Respective concentrations were determined in the basal state and 30 min following ip glucose injection. Glucose values exceeding the upper detection limit (600 mg/dl) were considered equal to 600 mg/dl. Data are presented as means ± SE. *P < 0.001 vs. basal levels; #P < 0.001 vs. respective concentrations in saline-treated mice. G: correlation between insulin and glucagon levels 30 min after ip glucose administration in CD-1 mice 28 days after first injection of either saline or STZ. Dashed line depicts the regression curve derived from nonlinear regression analysis (r2 = 0.99).
There was a significant inverse association between insulin and glucagon levels 30 min after ip glucose ingestion (Fig. 2G; r2 = 0.99), consistent with an inhibition of glucagon release by insulin.
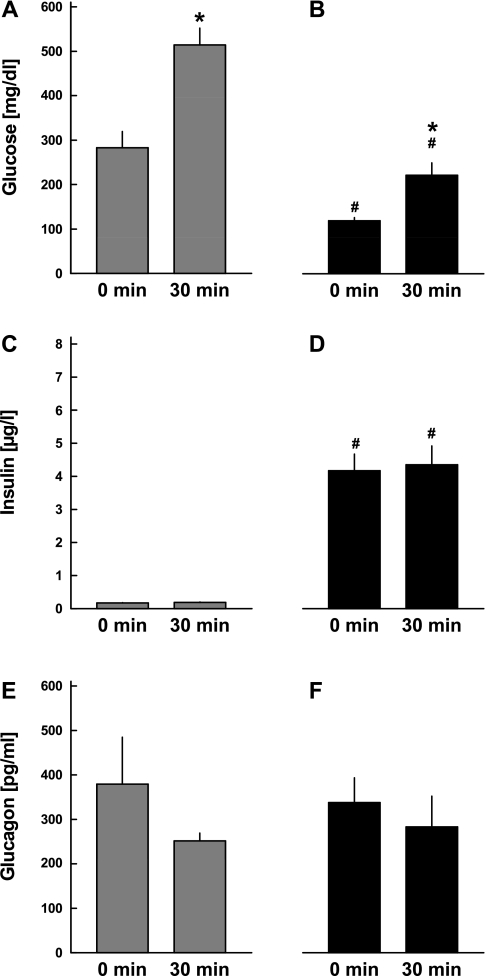
To determine whether the relationship between β-cells and α-cells was mediated by the release of insulin into the systemic circulation or the local effects of insulin and potentially other β-cell secretory products within the individual islets, four additional mice were treated with an osmotic insulin minipump over 12 days with the aim of restoring normoglycemia. Insulin treatment lowered blood glucose concentrations from 283.3 ± 36.3 to 119.3 ± 7.1 mg/dl (P < 0.01; see Fig. 3, A and B). At the same time, fasting insulin levels increased from 0.17 ± 0.01 to 4.17 ± 0.50 μg/l (P < 0.001; Fig. 3, C and D). Fasting glucagon concentrations were not different between the experiments before and after exogenous insulin administration (Fig. 3, E and F). Following ip glucose administration, blood glucose levels increased before and after the insulin treatment period (P < 0.01). Insulin concentrations were unchanged before and after insulin treatment in response to ip glucose administration (Fig. 3, C and D). The glucose-induced decline in glucagon levels was absent both before and after insulin treatment.
Fig. 3.
Plasma concentrations of glucose (A and B), insulin (C and D), and glucagon (E and F) in CD-1 mice treated with STZ. ip glucose tolerance tests were performed before (gray bars: A, C, E) and after (filled bars: B, D, F) 12 days of subcutaneous insulin treatment, and respective concentrations were determined in the basal state and 30 min following ip glucose injection. Glucose values exceeding the upper detection limit (600 mg/dl) were considered equal to 600 mg/dl. Data are presented as means ± SE. *P < 0.001 vs. basal levels; #P < 0.001 vs. respective concentrations before insulin treatment.
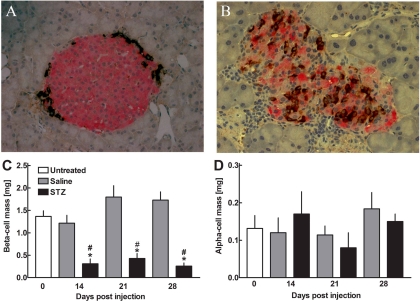
Pancreatic α- and β-cell area and pancreatic weight were determined in untreated animals, in saline-treated animals, and in STZ-treated animals 14, 21, and 28 days after the first injection. Fractional β-cell area was 1.0 ± 0.19% in untreated animals (n = 5) and 0.58 ± 0.11% (n = 6), 0.94 ± 0.18% (n = 6), and 0.65 ± 0.06% (n = 5) after 14, 21, and 28 days in saline-treated animals, respectively. In STZ-treated animals, the fractional β-cell areas amounted to 0.12 ± 0.3% (n = 6), 0.17 ± 0.03% (n = 4), and 0.081 ± 0.02% (n = 11) after 14, 21, and 28 days, respectively (P < 0.01 vs. saline-treated animals). Thus, β-cell mass was 1.37 ± 0.13 mg in untreated animals and 1.22 ± 0.18, 1.80 ± 0.25, and 1.73 ± 0.19 mg after 14, 21, and 28 days in saline-treated animals and 0.31 ± 0.11, 0.43 ± 0.11, and 0.26 ± 0.072 mg in STZ-treated animals, respectively (P < 0.01 vs. control animals; Fig. 4C).
Fig. 4.
Top: Histological sections of the pancreas in a saline-treated mouse (A) and an STZ-treated mouse (B) 28 days after first treatment stained for insulin (red) and glucagon (brown). Images were acquired at ×400 magnification. Bottom: pancreatic β-cell mass (C) and α-cell mass (D) in STZ-treated mice (filled bars: n = 6 at day 14, n = 4 at day 21, and n = 11 at day 28), saline-treated mice (gray bars; n = 6 at day 14, n = 6 at day 21, and n = 5 at day 28) and untreated mice (open bars; n = 5). Data are presented as means ± SE. *P < 0.01 vs. untreated mice; #P < 0.01 vs. saline-treated mice.
Fractional α-cell area was 0.08 ± 0.01% in untreated animals (n = 5) and 0.07 ± 0.02% (n = 6), 0.08 ± 0.01% (n = 6), and 0.05 ± 0.01% (n = 5) after 14, 21, and 28 days in saline-treated animals, respectively. In STZ-treated animals, the fractional α-cell area amounted to 0.05 ± 0.01% (n = 6), 0.04 ± 0.01% (n = 4), and 0.05 ± 0.01% (n = 11) after 14, 21, and 28 days, respectively. α-Cell mass was 0.13 ± 0.03 mg in untreated animals and 0.16 ± 0.06, 0.08 ± 0.04, and 0.15 ± 0.02 mg after 14, 21, and 28 days in saline-treated animals and 0.12 ± 0.04, 0.11 ± 0.02, and 0.18 ± 0.04 mg in STZ-treated animals, respectively (P = NS vs. control animals; Fig. 4D).
α-Cell apoptosis was rather infrequently noticed in either STZ- or saline-treated animals (Fig. 5, A and B). There were no differences in the frequencies of α-cell apoptosis between the groups (0.12 ± 0.073 and 0.26 ± 0.19%, respectively; P = 0.28). Likewise, α-cell replication, as measured by the expression frequencies of Ki67, was rarely detected in both groups (Fig. 5, C and D), and the abundance of α-cell replication was not different between STZ- and saline-treated animals (0.049 ± 0.049 and 0.072 ± 0.051%, P = 0.74).
Fig. 5.
A and B: pancreatic sections from a mouse 14 days after STZ administration (A) and a saline-treated mouse (B) stained for glucagon (green), apoptosis (TUNEL; red), and nuclei (DAPI; blue). C and D: pancreatic sections from a mouse 14 days after STZ administration (C) and a saline-treated mouse (D) stained for glucagon (green), replication (Ki67; red), and nuclei (DAPI; blue). Images were acquired at ×400 magnification.
DISCUSSION
The present study was designed to address whether the impaired suppression of glucagon release that occurs after a selective β-cell loss is primarily due to a diminished functional inhibition of α-cell secretion by insulin or rather due to an increase in α-cell mass and proliferation. We report that the glucose-induced decline in glucagon levels in mice is almost completely lost after an ∼75% loss of β-cell mass, and this defect cannot be reversed by exogenous insulin administration. The increased glucagon levels found in the STZ-treated mice were not associated with changes in either the mass or the turnover (replication and apoptosis) of α-cells. A tight inverse relationship between the circulating insulin levels and the respective glucagon concentrations after glucose administration suggests a functional interaction between α- and β-cells.
The present data lend strong support to the hypothesis that the hyperglucagonemia induced by a β-cell deficit is caused by the loss of intraislet inhibition of glucagon release. These findings are consistent with previous studies reporting elevations in glucagon levels after β-cell reduction in pigs (22), baboons (11), and humans (34). However, they extend these studies by demonstrating that the increase in glucagon concentrations is primarily driven by a functional upregulation of glucagon secretion rather than an adaptive increase in α-cell mass. Furthermore, the fact that exogenous insulin administration fails to normalize glucagon levels suggests that the local release of insulin or other β-cell secretory products rather than systemic insulin concentrations drive the suppression of glucagon secretion.
Hyperglucagonemia is a common finding in patients with both type 1 and type 2 diabetes (18, 37), but the reasons underlying this defect are less well understood. One hypothesis that has been expounded is that the increased glucagon levels in such patients are secondary to an increase in α-cell mass. Thus, Yoon et al. (38) have recently described a selective α-cell expansion in the pancreas of Korean patients with type 2 diabetes. However, because a large proportion of the patients included in that study had suffered from pancreatic carcinomas and other diseases known to independently alter pancreatic pathology, it is difficult to interpret those findings. Other studies in human pancreases from patients with type 2 diabetes have reported conflicting results with regard to the extent of α-cells in patients with diabetes (4, 16, 28). But overall there is clearly a paucity of well-controlled studies in this area, owing to the limited accessibility of human pancreatic specimens for histological analyses. However, a mere expansion in the number of α-cells appears rather implausible to explain the hyperglucagonemia in patients with diabetes, because the glucagon secretion rate in the fasting state and after glucose administration is far below the maximum secretory capacity of the α-cells and therefore likely independent of the total number of α-cells. Thus, although the maximum glucagon response to hypoglycemia or arginine administration likely depends on the total extent of α-cells in the pancreas, it is less likely that the suppression of glucagon levels after glucose administration is dependent on the respective α-cell mass as well.
In light of the paucity of well-controlled studies on α-cell mass in pancreases from patients with diabetes, we have tried to address the potential causes of the diabetic hyperglucagonemia in a rodent model. Although this approach clearly bears limitations, the metabolic defects induced in these mice were largely reminiscent of the alterations in diabetic patients. Thus, there is good evidence from autopsy studies that patients with type 2 diabetes exhibit a significant deficit in β-cell mass (∼30–65%) (3, 5, 16), they are typically characterized by a marked reduction of early insulin secretory response after intravenous glucose administration (19), and they typically fail to suppress their glucagon levels in response to glucose administration (26). In the present study, low-dose STZ treatment led to a selective ∼75% reduction of β-cell mass, a loss of early insulin secretion after intraperitoneal glucose injection, and a deficient suppression of glucagon secretion. These abnormalities are partly reminiscent of those in patients with type 2 diabetes, but clearly great caution needs to be taken regarding the translation of such findings to the situation in humans with diabetes Nevertheless, the present findings in mice are consistent with the hypothesis that a loss of insulin-mediated α-cell suppression contributes to the abnormal glucagon secretion in diabetic patients, although additional factors, such as the glucagonotropic actions of gut hormones, may still be involved (2, 17).
Even though the present study gives rise to the suggestion that the impaired glucagon suppression after glucose administration in β-cell deficient states is primarily due to functional reasons, the exact mediators of this interaction are still elusive (12). In fact, while the most obvious mediator of this relationship appears to be insulin itself, other secretory products of the β-cell, such as islet amyloid polypeptide (IAPP), or zinc might also be involved. Indeed, recent studies in rat β-cells have suggested a predominant role of zinc in the regulation of glucagon release (7, 36). Against this, Ravier and Rutter (30) argued that in mouse islets insulin and glucose, but not zinc, control the secretion of glucagon from α-cells. The present studies are consistent with an important role of β-cell secretory products other than insulin by showing that exogenous insulin treatment fails to normalize the glucose-induced suppression of glucagon secretion.
Another interesting finding of this study is the observation that, although ∼25% of the β-cells had resisted the low-dose STZ treatment, the functional response of insulin secretion to intraperitoneal glucose administration was completely absent in these animals. Interestingly, the endogenous insulin secretory response to glucose could be partly improved by exogenous insulin administration. This suggests that the functional impairment in insulin release even exceeds the actual extent of β-cell loss, possibly due to the detrimental effects of glucose toxicity. In line with this, the acute insulin response to intravenous glucose administration in hyperglycemic patients with newly-onset type 1 diabetes as well as in patients with type 2 diabetes is often completely lost despite a residual β-cell mass in the order of 20–30% and ∼50%, respectively (14, 23).
Even though this and other studies underline the importance of insulin for the control of glucagon release, it needs to be emphasized that under normal conditions the secretion of α-cells is regulated by numerous factors, including the direct actions of circulating glucose and amino acids (10, 15), the paracrine effects of somatostatin (18), the sympathetic nervous system (35), and other gut hormones such as glucagon-like peptide-1 (GLP-1) (27), GLP-2 (24), and gastric inhibitory polypeptide (21). Although changes in insulin secretion might be involved in the actions of some of these factors on α-cell function, they clearly cannot account for the effects of all modulators of insulin release.
The present findings in a rodent model of diabetes may have some relevance for the pharmacotherapy of diabetes. In fact, given the functional relationship between insulin and glucagon secretion, and considering the significant impairments in β-cell mass and function in patients with type 2 diabetes, the implication would be that therapeutic approaches aim to restore β-cell function, and potentially mass should also partly reverse the abnormalities in glucagon secretion in such patients. In line with such reasoning, a reduction in glucagon levels has been noted with therapies aiming to increase endogenous insulin release, such as sulfonylureas or glinides (6, 32). Furthermore, the GLP-1-based drugs, have been associated with a pronounced reduction in glucagon levels (1, 27). Enhancing β-cell function may therefore not only have immediate effects on insulin levels, but also secondarily lower the release of glucagon from pancreatic α-cells.
In conclusion, the present studies underline the importance of endogenous β-cell function for the suppression of glucagon secretion after glucose administration and suggest that the insufficient decline in glucagon levels after glucose administration is primarily due to a functional loss of intraislet inhibition of α-cell function rather than an expansion of α-cell mass. Therapeutic approaches aiming to increase β-cell function in patients with diabetes are therefore likely to result in secondary improvements in glucagon secretion as well.
GRANTS
These studies were supported by grants from the Deutsche Forschungsgemeinschaft (Me 2096/5-1 to J. J. Meier and SCHN 702/2-1 to S. Schneider) the Beta Cell-Biology Consortium of the National Institute of Diabetes and Digestive and Kidney Diseases (DK-072473 to S. Schneider).
DISCLOSURES
No conflicts of interest, financial or otherwise, are reported by the authors.
ACKNOWLEDGMENTS
The excellent technical assistance of Gudrun Müller is gratefully acknowledged.
REFERENCES
- 1. Ahren B, Landin-Olsson M, Jansson PA, Svensson M, Holmes D, Schweizer A. Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab 89: 2078–2084, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Burcelin R, Knauf C, Cani PD. Pancreatic α-cell dysfunction in diabetes. Diabetes Metab 34: S49–S55, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 52: 102–110, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Clark A, Wells CA, Buley ID, Cruickshank JK, Vanhegan RI, Matthews DR, Cooper GJ, Holman RR, Turner RC. Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: quantitative changes in the pancreas in type 2 diabetes. Diabetes Res 9: 151–159, 1988 [PubMed] [Google Scholar]
- 5. Clark A, Wells CA, Buley ID, Cruickshank JK, Vanhegan RI, Matthews DR, Cooper GJ, Holman RR, Turner RC. Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: quantitative changes in the pancreas in type 2 diabetes. Diabetes Res 9: 151–159, 1988 [PubMed] [Google Scholar]
- 6. Efendic S, Enzmann F, Nylen A, Uvnas-Wallensten K, Luft R. Sulphonylurea (glibenclamide) enhances somatostatin and inhibits glucagon release induced by arginine. Acta Physiol Scand 108: 231–233, 1980 [DOI] [PubMed] [Google Scholar]
- 7. Franklin I, Gromada J, Gjinovci A, Theander S, Wollheim CB. β-Cell secretory products activate α-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes 54: 1808–1815, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes 14: 619–633, 1965 [DOI] [PubMed] [Google Scholar]
- 9. Gerich JE. Abnormal glucagon secretion in type 2 (noninsulin-dependent) diabetes mellitus: causes and consequences. In: Diabetes Mellitus: Pathophysiology and Therapy, edited by Creutzfeldt W, Lef̀ ebvre P. Berlin, Heidelberg: Springer Verlag, 1989, p. 127–133 [Google Scholar]
- 10. Gerich JE, Tsalikian E, Lorenzi M, Schneider V, Bohannon NV, Gustafson G, Karam JH. Normalization of fasting hyperglucagonemia and excessive glucagon responses to intravenous arginine in human diabetes mellitus by prolonged infusion of insulin. J Clin Endocrinol Metab 41: 1178–1180, 1975 [DOI] [PubMed] [Google Scholar]
- 11. Goodner CJ, Koerker DJ, Weigle DS, McCulloch DK. Decreased insulin- and glucagon-pulse amplitude accompanying β-cell deficiency induced by streptozocin in baboons. Diabetes 38: 925–931, 1989 [DOI] [PubMed] [Google Scholar]
- 12. Gromada J, Franklin I, Wollheim CB. α-Cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev 28: 84–116 Epub 2007 Jan 2016, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Hope KM, Tran PO, Zhou H, Oseid E, Leroy E, Robertson RP. Regulation of α-cell function by the β-cell in isolated human and rat islets deprived of glucose: the “switch-off” hypothesis. Diabetes 53: 1488–1495, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Hramiak IM, Dupre J, Finegood DT. Determinants of clinical remission in recent-onset IDDM. Diabetes Care 16: 125–132, 1993 [DOI] [PubMed] [Google Scholar]
- 15. Ipp E, Dobbs RE, Arimura A, Vale W, Harris V, Unger RH. Release of immunoreactive somatostatin from the pancreas in response to glucose, amino acids, pancreozymin-cholecystokinin, and tolbutamide. J Clin Invest 60: 760–765, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kloppel G, Drenck CR, Oberholzer M, Heitz PU. Morphometric evidence for a striking B-cell reduction at the clinical onset of type 1 diabetes. Virchows Arch A Pathol Anat Histopathol 403: 441–452, 1984 [DOI] [PubMed] [Google Scholar]
- 17. Knop FK, Vilsboll T, Madsbad S, Holst JJ, Krarup T. Inappropriate suppression of glucagon during OGTT but not during isoglycaemic i.v. glucose infusion contributes to the reduced incretin effect in type 2 diabetes mellitus. Diabetologia 50: 797–805 Epub 2007 Jan 2016, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Lefèbvre PJ. Glucagon and its family revisited. Diabetes Care 18: 715–730, 1995 [DOI] [PubMed] [Google Scholar]
- 19. Meier JJ, Butler PC. Insulin secretion. In: Endocrinology (5th ed.), edited by DeGroot LJ, Jameson JL. Philadelphia, PA: Elsevier Saunders, 2005, p. 961–973 [Google Scholar]
- 20. Meier JJ, Deacon CF, Schmidt WE, Holst JJ, Nauck MA. Suppression of glucagon secretion is lower after oral glucose administration than during intravenous glucose administration in human subjects. Diabetologia 50: 806–813 Epub 2007 Feb 2016, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Meier JJ, Gallwitz B, Siepmann N, Holst JJ, Deacon CF, Schmidt WE, Nauck MA. Gastric inhibitory polypeptide (GIP) dose-dependently stimulates glucagon secretion in healthy human subjects at euglycaemia. Diabetologia 46: 798–801, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Meier JJ, Kjems LL, Veldhuis JD, Lefèbvre P, Butler PC. Post prandial suppression of glucagon secretion depends on intact pulsatile insulin secretion: further evidence for the intraislet insulin hypothesis. Diabetes 55: 1051–1056, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Meier JJ, Lin JC, Butler AE, Galasso R, Martinez DS, Butler PC. Direct evidence of attempted β-cell regeneration in an 89-year old patient with recent onset type 1 diabetes. Diabetologia 49: 1838–1844, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Meier JJ, Nauck MA, Pott A, Heinze K, Goetze O, Bulut K, Schmidt WE, Gallwitz B, Holst JJ. Glucagon-like peptide 2 stimulates glucagon secretion, enhances lipid absorption, and inhibits gastric acid secretion in humans. Gastroenterology 130: 44–54, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Menge BA, Tannapfel A, Belyaev O, Drescher R, Muller C, Uhl W, Schmidt WE, Meier JJ. Partial pancreatectomy in adult humans does not provoke β-cell regeneration. Diabetes 57: 142–149 Epub 2007 Oct 2024, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Mitrakou A, Kelley D, Mokan M, Veneman T, Pangburn T, Reilly J, Gerich J. Role of reduced suppression of glucose production and diminished early insulin release in impaired glucose tolerance. N Engl J Med 326: 22–29, 1992 [DOI] [PubMed] [Google Scholar]
- 27. Nauck MA, Kleine N, Ørskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7–36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 36: 741–744, 1993 [DOI] [PubMed] [Google Scholar]
- 28. Rahier J, Goebbels RM, Henquin JC. Cellular composition of the human diabetic pancreas. Diabetologia 24: 366–371, 1983 [DOI] [PubMed] [Google Scholar]
- 29. Raju B, Cryer PE. Loss of the decrement in intraislet insulin plausibly explains loss of the glucagon response to hypoglycemia in insulin-deficient diabetes: documentation of the intraislet insulin hypothesis in humans. Diabetes 54: 757–764, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Ravier MA, Rutter GA. Glucose or insulin, but not zinc ions, inhibit glucagon secretion from mouse pancreatic α-cells. Diabetes 54: 1789–1797, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Rizza R, Verdonk C, Miles J, Service FJ, Gerich J. Effect of intermittent endogenous hyperglucagonemia on glucose homeostasis in normal and diabetic man. J Clin Invest 63: 1119–1123, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rosenstock J, Hassman DR, Madder RD, Brazinsky SA, Farrell J, Khutoryansky N, Hale PM. Repaglinide versus nateglinide monotherapy: a randomized, multicenter study. Diabetes Care 27: 1265–1270, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Samols E, Stagner JI, Ewart RB, Marks V. The order of islet microvascular cellular perfusion is B—A—D in the perfused rat pancreas. J Clin Invest 82: 350–353, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schrader H, Menge BA, Breuer TG, Ritter PR, Uhl W, Schmidt WE, Holst JJ, Meier JJ. Impaired glucose-induced glucagon suppression after partial pancreatectomy. J Clin Endocrinol Metab 94: 2857–2863 Epub 2009 Jun 2852, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Schuit FC, Pipeleers DG. Differences in adrenergic recognition by pancreatic A and B cells. Science 232: 875–877, 1986 [DOI] [PubMed] [Google Scholar]
- 36. Slucca M, Harmon JS, Oseid EA, Bryan J, Robertson RP. ATP-sensitive K+ channel mediates the zinc switch-off signal for glucagon response during glucose deprivation. Diabetes 59: 128–134, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Unger RH, Aguilar-Parada E, Muller WA, Eisentraut AM. Studies of pancreatic α cell function in normal and diabetic subjects. J Clin Invest 49: 837–848, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB, Song KH, Yoo SJ, Kang MI, Cha BY, Lee KW, Son HY, Kang SK, Kim HS, Lee IK, Bonner-Weir S. Selective β-cell loss and α-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab 88: 2300–2308, 2003 [DOI] [PubMed] [Google Scholar]