Abstract
Neuronal cell death is an early pathological feature of diabetic retinopathy. We showed previously that insulin receptor signaling is diminished in retinas of animal models of diabetes and that downstream Akt signaling is involved in insulin-mediated retinal neuronal survival. Therefore, further understanding of the mechanisms by which retinal insulin receptor signaling is regulated could have therapeutic implications for neuronal cell death in diabetes. Here, we investigate the role of cholesterol-enriched membrane microdomains to regulate PKC-mediated inhibition of Akt-dependent insulin signaling in R28 retinal neurons. We demonstrate that PKC activation with either a phorbol ester or exogenous application of diacylglycerides impairs insulin-induced Akt activation, whereas PKC inhibition augments insulin-induced Akt activation. To investigate the mechanism by which PKC impairs insulin-stimulated Akt activity, we assessed various upstream mediators of Akt signaling. PKC activation did not alter the tyrosine phosphorylation of the insulin receptor or IRS-2. Additionally, PKC activation did not impair phosphatidylinositol 3-kinase activity, phosphoinositide-dependent kinase phosphorylation, lipid phosphatase (PTEN), or protein phosphatase 2A activities. Thus, we next investigated a biophysical mechanism by which insulin signaling could be disrupted and found that disruption of lipid microdomains via cholesterol depletion blocks insulin-induced Akt activation and reduces insulin receptor tyrosine phosphorylation. We also demonstrated that insulin localizes phosphorylated Akt to lipid microdomains and that PMA reduces phosphorylated Akt. In addition, PMA localizes and recruits PKC isotypes to these cholesterol-enriched microdomains. Taken together, these results demonstrate that both insulin-stimulated Akt signaling and PKC-induced inhibition of Akt signaling depend on cholesterol-enriched membrane microdomains, thus suggesting a putative biophysical mechanism underlying insulin resistance in diabetic retinopathy.
Keywords: diabetes, diabetic retinopathy, Akt, protein kinase C
increased vascular permeability and macula edema are hallmarks of diabetic retinopathy, whereas the loss of neuronal cells is less well recognized but may be a consequence of diabetes-induced insulin resistance and/or inflammation (2, 4). Insulin signaling maintains retinal neuronal cells, and insulin resistance contributes to neuronal cell death. In fact, insulin receptor substrate (IRS)-2 knockout mice exhibit apoptosis within the retinal ganglion cell layer, inner nuclear layer, and photoreceptors (48). We have demonstrated previously that the phosphatidylinositol 3-kinase (PI3K)/Akt pathway mediates insulin-stimulated survival of cultured retinal neurons (5, 32, 47) and that insulin receptor (IR), PI3K, and Akt activities are diminished in the diabetic retina from both pharmacological and molecular rodent models (3, 34). Despite strong evidence that diminished retinal insulin signaling may contribute to neurodegeneration in diabetic retinopathy, the underlying biochemical or biophysical mechanism(s) underlying impaired retinal insulin signaling has not been described. Thus, understanding the regulation of retinal IR signaling is important in understanding mechanisms of neurodegeneration in diabetic complications.
A large body of evidence suggests that activated protein kinase C (PKC) isotypes contribute to diminished insulin signaling in skeletal muscle and vascular tissues in diabetes (7, 12). Activated PKC isoforms have demonstrated multiple mechanisms of insulin signaling impairment in a wide variety of cell types. These mechanisms include direct inhibitory posttranslational modifications of IR, IRS-1, or Akt and activation of pathways such as mammalian target of rapamycin complex 1 (mTORC1) and MAPK, which will also negatively regulate IR signaling (43). Despite this evidence, the exact mechanisms by which PKC isotypes inhibit insulin signaling in diabetic retinas are still not well defined.
Besides posttranslational direct protein/protein interactions that regulate insulin signaling, recent studies have identified a more global biophysical mechanism by which IR “signalosomes” can be modified in diabetes (21). Lipid microdomains (a.k.a. rafts) have been speculated to control insulin signaling by regulating insulin receptor complex formation. Lipid microdomains are defined as small (10–200 nm), heterogeneous, highly dynamic sterol- and (glyco-)sphingolipid-enriched domains that compartmentalize cellular processes and that can sometimes stabilize to form larger platforms through protein-protein and protein-lipid interactions (27). The IR is localized within these lipid rafts in a variety of cell types. In adipose tissue, IR association with caveolae, a subset of lipid rafts, is necessary for IR stability and signaling (11). Also, in some cells, such as 3T3-L1 adipocytes, disruption of membrane rafts by cholesterol depletion can diminish insulin-induced IR phosphorylation (25), and glycosphingolipid clustering has also been demonstrated to inhibit insulin receptor localization within rafts (22). In fact, we have published previously that the concentration of the glycosphingolipid glucosylceramide increases in models of diabetic retinopathy associated with loss of retinal IR activity (13, 34). Furthermore, selective glucosylceramide synthase inhibitors increase insulin sensitivity in cultured retinal neuronal cells (13). Taken together, we now hypothesize that altered lipid microdomains influence PKC-induced insulin resistance in diabetic retinal neurons.
EXPERIMENTAL PROCEDURES
Materials.
Bovine insulin was purchased from Sigma (St. Louis, MO). Laminin and cell-permeable cAMP were from BD Biosciences (Franklin Lakes, NJ) and MP Biomedicals (Irvine, CA), respectively. Phorbol 12-myristate 13-acetate (PMA) and 4α-PMA were purchased from Promega (Madison, WI). The cell-permeable 1,2-dioctanoyl-sn-glycerol (DAG) was obtained from Avanti Polar Lipids (Alabaster, AL). Polyclonal rabbit anti-phosphorylated (phospho)-p42/44 MAPK (Thr202/Tyr204), rabbit anti-p42/44 MAPK, phospho-Akt, Akt, phospho-MARCKS, phospho-pyruvate dehydrogenase kinase (PDK), PDK, phosphatase and tensin homolog deleted on chromosome 10 (PTEN), phospho-PTEN, and phospho-tyrosine (PY102) antibodies were obtained from Cell Signaling Technology (Beverly, MA). Anti-rabbit and anti-mouse IgG horseradish peroxidase-conjugated, anti-IRβ, and anti-PKC antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The PKC inhibitor bisindolylmaleimide-I (BimI) and the mTOR inhibitor rapamycin were from EMD Biosciences (San Diego, CA). The MAPK/ERK kinase (MEK) inhibitors PD-98059 and U-0126 were purchased from Biomol (Plymouth Meeting, PA).
Cell culture.
R28 cells, a generous gift from Dr. Gail M. Seigel, State University of New York at Buffalo (39), were grown in Dulbecco's modified Eagle's medium containing 5.5 mM glucose supplemented with 10% newborn calf serum (Hyclone). The cells were differentiated to neurons on laminin-coated plates with the addition of 25 μM cell-permeable cAMP, as described previously (5). All treatments were performed after serum deprivation, typically 4–6 h.
Western blot analysis.
Western blot analyses were performed essentially as described previously with some modifications (9). Briefly, treated R28 cells were washed in ice-cold Dulbecco's phosphate-buffered saline solution, and lysis buffer [50 mM HEPES, 137 mM NaCl, 5 mM NaF, 1 mM EDTA, 1 mM EGTA, 1 mM Na3VO4, 1% NP-40, protease inhibitor cocktail (Roche)] was added. Cell lysates were cleared by centrifugation, and the Bio-Rad DC protein assay was utilized to determine protein concentration. Typically, 30 μg of protein lysate per sample was separated on a 4–12% NuPAGE gels (Invitrogen) and transferred to nitrocellulose membranes. The membranes were subsequently blocked in 5% BSA (1 h) and incubated with the primary overnight (1:1,000 dilution). Membranes were subsequently washed 3 × 10 min in Tris-buffered saline plus Tween 20 (TBST) and incubated with an horseradish peroxidase-conjugated secondary for 2 h (1:5,000 dilution), washed in TBST (3 × 10 min), and visualized by enhanced chemiluminescence (ECL) (GE Healthcare, Piscataway, NJ). The bands were visualized by ECL and quantified using ImageQuant (Molecular Dynamics, Sunnyvale, CA) or GeneTools SynGene (K & R Technology, Frederick, MD) software.
Cellular fractionation.
For Figs. 4, A–C, 6, and 7A, R28 cells were treated as described in results and the figure legends. After treatment, a detergent-free method of isolating lipid rafts/low-buoyant domains was performed as described by others (42). Briefly, cells were harvested in sodium carbonate buffer (pH 11.0) and homogenized. Homogenates were adjusted to 4 ml with 90% sucrose in MES-buffered saline (25 mM MES, pH 4.5, 150 mM NaCl, 1 mM sodium orthovanadate, 1 mM NaF, protease inhibitor tablet) to a final concentration of 45% sucrose. Samples were layered at the bottom of a 14 × 89 mm tube containing 4 ml each of 35 and 5% sucrose in sodium carbonate-MES-buffered saline and centrifuged in a Beckman SW41ti rotor at 200,000 g for 16–20 h at 4°C. Serial fractions of 1 ml were removed. Equal volumes of each fraction were analyzed by Western blotting.
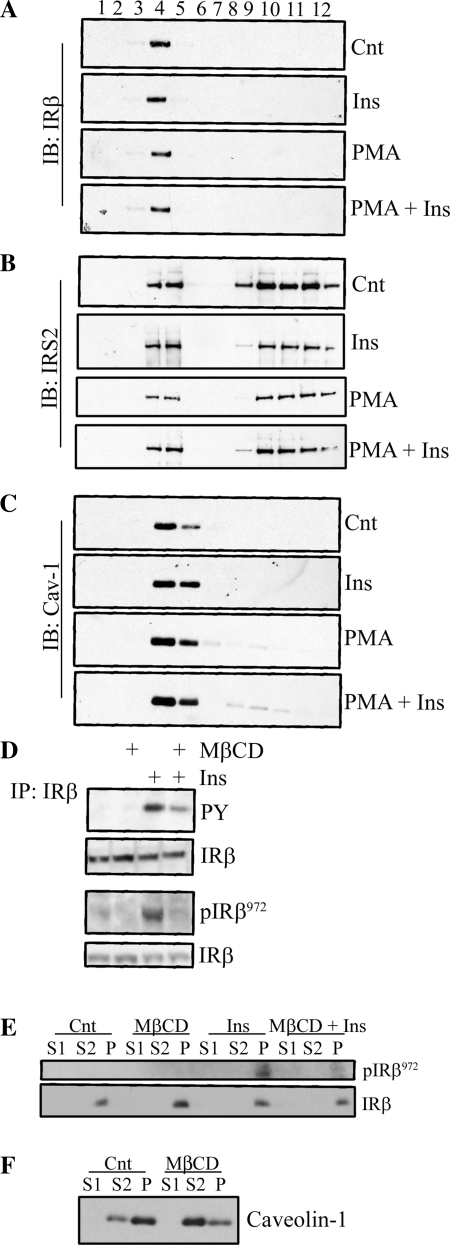
Fig. 4.
Cholesterol depletion disrupts IR activation and signaling. Discontinuous sucrose gradients were performed on R28 cell lysates after treatment with Ins (15 min, 10 nM) and/or PMA (100 nM, 30 min) to assess IR (A), IRS-2 (B), and caveolin-1 (Cav-1; C) localization within lipid rafts. D: R28 cells were treated with Ins and/or PMA after prior treatment with 1% methyl-β-cyclodextrin (MβCD) for 60 min. The IRβ was immunoprecipitated and its phosphorylation state assessed by a PY antibody (n = 3), and total lysate was assessed using a phospho-IRβ972 antibody. E: a cytosolic (S1), lubrol-solubilized (S2), and lubrol-resistant fraction from R28 cells treated in the presence or absence of MβCD with further treatment with vehicle or Ins was prepared and assessed for total and phosphorylated IR. F: similarly, Cav-1 localization to these fractions with or without MβCD treatment was assessed.
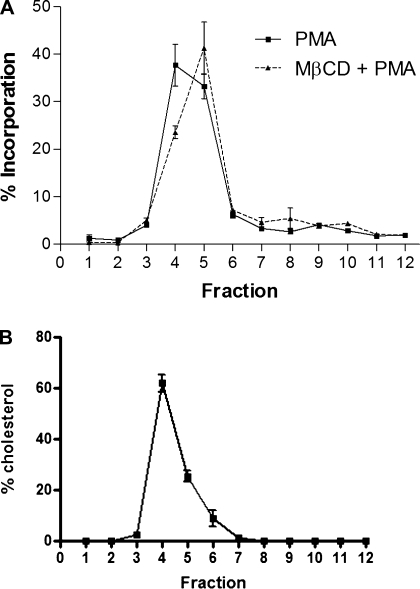
Fig. 6.
PMA localizes to low-density domains. A: R28 cells were treated with 1% MβCD for 60 min. R28 cells were subsequently treated with a 3H-labeled PMA (15 min) prior to the isolation of low-buoyant fractions (lipid rafts) on a discontinuous sucrose gradient. Radioactivity was assessed in each fraction by scintillation counting. B: as further validation of successful fractionation, fractions were assessed for cholesterol content (n = 4).
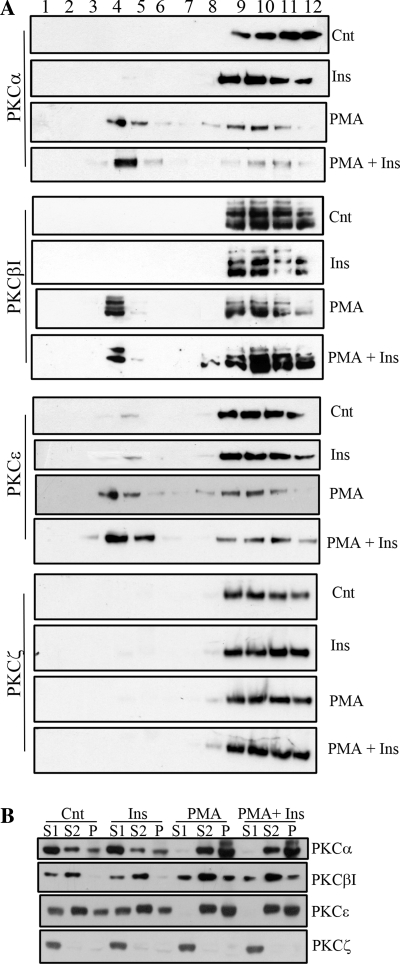
Fig. 7.
PMA recruits PKC isoform localization in lipid microdomains. A: PKC localization within low-buoyant fractions of Cnt, insulin-treated, and PMA-treated R28 cells was assessed by discontinuous sucrose gradient to isolate these domains (n = 3). Equivalent aliquots of each fraction were assessed for the presence of PKC isotypes by Western blotting. B: PKC localization was also assessed from cytosolic (S1), lubrol-solubilized (S2), and lubrol-resistant (P) fractions after Cnt, insulin-treated, and PMA-treated R28 cells (n = 3).
Two alternative approaches for microdomain isolation based on detergent resistance were also utilized. For these studies in Fig. 4, E and F, 5, A, and B, and 7B, R28 cells were treated as described in results and the figure legends. Experiments examining Akt localization were performed at 22°C to retard the translocation. Cells were passed through a 27-gauge needle 20 times and subsequently processed for microsome isolation by spinning at 100,000 g for 45 min. The resultant pellet was incubated with 0.5% Lubrol (a polyoxyethylene nonionic detergent) or 0.08% Triton X-100 on ice for 30 min. The lysate was centrifuged again at 100,000 g and the resultant pellet resuspended in 1% SDS. Thus, for each sample, three fractions were obtained: 1) soluble, 2) detergent soluble, and 3) detergent resistant. Equal fraction volumes were then assessed by Western blotting.
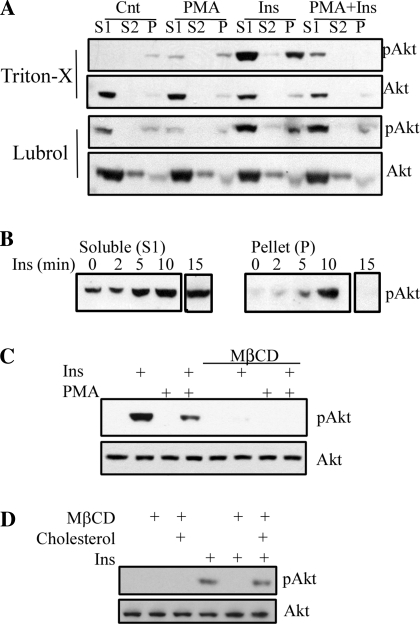
Fig. 5.
Akt is regulated by PMA and cholesterol within lipid microdomains. A: cytosolic (S1), lubrol, or Triton X-solubilized (S2) and detergent-resistant fractions from R28 cells treated in the presence or absence of a 30-min pretreatment with PMA (100 nM) followed by insulin (10 nM, 10 min) or vehicle were prepared and assessed for total and phosphorylated Akt by Western blotting. B: phosphorylated Akt (p-Akt) was assessed in the S1 and P fractions after Ins treatment (10 nM) at the time points indicated. The phosphorylation state and levels of Akt were assessed by Western blotting after cholesterol depletion (C) and repletion (D) in the presence or absence of Ins.
Cholesterol assay.
Sucrose gradient fractions (800 μl) were diluted to 9 ml with cold PBS. The samples were centrifuged at 100,000 g for 1 h at 4°C in a 50Ti rotor (Beckman Instruments, Palo Alto, CA). Resulting pellets were resuspended in 100 μl of 10 mM HEPES, pH 7.4. Cholesterol assays (Cholesterol Assay Kit 10007640; Cayman Chemical) were performed on 50 μl of each fraction according to the manufacturer's protocol.
Cholesterol repletion assay.
Cholesterol repletion experiments were performed as described previously (15). Briefly, 200 mg of methyl-β-cyclodextrin (MβCD) dissolved in 2.2 ml of H2O and 6 mg of cholesterol dissolved in 80 μl of isopropanol were mixed to give a 6.8-mM stock of cholesterol in 70 mM MβCD. The solution mixture was maintained at 80°C until clear and used for cell treatment at appropriate concentrations by dissolving the stock solution in serum-free media.
Akt isoform-specific kinase assays.
Akt isoform-specific kinase assays were performed essentially as described previously (38) with some modifications (20). The supernatants (200 μg of protein) of R28 cell homogenates were subjected to immunoprecipitation (overnight at 4°C) with 2 μg of anti-Akt1, -2 (Santa Cruz Biotechnology), and -3 (Upstate Biotechnology) primary antibodies. The antibody-antigen complex was then incubated with Gammabind G-Sepharose (GE Healthcare) for 1 h at 4°C. The immunoprecipitates were washed and incubated in assay buffer [20 mM HEPES (pH 7.2), 25 mM β-glycerophosphate, 1 mM sodium orthovanadate, 10 μM cold ATP, 5 mM MgCl2, and 1 mM dithiothreitol] at 35°C for 10 min in the presence of a PKA inhibitor peptide (1 μM; Upstate Biotechnology), GST-GSK-3 (3 μg/assay; Cell Signaling Technology), and [γ-32P]ATP (10 μCi/assay). The amount of 32P incorporated into GSK-3 was determined by SDS-PAGE and transferred to nitrocellulose and exposed to film. The radioactive bands corresponding to GSK-3 were cut out and measured by scintillation counting. The observed counts/min values were corrected for nonspecific binding by subtracting the background values (no primary antibody immunoprecipitation) and normalized to the total amount of Akt immunoprecipitated by reprobing the blots for Akt.
PI3K activity assay.
The PI3K assay was performed essentially as described before (8), but with the utilization of a phospho-tyrosine antibody. Briefly, R28 cell lysates were immunoprecipitated with a phospho-tyrosine antibody (PY102; Cell Signaling Technology). Washed immunoprecipitates were then incubated with phosphoinositol in the presence of [γ-32P]ATP, and the incorporation of this radiolabel was determined by thin-layer chromatrography.
Statistical methods.
One-way analysis of variance with Bonferroni multiple-comparison posttest and t-test analysis were performed using GraphPad Prism 4.0 software, with statistical significance considered if P < 0.05. All data shown are from a minimum of n = 3 experiments.
RESULTS
PKC activation inhibits Akt phosphorylation in retinal neurons.
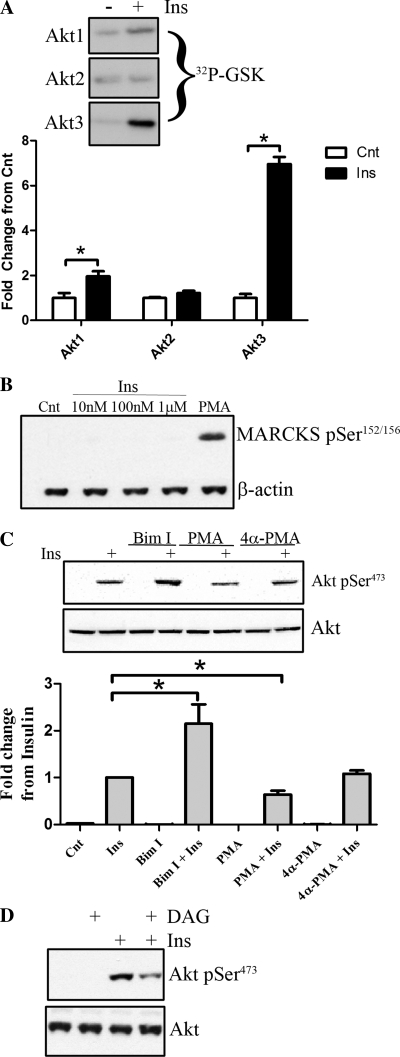
Insulin-stimulated Akt signaling promotes retinal cell survival following serum deprivation (5), so we initially validated that insulin activates Akt isotypes in cultured rat retinal neurons. R28 cells were stimulated with 10 nM insulin after serum deprivation to measure Akt isotype-specific activation (Fig. 1A). Isotype-specific kinase activities, via immunoprecipitation of individual Akt isotypes, reveal that Akt3 shows the greatest increase (P < 0.01), followed by Akt1 (P < 0.05), whereas no change in Akt2 activity was detected. These results are consistent with the diabetes-induced reduction of Akt1 and Akt3 activities in diabetic rat retinas (34). PKCs have gained much attention for the ability to contribute to insulin resistance in diabetes, so we next determined whether activated PKCs influence insulin-stimulated Akt activity in retinal neurons. We initially determined whether phorbol esters activate PKC in R28 cells by assessing the phosphorylation state of MARCKS (myristoylated alanine-rich C kinase), a major substrate of activated-PKCs. When R28 cells are stimulated with PMA, but not varying dosages of insulin, considerable phosphorylation of MARCKS is observed (Fig. 1B). These data demonstrate that PMA, but not insulin, activated PKCs in R28 retinal neurons. Pretreatment with PMA, prior to insulin stimulation, leads to significant inhibition of Akt activation (P < 0.05; Fig. 1C). The inactive analog of PMA, 4α-PMA, has no effect on insulin stimulation of Akt phosphorylation (P > 0.05; Fig. 1C). In contrast, when the PKC inhibitor BimI is utilized prior to insulin stimulation, a marked increase in insulin-stimulated Akt phosphorylation is observed (P < 0.01; Fig. 1C) . We next assessed whether treatment with exogenous diacylglycerides would recapitulate the results observed with PMA. Similar to PMA, exogenous diacylglycerides significantly inhibit insulin-stimulated Akt activation (Fig. 1D). Taken together, these data demonstrate that PKC activation negatively regulates insulin-stimulated Akt in R28 retinal neurons.
Fig. 1.
PKC activation negatively regulates Akt phosphorylation. A: R28 cells were treated with insulin (Ins; 10 nM, 15 min), and Akt isotypes were immunoprecipitated and subjected to kinase activity assays as described in experimental procedures (a representative autoradiograph from n = 3 experiments is shown). B: as a marker of PKC activation, R28 cells were stimulated with varying concentrations of Ins (15 min) or phorbol 12-myristate 13-acetate (PMA; 100 nM, 30 min), and the phosphorylation state of myristoylated alanine-rich C kinase (MARCKS) was assessed (representative blots of an n = 3 study is shown). C: R28 cells were serum starved and then stimulated with either phorbol 12-myristate 13-acetate (PMA; 100 nM) or 4α-PMA (100 nM) for 30 min with or without further stimulation with insulin (10 nM) for an additional 15 min. Where bisindolylmaleimide I (BimI) was utilized, cells were treated with 5 μM prior to insulin stimulation. The phosphorylation state and total expression of Akt were then assessed by Western blotting. D: similarly, R28 cells were pretreated with 50 μM 1,2-dioctanoyl-sn-glycerol (DAG) for 15 min, with successive stimulation with 10 nM Ins for 15 min, and Akt phosphorylation was analyzed. *P < 0.05. Cnt, control.
PKC activation does not inhibit upstream regulators of Akt.
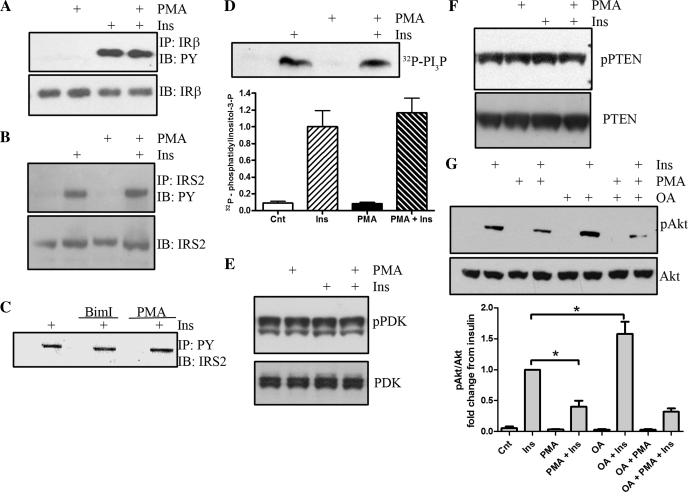
To define the putative biochemical mechanism(s) of action of PKC on Akt signaling, we initially analyzed immunoprecipitated IRβ subunit for tyrosine phosphorylation (Fig. 2A). Insulin treatment leads to increased tyrosine phosphorylation, but this phosphorylation is unaffected by the presence or absence of PMA, demonstrating that PKC-dependent Akt inhibition occurs downstream of the IR. We next assessed the tyrosine phosphorylation of IRS-2, the predominant IRS isoform utilized in the retina (30, 33). Similar to the insulin receptor, immunoprecipitated IRS-2 demonstrates a robust increase in tyrosine phosphorylation that is unaffected by PKC activation (Fig. 2B). We also assessed IRS-2 in an alternative manner by immunoprecipitation with a phosphotyrosine antibody first and then by performing Western blot analysis to detect IRS-2 precipitation (Fig. 2C). IRS-2 is detected only in insulin-stimulated R28 cells. The amount of tyrosine-phosphorylated IRS-2 immunoprecipitated is unaffected by treatment with the PKC inhibitor BimI or the PKC activator PMA.
Fig. 2.
PMA-induced inhibition of Akt signaling is independent of upstream insulin receptor (IR), insulin receptor substrate-2 (IRS-2), and phosphatidylinositol 3-kinase (PI3K). R28 cells were treated with 10 nM insulin (15 min) and/or 100 nM PMA (30 min). A: immunoprecipitated (IP) IRb was assessed for phosphotyrosine. B and C: IP IRS-2 was assessed for phosphotyrosine (B) in addition to detection of IRS-2 after immunoprecipitation with a phosphotyrosine antibody (C). D: PI3K activity was assessed by immunoprecipitation with a phospho-tyrosine antibody and by assessing the ability of the IP to phosphorylate phosphatidylinositol. The reaction was separated by thin-layer chromatography and exposed to film. Representative autoradiograph of a representative experiment (n = 4; P = 0.54). E and F: phosphorylation of pyruvate dehydrogenase kinase (PDK) was assessed (E), as was the phosphorylation state of phosphatase and tensin homologs deleted on chromosome 10 (PTEN; F) after PMA and/or insulin treatments. G: the effects of pretreatment of okadaic acid (OA), an inhibitor of protein phosphatase 2A, on insulin and PMA-regulated Akt phosphorylation was assessed (n = 3). *P < 0.05.
Next, we assessed the activity of PI3K after immunoprecipitation with a phosphotyrosine antibody (Fig. 2D). PI3K is downstream of IRS isoforms, although it may also signal independently through direct IR association (45). Insulin administration elevates PI3K activity, and pretreatment with PMA did not significantly alter insulin-induced activity. We next assessed the phosphorylation of PDK at Ser241 (Fig. 2E) . Phosphorylation at this site is essential for PDK activity, which in part is regulated by PI3K activity. Ser241 can be constitutively phosphorylated, and phosphorylation can be upregulated by agonists such as angiotensin II (36) and insulin (10). Examination of this phosphorylation site revealed that serum-deprived R28 cells exhibit substantial basal Ser241 phosporylation, and neither insulin treatment nor PMA alters the phosphorylation at this site, and thus PDK may be constitutively phosphorylated in these cells (Fig. 2E).
We next assessed the phosphorylation state of PTEN at Ser380/Thr382/383 (Fig. 2F). PTEN is a major negative regulator of Akt signaling though the dephosphorylation of phosphatidylinositol 3-phosphates. When R28 cells are stimulated with either insulin or PMA, no alterations in PTEN phosphorylation are observed (Fig. 2F). Thus, PTEN does not appear to contribute to reduced Akt activity via PKC activation. In addition to lipid-regulating phosphatases, such as PTEN, the phosphorylation state of Ser473 on Akt can be regulated by protein phosphatases, including protein phosphatase 2A (PP2A). When R28 cells are pretreated with okadaic acid, a PP2A inhibitor prior to treatment with insulin, a significant increase in insulin-stimulated Akt activation is observed (Fig. 2G). However, in the presence of PMA, okadaic acid treatment does not increase the PMA-inhibited insulin response. Taken together, these data demonstrate that PKC-induced inhibition of insulin-stimulated Akt is independent of the IR signaling phosphoinositide 3-phosphate-dependent signaling cascade.
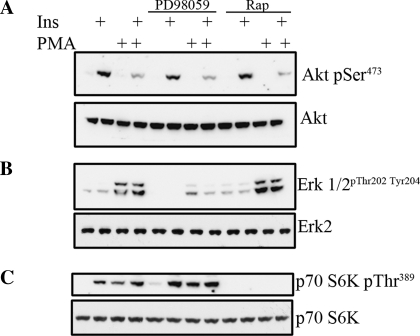
Although PKCs are not inhibiting Akt via direct inhibition of the IR or phosphoinositide 3-phosphate-dependent signaling cascades, several other PKC-activated pathways have been implicated in the negative control of insulin signaling, i.e, insulin resistance. For example, the MEK pathway and rapamycin-sensitive mTOR signaling have been shown to inhibit insulin signaling through phosphorylation of serine residues of IRS-1 to diminish tyrosine phosphorylation (24, 37). To test these possibilities, we utilized the MEK inhibitor PD-98059 and the mTOR inhibitor rapamycin to determine whether this would restore insulin signaling in response to PKC activation. We again found that insulin-activated Akt is impaired by PMA (Fig. 3A), and this inhibition is unaffected by either PD-98059 or rapamycin pretreatment. Examination of ERK1/2, a downstream target of MEK, reveals that PMA, but not insulin, increases ERK1/2 phosphorylation (Fig. 3B), consistent with our previous findings from ex vivo retinas (33). Both basal and PMA-activated ERK1/2 are diminished in the presence of PD-98059. To validate that rapamycin inhibits mTOR signaling, we examined p70 S6 kinase, a downstream effector of mTOR (Fig. 3C). Here, both insulin and PMA elevate p70 S6 kinase phosphorylation, which is subsequently blocked with rapamycin pretreatment. Taken together, PKC inhibition of Akt is also independent of MEK and mTOR kinases.
Fig. 3.
S8 cells were serum-starved and pretreated with PD-98059 [MAPK/ERK kinase (MEK) inhibitor] or rapamycin [mammalian target of rapamycin (mTOR) inhibitor] for 30 min prior to PMA and/or insulin treatment. Western blots were performed to assess the phosphorylation state of Akt Ser473 (A) and Akt, phosphorylated ERK and ERK (B), and phospho-p70 at Thr389 and total p70 (C). A representative blot of an n = 3 experiment is shown.
IR signaling is localized to cholesterol-enriched lipid microdomains in R28 retinal neurons.
Our data indicate that PKC-dependent inactivation of Akt may be independent of direct interactions with the IR and upstream effectors that regulate Akt activity, so we next investigated a biophysical mechanism of action by which insulin signaling could be regulated. Compartmentalization of IR signaling into lipid microdomains has been studied in various model systems, particularly adipocytes and liver, but retinal IR signaling is less understood. Using again the R28 retinal neuronal cell line, we determined whether the IR localizes to lipid microdomains. Isolation of lipid microdomains by sucrose gradient centrifugation reveals that the IR localizes within caveolin-enriched fractions 3–5, consistent with lipid microdomains, which are unaltered by insulin and/or PMA treatment (Fig. 4A). Similarly, a portion of the IRS-2 also localizes to these lipid microdomains, and the percentage localized to fractions 4 and 5 is unaffected by insulin and/or PMA treatment (Fig. 4B). To validate successful fractionation and isolation of lipid microdomains, we confirm that caveolin-1 preferentially localizes within the buoyant fractions, which are unaltered by insulin or PMA treatment (Fig. 4C).
We next examined the role of disruption of lipid microdomains on retinal cell insulin receptor signaling. Sixty-minute treatment with 1% MβCD does not change the gross morphology of cultured cells at these concentrations (data not shown). Phosphotyrosine analysis of the immunoprecipitated IRβ subunit reveals that cholesterol depletion with MβCD, which can disrupt lipid microdomains by extracting cholesterol from cellular membranes, significantly diminishes insulin-induced IR autophosphorylation (Fig. 4D). We also demonstrated that insulin-stimulated tyrosine phosphorylation of IRβ subunit amino acid 972 is significantly impaired with pretreatment of MβCD (Fig. 4D), which is in stark contrast to the lack of effect with PMA upon IRβ (Fig. 2A).
Because there are known variations between different lipid microdomain preparations, we confirmed and expanded these data by subjecting R28s to membrane extraction with the nonionic detergent lubrol, followed by centrifugation. We found enrichment of the IR in the lubrol-resistant fraction (P) but not in the cytosolic fraction (S1) or lubrol-solubilized pellet fraction (S2) (Fig. 4E). Upon treatment with MβCD, we observed that the phosphorylated tyrosine 972 on the IR is impaired under insulin treatment within this lubrol-resistant pellet fraction. To further support the efficacy of MβCD, we demonstrate that caveolin-1 localization in the detergent-resistant pellet (P) is reduced with MβCD treatment, with a corresponding increase in the S2 fraction (Fig. 4F). Taken together, both detergent-free and detergent-resistant isolation procedures for membrane microdomains confirm that IR signaling is localized and regulated within lipid microdomains.
To further examine the role of these microdomains on insulin receptor signaling, we examined the effect of PMA treatment on the phosphorylation of insulin-activated Akt within detergent-solubilized fractions, consistent with lipid rafts (Fig. 5A). We utilized both Triton X and lubrol as the detergent choices for creating a detergent-resistant pellet fraction. We observe that, with both preparations, Akt is predominantly localized to the cytosolic fraction (S1). Upon stimulation with insulin, we observe the phosphorylated Akt predominantly in the S1 and detergent-insoluble (P) fractions, which was subsequently impaired by PMA treatment. These data demonstrate that a portion of the activated Akt is localized within the lipid microdomain fraction, which is impaired by PMA treatment. More compelling evidence to support Akt localization within lipid microdomains is depicted in Fig. 5B. In this time course experiment, we observed that insulin treatment resulted in sustained phosphorylation of Akt in the soluble fraction, with transient phosphorylated protein in the pellet, peaking at 10 min. Thus our data suggest that Akt translocates to lipid rafts and, once there, upon phosphorylation, leaves this fraction.
We also assessed the effects of cholesterol depletion to disrupt lipid rafts on Akt activation. After cholesterol depletion with MβCD and subsequent treatment with insulin, Akt phosphorylation is drastically reduced, similar to the effects of PMA treatment (Fig. 5C). This inhibition occurs despite the ability of PMA to still induce p42/p44 (ERK1/2) phosphorylation. In fact, cholesterol depletion slightly increases p42/p44 phosphorylation (data not shown). It is of interest that the MβCD inhibition of Akt is greater than the MβCD-induced inhibition of IRβ phosphorylation, suggesting that intermediate steps in signaling between the IR and Akt are further impaired by cholesterol depletion and/or lipid microdomain dysfunction. We next utilized cholesterol repletion after MβCD treatment to determine whether this would restore insulin-stimulated Akt phosphorylation (Fig. 5D). Again, we observed that cholesterol depletion inhibits insulin-stimulated Akt. When cholesterol is added back after cholesterol depletion, insulin is able to stimulate Akt phosphorylation again. Taken together, these data support the notion that regulation of IR and Akt signaling depends on cholesterol-enriched microdomains.
PMA localizes and recruits PKC isoforms to lipid microdomains.
Because insulin signaling is negatively regulated presumably within lipid microdomains in R28 cells, we next assessed whether PMA itself localizes to these low-buoyant fractions, consistent with lipid microdomains. When R28 cells are treated with radiolabeled 3H-PMA and low-buoyant fractions are subsequently isolated, we show that >70% of the radiolabeled PMA is localized within fractions 4 and 5 (Fig. 6A), consistent with lipid-raft enriched fractions, as evidenced by strong localization of the lipid raft marker caveolin-1 (Fig. 4C) and cholesterol (Fig. 6B) to these fractions. MβCD-induced cholesterol depletion has no significant effect on the distribution of PMA within lipid microdomains (fractions 4 and 5, P = 0.225), and thus PMA localization is independent of sterols.
Because PMA is localized to the lipid raft fractions (Fig. 6A), we next examined whether PMA recruits PKC isotypes to these lipid microdomains (Fig. 7). Lipid microdomains dynamically associate to form platforms important for membrane protein sorting and construction of signaling complexes to allow for efficient downstream signaling in response to many ligands. We isolated lipid microdomains from R28 cells on a discontinuous sucrose gradient without detergent and observed that PMA, but not insulin, significantly increases the localization of classical (α, β1) and novel (ε) PKCs but not the atypical PKCζ within fractions 4 and 5, which are consistent with low-buoyant microdomains (Fig. 7A). We next confirmed these data by utilizing the detergent resistance methodology. Like the sucrose gradient centrifugation, we again observed a PMA but not insulin recruitment of classical and novel PKC isotypes to the detergent-resistant pellet (Fig. 7B). These data suggest that PMA induces the translocation of PKCs to lipid microdomains, where they negatively regulate insulin-induced Akt activation. In conclusion, the mechanism underlying PKC-induced negative regulation of Akt may indeed reflect localization of PKC isotypes to preformed Akt signalosomes within lipid microdomains.
DISCUSSION
Deficient insulin signaling in both type 1 and type 2 diabetes results in diabetes and also contributes directly to complications. Insulin receptors are widely expressed in the retina with the most abundant expression levels within neuronal cell bodies, including photoreceptors, and in the plexiform layers, comprised of dendrites, and Müller cells (16, 17, 35). Under normal physiological conditions, the retina exhibits a high basal activity/phosphorylation of the insulin receptor, IRS-2, PI3K, and Akt proteins (33). In animal models of diabetic retinopathy, insulin receptor kinase activity, IRS-2 phosphorylation, PI3K activity, and Akt activity are all progressively diminished with prolonged duration of diabetes in the retina (34). Rajala et al. (29) has shown that photoreceptor-specific insulin receptor deletion increases susceptibility to light stress and increases caspase-3 activity. Furthermore, photoreceptor-specific knockout of the protein-tyrosine phosphatase 1B, a negative regulator of insulin receptor signaling, confers neuroprotective activity (31). These data support that impaired insulin receptor signaling may contribute to the pathogenesis of diabetic retinopathy since intraocular administration of insulin restores retinal insulin receptor kinase activity (33) and diminishes diabetes-induced neuronal cell death (Gardner TW, unpublished observations). PKC activation has received substantial attention in contributing to the pathogenesis of systemic insulin resistance and diabetic complications, including retinopathy, so understanding these mechanisms may shed new light on the role of impaired retinal insulin signaling in diabetic retinopathy.
The major finding of this study is that PKC-induced negative regulation of Akt-dependent insulin recceptor signaling occurs within lipid microdomains. In fact, both PKCs and lipid raft modulation inhibit insulin receptor signaling in retinal neural cells. To the best of our knowledge, this is the first demonstration of this mechanism in cells from an organ that exhibits complications of long-term diabetes. Assessing PKC regulation of Akt, we demonstrate that PKC inhibition augments insulin-stimulated Akt, whereas PKC activation via exogenous DAG or phorbol ester treatment impairs Akt activation (Fig. 1, A–D). Biochemically, it is unclear how increased PKC activation inhibits Akt, and we demonstrate that it is independent of MEK, mTOR, and PP2A activation (Figs. 2 and 3). Furthermore, the upstream effectors of Akt, the insulin receptor, IRS-2, PI3K, PDK, and PTEN, do not demonstrate changes in phosphorylation/activity (Fig. 2), suggesting that the regulation is more directed at the level of Akt. Because functional insulin receptor signalosomes may reside within lipid microdomains, we also examined whether lipid microdomains regulate PKC-impaired insulin signaling at the level of Akt. Disruption of lipid microdomains via cholesterol depletion significantly impairs Akt activity (Fig. 5C), and PMA diminishes phosphorylated Akt within lipid microdomains (Fig. 5A). Consistent with a functional insulin-responsive Akt signalosome within lipid microdomains, we also demonstrate that the insulin receptor localizes to lipid microdomains, as does a portion of IRS-2 (Fig. 4, A and B). Disruption of lipid rafts via cholesterol depletion also impairs the tyrosine phosphorylation of the insulin receptor (Fig. 4, D and E). On the basis of these data, it can be inferred that an intact Akt-dependent insulin receptor signalosome resides within these functional lipid microdomains. Further examination of the mechanism of PMA-induced Akt inactivation, in the context of lipid microdomains, reveals that PMA itself is localized to these domains (Fig. 6) and results in the recruitment of classical and novel PKC isotypes to these domains (Fig. 7, A and B). Taken together, the data implicate altered membrane microdomains, in addition to PKC recruitment to these microdomains, as potential contributors to reduced insulin signaling or insulin resistance in diabetic retinopathy.
Alternate explanations besides recruitment of PKC isotypes to membrane microdomains can also explain PKC-induced insulin resistance. In the present study, we exclude several pathways, including MAPK and TORC1, that downregulate Akt activation. Yet this effect may reflect the preferential use of the IRS-2 isoform in retinal tissue, in contrast to IRS-1 in muscle and liver. Many studies have demonstrated that serine phosphorylation of IRS-1 by pathways such as PKC, MAPK, and TOR can limit downstream activation of insulin-regulated signaling elements. Less evidence exists for IRS-2 serine phosphorylation in decreasing insulin sensitivity (26). Our data also demonstrate that several intermediates between the insulin receptor and Akt (IRS-2, PI3K, PDK) are not altered by PMA treatment, so the inactivation of Akt by PKCs may be direct. In fact, our studies do not exclude the possibility that Akt can be directly phosphorylated by PKC isotypes at Ser/Thr34 (14, 18, 28), which we and others have previously shown to inactivate Akt. These studies, however, implicate PKCζ (which is not be stimulated by PMA) to phosphorylate and inactivate Akt. It is unclear whether classical or novel isotypes can inhibit Akt via phosphorylation at this site.
Several groups have previously published reports of increased PKC expression, translocation, and/or activation in the diabetic retina (19, 23, 40). Based on these findings, two multicenter clinical trials, the PKC-Diabetic Macular Edema Study and the PKC-Diabetic Retinopathy Study, examined the therapeutic efficacy of the PKCβ inhibitor ruboxistaurin for diabetic retinopathy. The PKC-Diabetic Macular Edema Study reported that ruboxistaurin significantly delayed the occurrence of moderate visual loss (27a). Moreover, PKC inhibitors inhibited diabetes-induced changes of retinal hemodynamic properties (1). Our studies now suggest that, in addition to regulating vascular injury in diabetic retinopathy, PKCs may induce neuronal retinal loss via inhibition of insulin-stimulated prosurvival Akt signaling possibly within altered lipid microdomains. Indeed, PKCs are expressed in retinal neurons (41, 46) as well as vascular cells.
In conclusion, our data clearly demonstrate impaired activation of the insulin receptor and downstream signaling due to lipid raft disruption through cholesterol depletion. It is unclear whether this response is a consequence of triggering receptor internalization or by altering interactions with other kinases and phosphatases within lipid microdomains that can modulate receptor activity. Regardless, we demonstrate an importance of lipid microdomains in regulating insulin and Akt signaling, and these observations are consistent with the recent finding that polyunsaturated retinal fatty acid metabolism is reduced by diabetes (44). Taken together, our studies demonstrate that insulin receptor signaling is regulated in lipid microdomains in retinal neurons and may be a putative mechanism of impaired insulin signaling and neuronal cell death in diabetic retinopathy.
GRANTS
This work was supported by the Juvenile Diabetes Research Foundation (JDRF) Diabetic Retinopathy Center (no. 4-2002-455 to M. Kester and T. W. Gardner), a JDRF postdoctoral fellowship (to T. E. Fox), EY015800 (to M. Kester and T. E. Fox) and EY020582 (T. W. Gardner) from the National Eye Institute, and the American Diabetes Association. T. W. Gardner is the Jack and Nancy Turner Professor of Ophthalmology.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Aiello LP, Clermont A, Arora V, Davis MD, Sheetz MJ, Bursell SE. Inhibition of PKC beta by oral administration of ruboxistaurin is well tolerated and ameliorates diabetes-induced retinal hemodynamic abnormalities in patients. Invest Ophthalmol Vis Sci 47: 86–92, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Antonetti DA, Barber AJ, Bronson SK, Freeman WM, Gardner TW, Jefferson LS, Kester M, Kimball SR, Krady JK, LaNoue KF, Norbury CC, Quinn PG, Sandirasegarane L, Simpson IA. Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes 55: 2401–2411, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Barber AJ, Antonetti DA, Kern TS, Reiter CE, Soans RS, Krady JK, Levison SW, Gardner TW, Bronson SK. The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest Ophthalmol Vis Sci 46: 2210–2218, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest 102: 783–791, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barber AJ, Nakamura M, Wolpert EB, Reiter CE, Seigel GM, Antonetti DA, Gardner TW. Insulin rescues retinal neurons from apoptosis by a phosphatidylinositol 3-kinase/Akt-mediated mechanism that reduces the activation of caspase-3. J Biol Chem 276: 32814–32821, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Bhattacharya S, Dey D, Roy SS. Molecular mechanism of insulin resistance. J Biosci 32: 405–413, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Bourbon NA, Sandirasegarane L, Kester M. Ceramide-induced inhibition of Akt is mediated through protein kinase Czeta: implications for growth arrest. J Biol Chem 277: 3286–3292, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Bourbon NA, Yun J, Kester M. Ceramide directly activates protein kinase C zeta to regulate a stress-activated protein kinase signaling complex. J Biol Chem 275: 35617–35623, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Chen H, Nystrom FH, Dong LQ, Li Y, Song S, Liu F, Quon MJ. Insulin stimulates increased catalytic activity of phosphoinositide-dependent kinase-1 by a phosphorylation-dependent mechanism. Biochemistry 40: 11851–11859, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Cohen AW, Razani B, Wang XB, Combs TP, Williams TM, Scherer PE, Lisanti MP. Caveolin-1-deficient mice show insulin resistance and defective insulin receptor protein expression in adipose tissue. Am J Physiol Cell Physiol 285: C222–C235, 2003 [DOI] [PubMed] [Google Scholar]
- 12. DasEvcimen N, King GL. The role of protein kinase C activation and the vascular complications of diabetes. Pharmacol Res 55: 498–510, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Fox TE, Han X, Kelly S, Merrill AH, 2nd, Martin RE, Anderson RE, Gardner TW, Kester M. Diabetes alters sphingolipid metabolism in the retina: a potential mechanism of cell death in diabetic retinopathy. Diabetes 55: 3573–3580, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fox TE, Houck KL, O'Neill SM, Nagarajan M, Stover TC, Pomianowski PT, Unal O, Yun JK, Naides SJ, Kester M. Ceramide recruits and activates protein kinase C zeta (PKC zeta) within structured membrane microdomains. J Biol Chem 282: 12450–12457, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Ganapathi SB, Fox TE, Kester M, Elmslie KS. Ceramide modulates HERG potassium channel gating by translocation into lipid rafts. Am J Physiol Cell Physiol 299: C74–C86, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gosbell AD, Favilla I, Baxter KM, Jablonski P. Insulin receptor and insulin receptor substrate-I in rat retinae. Clin Experiment Ophthalmol 28: 212–215, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Gosbell AD, Favilla I, Jablonski P. The location of insulin receptors in bovine retina and isolated retinal cells. Clin Experiment Ophthalmol 30: 124–130, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Hajduch E, Turban S, Le Liepvre X, Le Lay S, Lipina C, Dimopoulos N, Dugail I, Hundal HS. Targeting of PKCzeta and PKB to caveolin-enriched microdomains represents a crucial step underpinning the disruption in PKB-directed signalling by ceramide. Biochem J 410: 369–379, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Hammes HP, Du X, Edelstein D, Taguchi T, Matsumura T, Ju Q, Lin J, Bierhaus A, Nawroth P, Hannak D, Neumaier M, Bergfeld R, Giardino I, Brownlee M. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med 9: 294–299, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Hoehn KL, Summers SA. Assaying AKT/protein kinase B activity. Methods Mol Med 83: 137–144, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Inokuchi J. Insulin resistance as a membrane microdomain disorder. Biol Pharm Bull 29: 1532–1537, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Kabayama K, Sato T, Kitamura F, Uemura S, Kang BW, Igarashi Y, Inokuchi J. TNFalpha-induced insulin resistance in adipocytes as a membrane microdomain disorder: involvement of ganglioside GM3. Glycobiology 15: 21–29, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Kim YH, Kim YS, Kang SS, Noh HS, Kim HJ, Cho GJ, Choi WS. Expression of 14-3-3 zeta and interaction with protein kinase C in the rat retina in early diabetes. Diabetologia 48: 1411–1415, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Ozes ON, Akca H, Mayo LD, Gustin JA, Maehama T, Dixon JE, Donner DB. A phosphatidylinositol 3-kinase/Akt/mTOR pathway mediates and PTEN antagonizes tumor necrosis factor inhibition of insulin signaling through insulin receptor substrate-1. Proc Natl Acad Sci USA 98: 4640–4645, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Parpal S, Karlsson M, Thorn H, Stralfors P. Cholesterol depletion disrupts caveolae and insulin receptor signaling for metabolic control via insulin receptor substrate-1, but not for mitogen-activated protein kinase control. J Biol Chem 276: 9670–9678, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Paz K, Hemi R, LeRoith D, Karasik A, Elhanany E, Kanety H, Zick Y. A molecular basis for insulin resistance. Elevated serine/threonine phosphorylation of IRS-1 and IRS-2 inhibits their binding to the juxtamembrane region of the insulin receptor and impairs their ability to undergo insulin-induced tyrosine phosphorylation. J Biol Chem 272: 29911–29918, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Pike LJ. Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function. J Lipid Res 47: 1597–1598, 2006 [DOI] [PubMed] [Google Scholar]
- 27a. PKC-DRS Study Group The effect of ruboxistaurin on visual loss in patients with moderately severe to very severe nonproliferative diabetic retinopathy: initial results of the Protein Kinase C beta Inhibitor Diabetic Retinopathy Study (PKC-DRS) multicenter randomized clinical trial. Diabetes 54: 2188–2197, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Powell DJ, Hajduch E, Kular G, Hundal HS. Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCzeta-dependent mechanism. Mol Cell Biol 23: 7794–7808, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rajala A, Tanito M, Le YZ, Kahn CR, Rajala RV. Loss of neuroprotective survival signal in mice lacking insulin receptor gene in rod photoreceptor cells. J Biol Chem 283: 19781–19792, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rajala RV, McClellan ME, Chan MD, Tsiokas L, Anderson RE. Interaction of the retinal insulin receptor beta-subunit with the p85 subunit of phosphoinositide 3-kinase. Biochemistry 43: 5637–5650, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Rajala RV, Tanito M, Neel BG, Rajala A. Enhanced retinal insulin receptor-activated neuroprotective survival signal in mice lacking the protein-tyrosine phosphatase-1B gene. J Biol Chem 285: 8894–8904, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rajala RV, Wiskur B, Tanito M, Callegan M, Rajala A. Diabetes reduces autophosphorylation of retinal insulin receptor and increases protein-tyrosine phosphatase-1B activity. Invest Ophthalmol Vis Sci 50: 1033–1040, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reiter CE, Sandirasegarane L, Wolpert EB, Klinger M, Simpson IA, Barber AJ, Antonetti DA, Kester M, Gardner TW. Characterization of insulin signaling in rat retina in vivo and ex vivo. Am J Physiol Endocrinol Metab 285: E763–E774, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Reiter CE, Wu X, Sandirasegarane L, Nakamura M, Gilbert KA, Singh RS, Fort PE, Antonetti DA, Gardner TW. Diabetes reduces basal retinal insulin receptor signaling: reversal with systemic and local insulin. Diabetes 55: 1148–1156, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Rodrigues M, Waldbillig RJ, Rajagopalan S, Hackett J, LeRoith D, Chader GJ. Retinal insulin receptors: localization using a polyclonal anti-insulin receptor antibody. Brain Res 443: 389–394, 1988 [DOI] [PubMed] [Google Scholar]
- 36. Rölz W, Xin C, Ren S, Pfeilschifter J, Huwiler A. Interleukin-1 inhibits angiotensin II-stimulated protein kinase B pathway in renal mesangial cells via the inducible nitric oxide synthase. Eur J Pharmacol 442: 195–203, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Rui L, Aguirre V, Kim JK, Shulman GI, Lee A, Corbould A, Dunaif A, White MF. Insulin/IGF-1 and TNF-alpha stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J Clin Invest 107: 181–189, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sandirasegarane L, Kester M. Enhanced stimulation of Akt-3/protein kinase B-gamma in human aortic smooth muscle cells. Biochem Biophys Res Commun 283: 158–163, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Seigel GM. Establishment of an E1A-immortalized retinal cell culture. In Vitro Cell Dev Biol Anim 32: 66–68, 1996 [DOI] [PubMed] [Google Scholar]
- 40. Shiba T, Inoguchi T, Sportsman JR, Heath WF, Bursell S, King GL. Correlation of diacylglycerol level and protein kinase C activity in rat retina to retinal circulation. Am J Physiol Endocrinol Metab 265: E783–E793, 1993 [DOI] [PubMed] [Google Scholar]
- 41. Shin T, Kim S, Ahn M, Kim H. An immunohistochemical study of protein kinase C in the bovine retina. J Vet Med Sci 68: 71–74, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Song KS, Li S, Okamoto T, Quilliam LA, Sargiacomo M, Lisanti MP. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae microdomains. J Biol Chem 271: 9690–9697, 1996 [DOI] [PubMed] [Google Scholar]
- 43. Tanti JF, Jager J. Cellular mechanisms of insulin resistance: role of stress-regulated serine kinases and insulin receptor substrates (IRS) serine phosphorylation. Curr Opin Pharmacol 9: 753–762, 2009 [DOI] [PubMed] [Google Scholar]
- 44. Tikhonenko M, Lydic TA, Wang Y, Chen W, Opreanu M, Sochacki A, McSorley KM, Renis RL, Kern T, Jump DB, Reid GE, Busik JV. Remodeling of retinal Fatty acids in an animal model of diabetes: a decrease in long-chain polyunsaturated fatty acids is associated with a decrease in fatty acid elongases Elovl2 and Elovl4. Diabetes 59: 219–227, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Van Horn DJ, Myers MG, Jr, Backer JM. Direct activation of the phosphatidylinositol 3′-kinase by the insulin receptor. J Biol Chem 269: 29–32, 1994 [PubMed] [Google Scholar]
- 46. Wood JP, McCord RJ, Osborne NN. Retinal protein kinase C. Neurochem Int 30: 119–136, 1997 [DOI] [PubMed] [Google Scholar]
- 47. Wu X, Reiter CE, Antonetti DA, Kimball SR, Jefferson LS, Gardner TW. Insulin promotes rat retinal neuronal cell survival in a p70S6K-dependent manner. J Biol Chem 279: 9167–9175, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Yi X, Schubert M, Peachey NS, Suzuma K, Burks DJ, Kushner JA, Suzuma I, Cahill C, Flint CL, Dow MA, Leshan RL, King GL, White MF. Insulin receptor substrate 2 is essential for maturation and survival of photoreceptor cells. J Neurosci 25: 1240–1248, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]