Abstract
Aims
We tested whether on-statin C-reactive protein is associated with cardiovascular (CV) outcome in the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT).
Methods and results
ASCOT randomized a subset of 4853 patients with total cholesterol ≤6.5 mmol/L (250 mg/dL) to atorvastatin or placebo. In a case–control study during 5.5-year follow-up, 485 CV cases were age- and sex-matched with 1367 controls. Baseline LDL-cholesterol (LDL-c) and log-transformed C-reactive protein predicted CV events [odds ratio (OR) per 1 standard deviation (SD) 1.31 (95% confidence interval {CI}: 1.10, 1.56), P = 0.002 and OR 1.19 (1.05, 1.34), P = 0.006, respectively]. Including baseline C-reactive protein into a Framingham risk model very modestly improved risk prediction. Baseline C-reactive protein did not indicate the magnitude of the atorvastatin effect on CV outcome (P = 0.54). At 6 months, atorvastatin reduced median LDL-c by 40.3% and median C-reactive protein by 27.4%. In those randomized to atorvastatin, lower on-treatment LDL-c at 6 months was associated with a significant reduction in subsequent CV events [OR 0.41 (0.22, 0.75), P = 0.004] comparing those above and below the median (2.1 mmol/L). In contrast, C-reactive protein below the median (1.83 mg/L) compared with C-reactive protein above the median was not associated with a significant reduction in CV events [OR 0.86 (0.49, 1.51), P = 0.60]. Consequently, addition of on-treatment C-reactive protein to LDL-c did not improve prediction of statin efficacy.
Conclusion
Among these hypertensive patients selected on the basis of traditional CV risk factors, C-reactive protein did not usefully improve the prediction of CV events and, critically, reduction in C-reactive protein associated with statin therapy was not a predictor of CV outcome alone or in combination with LDL-c.
Keywords: C-reactive protein, Atorvastatin, ASCOT
See page 430 for the editorial comment on this article (doi:10.1093/eurheartj/ehr310)
Introduction
Certain inflammatory markers, such as C-reactive protein, have been shown to be associated with risk for cardiovascular (CV) disease independent of classical risk markers, including LDL-cholesterol (LDL-c).1,2 In several trials, statin therapy has been shown to lower circulating C-reactive protein.3,4 In the recent JUPITER (Justification for the Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) trial, the extent to which C-reactive protein is lowered by statins was reported to predict CV outcomes independently of LDL-c lowering.5 Indeed, authors have argued that the JUPITER trial provides confirmation of the value of C-reactive protein in determining CV risk.6 This finding is at variance with data from existing meta-analyses, which suggest that the ‘anti-inflammatory’ effect of statins may be a secondary effect of LDL-c reduction and that LDL-c reduction accounts for most of the benefit of statins.7,8 To further investigate this controversial issue, we have conducted a nested case–control study on participants recruited into ASCOT. Specifically, we tested the hypotheses that (i) baseline C-reactive protein (i.e. prior to randomization) is associated with and improves CV disease risk prediction, (ii) that baseline C-reactive protein modifies the efficacy of statins in reducing CV events, and (iii) that C-reactive protein levels during statin use predict statin efficacy independently of LDL-c reduction.
Methods
A nested case–control study based on the ASCOT-Blood Pressure-Lowering Arm (BPLA) cohort was used to determine the association of baseline C-reactive protein with subsequent events. In a separate analysis, the ASCOT-Lipid-Lowering Arm (LLA) subgroup was used to assess whether C-reactive protein levels following 6-month treatment with atorvastatin 10 mg was an independent predictor of CV outcomes.
For the purpose of the present study, those with any history of CV disease were excluded.
Patients and recruitment
The detailed ASCOT protocol has been published previously9 and further information is available at http://www.ascotstudy.org. Hypertensive patients, with three or more other risk factors for cardiovascular disease but no history of prior myocardial infarction or currently treated angina, were eligible.
In ASCOT-BPLA, in the UK and Ireland, 9098 patients were randomized to either amlodipine adding perindopril as required (amlodipine-based) or atenolol adding bendroflumethiazide as required (atenolol-based).
In addition to randomization into ASCOT-BPLA, those with a fasting total cholesterol of ≤6.5 mmol/L (250 mg/dL) were further randomized, using a factorial design, to either 10 mg atorvastatin daily or matching placebo (ASCOT-LLA).
ASCOT-LLA was stopped prematurely after a median follow-up of 3.3 years owing to highly significant benefits in favour of atorvastatin over placebo on the primary coronary endpoint. All patients in ASCOT-LLA were offered open-label atorvastatin and continued in the ASCOT-BPLA until its termination after a median 5.5-year follow-up.
Baseline characteristics of participants and primary outcomes of each arm of the trial have been reported previously.9–11
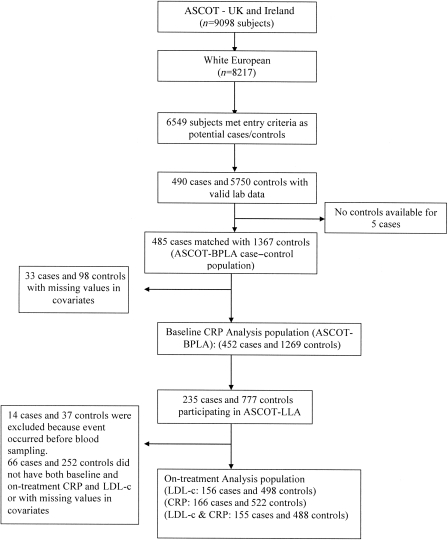
All events of fatal coronary heart disease (CHD), symptomatic non-fatal myocardial infarction (MI), coronary revascularization, and fatal and non-fatal stroke occurring in the UK and Ireland during the ASCOT-BPLA study period between February 1998 and October 2005 were identified as cases. During the median follow-up period of 5.5 years, 485 CV events (355 CHD and 130 strokes) were eligible for case–control analyses. Controls were selected from the UK and Ireland ASCOT study population who were alive at the time the case was diagnosed and free from CV disease in the study period. Up to three controls from the same risk set were matched to each case by age (±1 year), sex, and study entry time ±90 days. In total, the 485 cases were matched to 1367 controls. Of the 4853 subjects from UK and Ireland, who were randomized to either atorvastatin or placebo in ASCOT-LLA, there were 235 cases and 777 controls (Figure 1).
Figure 1.
ASCOT C-reactive protein nested case–control trial profile.
Laboratory methods
Fasting lipids were routinely measured during the trial (www.ascotstudy.org). C-reactive protein samples were collected at baseline and 6 months and subsequently all stored serum samples were measured by a high-sensitivity method, on an Abbott Architect, by technicians blinded to the case–control status of the samples. The lower limit of sensitivity was 0.1 mg/L and coefficient of variation <4%.
Statistical methods
Age-adjusted partial correlations between loge transformed baseline C-reactive protein and baseline clinical characteristics were estimated. The association between C-reactive protein and the risk of each of CHD or stroke event was reported as an odds ratio (OR) obtained from a conditional logistic regression model, first by treating log-transformed baseline C-reactive protein as a continuous variable giving the odds of having an event per 1 SD change with 95% confidence intervals (CIs) and secondly, by categorizing C-reactive protein into tertiles with the lowest as a reference. Three models were used: Model 1: unadjusted; Model 2 (modified Framingham CV risk factors): adjusted for current smoking status, diabetes mellitus, left ventricular hypertrophy, baseline systolic blood pressure (SBP), total cholesterol and HDL, randomized atorvastatin/placebo/not in ASCOT-LLA, randomized atenolol/amlodipine; Model 3 (extended CV risk factors): adjusted for Model 2 plus body mass index (BMI), logetransformed fasting glucose, family history of CHD (FHCHD), creatinine, and educational attainment. To examine the extent to which log C-reactive protein predicted CV events, unconditional logistic regression models with adjustment for age and sex were used on Framingham-based (Model 2) with and without loge C-reactive protein; and the fully adjusted model (Model 3) with and without loge C-reactive protein. Akaike's information criterion and the Bayes information criterion were used to assess global fit of these models (Models 2 and 3). We also performed likelihood-ratio tests to evaluate whether the global model fit improved after the addition of loge C-reactive protein. Areas under the receiver operator characteristic (AUROC) curve for Model 2 ± loge C-reactive protein and for Model 3 ± loge C-reactive protein were calculated. Discrimination was assessed by the AUROC curve. Model calibration was assessed with the Hosmer–Lemeshow goodness-of-fit test. We computed the net reclassification improvement (NRI), which compares the shifts in reclassified categories by observed outcome. The integrated discrimination improvement (IDI) was also calculated.12
To assess the role of on-treatment LDL-c and C-reactive protein in determining the likely impact of statin on CV events, cases and controls assigned atorvastatin in ASCOT-LLA were divided into two groups according to whether or not their on-treatment LDL-c was less than or more than the median value achieved at 6-month in-trial. A similar analysis was performed after subdividing on the basis of being above or below the median achieved C-reactive protein. These groups potentially gave rise to 235 cases and 777 controls for analyses (Figure 1). A multiple conditional logistic regression model (Model 3) was used to estimate ORs for CV events in these groups compared with the placebo group. A similar analysis was performed after subdividing into two groups on the basis of being above or below the median achieved C-reactive protein. Subjects with an event which occurred before the on-treatment C-reactive protein or LDL-c blood sample was obtained were excluded from these analyses.
In order to investigate the effect of the on-treatment C-reactive protein across the categories of on-treatment LDL-c, the above process was repeated after dividing the subjects allocated atorvastatin into four groups on the basis of the LDL-c cut-off value and the C-reactive protein cut-off value. For comparison with data derived from the recently reported JUPITER trial,5 we also repeated the analyses using the cut-off values for LDL-c (1.8 mmol/L) and C-reactive protein (1 and 2 mg/L).
Sensitivity analyses were performed on data restricted to patients with baseline C-reactive protein of <3 SDs. Two-sided P-values of ≤0.5 were considered statistically significant. All statistical analyses were performed with SAS V9.1 (SAS Institute, Cary, NC, USA) and STATA V11 (STATA Corporation, College Station, TX, USA).
Results
Baseline characteristics
During a median follow-up period of 5.5 years [inter-quartile range (IQR): 5.0, 6.0], a total of 1852 cases and controls were eligible for inclusion in these analyses. Of these, 131 were excluded for missing values of baseline C-reactive protein or covariates used in the models (Figure 1). The mean age was 64.7 ± 7.7 years and 84.7% were male. A comparison of the baseline characteristics between cases and controls showed that cases had a generally worse clinical profile than the controls (Table 1).
Table 1.
Baseline characteristics of study population
| Variable | Cases (n= 485) | Controls (n = 1367) | P-value |
|---|---|---|---|
| Male | 409 (84.3%) | 1159 (84.8%) | 0.81 |
| Age (years) | 64.80 (7.83) | 64.65 (7.66) | 0.71 |
| Current smokers | 127 (26.2%) | 297 (21.7%) | 0.04 |
| Alcohol | |||
| Never | 124 (25.6%) | 298 (21.8%) | |
| ≤14/21 U/week | 270 (55.7%) | 803 (58.7%) | |
| >14/21 U/week | 91 (18.8%) | 266 (19.5%) | 0.23 |
| Completed education | 182 (37.5%) | 452 (33.1%) | |
| Age: ≤15 | 233 (48.0%) | 632 (46.2%) | |
| Age: ≤18 | 46 (9.5%) | 154 (11.3%) | |
| Age: 19+ | 24 (4.9%) | 129 (9.4%) | 0.007 |
| SBP (mmHg) | 164.61 (17.81) | 161.70 (17.48) | 0.002 |
| DBP (mmHg) | 92.95 (10.37) | 92.13 (9.83) | 0.12 |
| Heart rate (b.p.m.) | 69.65 (12.91) | 70.35 (11.85) | 0.28 |
| BMI (kg/m2) | 28.71 (4.05) | 29.00 (4.50) | 0.21 |
| Total cholesterol (mmol/L) | 5.99 (1.07) | 5.92 (1.07) | 0.21 |
| LDL-c (mmol/L) | 3.92 (0.97) | 3.79 (0.96) | 0.02 |
| HDL-cholesterol (mmol/L) | 1.26 (0.33) | 1.30 (0.34) | 0.02 |
| Loge triglyceride (mmol/L) | 0.52 (0.46) | 0.50 (0.49) | 0.44 |
| Loge C-reactive protein (mg/L) | 1.13 (0.98) | 0.95 (0.98) | 0.0006 |
| Loge glucose (mmol/L) | 1.82 (0.31) | 1.78 (0.28) | 0.03 |
| Creatinine (mmol/L) | 102.34 (19.31) | 99.23 (16.57) | 0.0008 |
| Diabetes | 150 (30.9%) | 356 (26.0%) | 0.038 |
| Family history CHD | 85 (17.5%) | 233 (17.0%) | 0.81 |
| Amlodipine | 227 (46.8%) | 694 (50.8%) | 0.13 |
| Atorvastatina | 101 (43.0%) | 407(52.4%) | 0.01 |
Values are mean (SD) or n (%). Missing: LDL-c, 121; triglycerides, 79; fasting glucose, 84; creatinine, 45; C-reactive protein, 4.
aTwo hundred and thirty-five cases and 777 controls participated in lipid-lowering arm.
Baseline C-reactive protein and risk of cardiovascular events in follow-up
Baseline C-reactive protein was associated with baseline LDL-c (r = 0.11, P < 0.0001) among cases and controls combined. C-reactive protein showed a direct linear association with the risk of CHD and stroke combined and CHD alone but not with stroke. The ORs for CV disease, CHD, and stroke were 1.19 (95% CI: 1.05, 1.34; P = 0.006), 1.23 (1.06, 1.42; P = 0.005) and 1.06 (0.82, 1.37; P = 0.64) per 1 SD increase in loge-transformed C-reactive protein, respectively, after adjusting for extensive CV risk factors (Model 3; Table 2). Similar results were found when analysing by tertiles of C-reactive protein (although only the combined CHD and stroke endpoint showed significant associations) (Table 2).
Table 2.
Odds ratios (95% confidence interval) of cardiovascular event (coronary heart disease or stroke) in relation to baseline C-reactive protein (per standard deviation increase in log-transformed C-reactive protein and in C-reactive protein tertiles)
| Cases/controls | Model 1 | P-value | Model 2 | P-value | Model 3 | P-value | |
|---|---|---|---|---|---|---|---|
| CHD or stroke | |||||||
| Per 1 SD increase in loge C-reactive protein | 452/1269 | 1.21 (1.09,1.35) | 0.0004 | 1.16 (1.04,1.30) | 0.009 | 1.19 (1.05,1.34) | 0.006 |
| Tertile 1 C-reactive protein: <1.74 mg/L | 131/448 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | |||
| Tertile 2 C-reactive protein: 1.74–4.09 mg/L | 153/417 | 1.26 (0.97,1.64) | 0.08 | 1.16 (0.88,1.51) | 0.29 | 1.25 (0.93,1.67) | 0.14 |
| Tertile 3 C-reactive protein: >4.09 mg/L | 168/404 | 1.45 (1.12,1.89) | 0.005 | 1.30 (0.99,1.70) | 0.06 | 1.35 (1.00,1.82) | 0.05 |
| Trend | 0.005 | 0.057 | 0.05 | ||||
| CHD Only | |||||||
| Per 1 SD increase in loge C-reactive protein | 331/939 | 1.26 (1.11,1.42) | 0.0003 | 1.19 (1.04,1.35) | 0.009 | 1.23 (1.06,1.42) | 0.005 |
| Tertile 1 C-reactive protein: <1.74 mg/L | 87/330 | 1.00 (ref) | 0.02 | 1.00 (ref) | 0.14 | 1.00 (ref) | |
| Tertile 2 C-reactive protein: 1.74–4.09 mg/L | 119/310 | 1.43 (1.05,1.94) | 0.024 | 1.28 (0.93,1.76) | 0.13 | 1.35 (0.95,1.91) | 0.09 |
| Tertile 3 C-reactive protein: >4.09 mg/L | 125/299 | 1.50 (1.10,2.04) | 0.01 | 1.27 (0.92,1.75) | 0.15 | 1.36 (0.95,1.94) | 0.09 |
| Trend | 0.01 | 0.16 | 0.10 | ||||
| Stroke only | |||||||
| Per 1 SD increase in loge C-reactive protein | 121/330 | 1.09 (0.88,1.34) | 0.44 | 1.08 (0.86,1.36) | 0.50 | 1.06 (0.82,1.37) | 0.64 |
| Tertile 1 C-reactive protein: <1.74 mg/L | 44/118 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | |||
| Tertile 2 C-reactive protein: 1.74–4.09 mg/L | 34/107 | 0.91 (0.55,1.50) | 0.70 | 0.88 (0.52,1.50) | 0.63 | 1.03 (0.57,1.87) | 0.91 |
| Tertile 3 C-reactive protein: >4.09 mg/L | 43/105 | 1.38 (0.84,2.28) | 0.20 | 1.37 (0.81,2.32) | 0.25 | 1.36 (0.74,2.48) | 0.32 |
| 0.22 | 0.24 | 0.32 | |||||
Model 1: unadjusted. Model 2: adjusted for current smoking status, diabetes mellitus, randomized BP treatment (atenolol/amlodipine), randomized
atorvastatin/placebo/not in LLA, left ventricular hypertrophy, baseline SBP, total cholesterol, and HDL-cholesterol. Model 3: adjusted as for in Model 2 plus BMI, loge glucose, family history of CHD, creatinine, and educational attainment.
Predictive ability of baseline C-reactive protein in cardiovascular disease prediction beyond classical risk factors
There was no significant difference in the AUROC curve in either the modified Framingham model (Model 2) (P = 0.2), or the fully adjusted Model 3 (P = 0.18), after addition of C-reactive protein (see Supplementary material online, Table S1). The Hosmer–Lemeshow test indicated that C-reactive protein did not improve calibration of either model.
We then considered the NRI across risk categories (see Supplementary material online, Table S2). In 485 subjects who experienced events, the Framingham model with C-reactive protein increased (improved) risk category classification in 54 subjects but decreased classification in 39, a net improvement in reclassification of +3.3%. In 1367 subjects who did not experience an event, the model with C-reactive protein reclassified 131 increased (worsened) risk categories and 116 decreased risk categories, a net worsening in reclassification of 1.2%. The NRI for the model including C-reactive protein over the model without C-reactive protein was therefore estimated to be 2.1% (P = 0.32). Similar results were obtained in the fully adjusted model (Model 3).
The estimate of IDI was then assessed. There was a small increase in the IDI of 0.38% (P = 0.015) in Model 2, and 0.49% (P = 0.013) in the fully adjusted Model 3 when C-reactive protein was included.
Baseline C-reactive protein and outcome in the on-statin group
There was no evidence of an interaction between baseline LDL-c or C-reactive protein and treatment effect (statin/placebo or atenolol/amlodipine-based) on CV events. The statin effect did not differ according to the tertile of baseline C-reactive protein, despite the lowest tertile having a somewhat greater benefit (Table 3).
Table 3.
Atorvastatin effect by tertile of baseline C-reactive protein
| ORs (95% CI) by tertile of baseline C-reactive protein |
||||
|---|---|---|---|---|
| Low <1.74 mg/L | Middle 1.74–4.09 mg/L | High >4.09 mg/L | Interactiona | |
| Case/control | 67/259 | 72/239 | 80/219 | |
| CHD or stroke | 0.74 (0.38, 1.45) | 0.81 (0.42, 1.57) | 1.09 (0.56, 2.14) | 0.70 |
Adjusted for current smoking status, diabetes mellitus, randomized BP treatment (atenolol/amlodipine), randomized atorvastatin/placebo, left ventricular hypertrophy, baseline SBP, total cholesterol, HDL- cholesterol, BMI, loge-glucose, family history of CHD, creatinine, and educational attainment.
aInteraction between statin treatment and tertile baseline C-reactive protein.
Atorvastatin reduced the median LDL-c at 6 months by 40.3% [from 3.55 mmol/L IQR: (2.98, 4.01) to 2.12 mmol/L (1.75, 2.52)], while in the placebo group, the median fell by 2% [from 3.44 mmol/L (2.99, 4.00) to 3.37 mmol/L (2.87, 3.87); comparing change, P < 0.0001]. The concomitant changes for C-reactive protein were a 27.4% reduction on atorvastatin [from 2.52 mg/L (1.27, 5.02) to 1.83 mg/L (0.91, 3.62)] and a 6% increase on placebo [from 2.43 mg/L (1.21, 4.90) to 2.57 mg/L (1.39, 5.02); P < 0.0001]. This on-treatment change in C-reactive protein was independent of baseline classical risk factors, baseline C-reactive protein, and change in LDL-c (P = 0.02). In ASCOT-LLA, in those assigned atorvastatin, age-adjusted Spearman's correlation between percentage change in C-reactive protein and percentage change in LDL was modest (r = 0.19 P = 0.0006).
Baseline profiles of statin recipients who did and did not achieve 6-month C-reactive protein or LDL-c levels below the median
Despite reduction in C-reactive protein being independent of baseline classical risk factors, there were baseline differences in statin recipients based on achieved C-reactive protein above and below 1.83 mg/L. Those not achieving this C-reactive protein concentration had a higher baseline C-reactive protein, were more commonly smokers, had around a 2 U higher BMI, a far greater prevalence of diabetes, and family history of CHD, as well as higher triglycerides (see Supplementary material online, Table S3). Statin recipients who did achieve lower LDL-c values were broadly similar to those who did not, other than a higher baseline cholesterol level and a slight age difference (see Supplementary material online, Table S4).
Six-month reduction in LDL-cholesterol and C-reactive protein and risk of cardiovascular events in ASCOT-LLA
Compared with placebo, subjects allocated to atorvastatin who did not achieve an LDL-c of <2.1 mmol/L (median value, atorvastatin group) did not have a lower risk of CV disease [OR 1.10 (0.69–1.76); Table 4]. However, there was a 55% reduction in the risk of CV events in those who did achieve a reduction in LDL-c to <2.1 mmol/L [OR 0.45 (0.27–0.77); P= 0.003] in the multivariable-adjusted Model 3. Consequently, CV event risk was reduced by 59% in those whose LDL-c reduction was less than compared with those greater than the median [OR 0.41 (0.22–0.75)]. In contrast, those who achieved C-reactive protein below the median did not have a significantly reduced risk of CV events relative to those who did not [OR 0.86 (0.49–1.51)]. The effects of percentage change in LDL-c and C-reactive protein on CV events showed only borderline significance for LDL-c change and no association with C-reactive protein change (see Supplementary material online, Table S5). The risk of CHD events was much lower in those who achieved the LDL-c level below the median at 6 months (see Supplementary material online, Table S6). There was little change in these results when restricted to those with a baseline C-reactive protein of <3 SD (see Supplementary material online, Tables S7 and S8).
Table 4.
Odds ratios (95% confidence interval) for cardiovascular (coronary heart disease or stroke) events in relation to achieved (6-month in-trial) and median reductions in LDL-c and C-reactive protein
| Cases/controls | Model 1 |
P-value |
Model 2 |
P-value |
Model 3 |
P-value |
|
|---|---|---|---|---|---|---|---|
| Target lipid or C-reactive protein concentration |
|||||||
| Lipid | |||||||
| Placebo | 89/232 | 1 (ref.) | 1 (ref.) | 1 (ref.) | |||
| Atorvastatin | |||||||
| LDL ≥2.1 mmol/La | 44/126 | 0.97 (0.64, 1.46) | 0.87 | 1.09 (0.70, 1.67) | 0.71 | 1.10 (0.69, 1.76) | 0.68 |
| LDL <2.1 mmol/La | 23/140 | 0.45 (0.27, 0.73) | 0.001 | 0.45 (0.27, 0.76) | 0.003 | 0.45 (0.27, 0.77) | 0.003 |
| LDL <2.1 vs. ≥2.1 mmol/L | 0.40 (0.22, 0.71 | 0.002 | 0.41 (0.23, 0.74) | 0.003 | 0.41 (0.22, 0.75) | 0.004 | |
| C-reactive protein | |||||||
| Placebo | 93/245 | 1 (ref.) | 1 (ref.) | 1 (ref.) | |||
| Atorvastatin | |||||||
| C-reactive protein ≥1.83 mg/La | 41/137 | 0.86 (0.58, 1.29) | 0.47 | 0.83 (0.54, 1.27) | 0.39 | 0.82 (0.52, 1.28) | 0.38 |
| C-reactive protein <1.83 mg/La | 32/140 | 0.61 (0.39, 0.94) | 0.03 | 0.71 (0.45, 1.12) | 0.14 | 0.70 (0.44, 1.13) | 0.15 |
| C-reactive protein <1.83 vs. ≥1.83 mg/L | 0.70 (0.43, 1.16) | 0.17 | 0.86 (0.50, 1.46) | 0.56 | 0.86 (0.49, 1.51) | 0.60 | |
Model 1: unadjusted. Model 2: adjusted for baseline LDL cholesterol and logebaseline C-reactive protein. Model 3: current smoking status, diabetes mellitus, randomized BP treatment (atenolol/amlodipine), left ventricular hypertrophy, baseline SBP, HDL-cholesterol, BMI, loge-glucose, family history of CHD, creatinine, educational attainment, baseline LDL-c or total cholesterol, and loge baseline C-reactive protein.
aMedian LDL and C-reactive protein concentrations in the atorvastatin group.
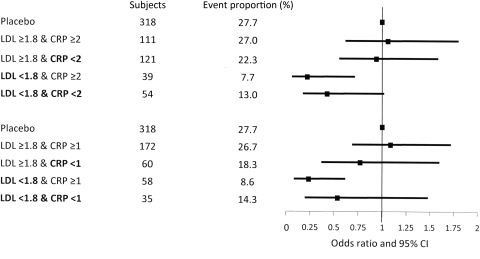
There was no clear evidence that the achievement of various ‘target’ C-reactive protein levels after the LDL-c target was achieved, resulted in any further reduction in risk using a number of models (Figure 2 and Table 5). For instance, if LDL-c was lowered to <2.1 mmol/L but C-reactive protein remained above the median (1.83 mg/L), the risk of CV events was reduced by 57% relative to placebo. In contrast if both LDL-c and C-reactive protein achieved the ‘target’, the risk of CV events was reduced 51% relative to placebo (Table 5). Similar trends were also noted for the LDL-c and C-reactive protein cut-offs presented in JUPITER. Similar findings were also observed when only CHD events were considered (see Supplementary material online, Table S9).
Figure 2.
Odds ratios for incident cardiovascular events according to achieved LDL-cholesterol and C-reactive protein after 6 months on atorvastatin. LDL-cholesterol achieved in mmol/L and C-reactive protein in mg/L. Adjusted for current smoking status, diabetes mellitus, randomized blood pressure treatment (atenolol/amlodipine), randomized atorvastatin/placebo/not in lipid-lowering arm, left ventricular hypertrophy, baseline systolic blood pressure, total cholesterol, HDL, body mass index, log-glucose, family history of coronary heart disease, creatinine and educational attainment, baseline LDL or total cholesterol, and log baseline C-reactive protein.
Table 5.
Odds ratios (95% confidence interval) for cardiovascular events (coronary heart disease or stroke) in relation to achieved concentrations at 6-month in-trial of both cholesterol and C-reactive protein in subjects allocated to atorvastatin
| Cases/controls | Odds ratios | P-value | |
|---|---|---|---|
| Placebo (ref.) | 88/230 | 1.00 (ref.) | |
| (ASCOT medians) | |||
| LDL ≥2.1 and C-reactive protein ≥1.83 | 27/65 | 1.28 (0.72, 2.26) | 0.40 |
| LDL ≥2.1 and C-reactive protein <1.83 | 17/55 | 0.99 (0.52, 1.88) | 0.98 |
| LDL <2.1 and C-reactive protein ≥1.83 | 11/62 | 0.43 (0.21, 0.88) | 0.02 |
| LDL <2.1 and C-reactive protein <1.83 | 12/76 | 0.49 (0.25, 0.99) | 0.05 |
| (JUPITER cut-offs) | |||
| LDL ≥1.8 and C-reactive protein ≥2 | 30/81 | 1.05 (0.61, 1.81) | 0.85 |
| LDL ≥1.8 and C-reactive protein <2 | 27/94 | 0.93 (0.55, 1.59) | 0.80 |
| LDL <1.8 and C-reactive protein ≥2 | 3/36 | 0.21 (0.06, 0.71) | 0.01 |
| LDL <1.8 and C-reactive protein <2 | 7/47 | 0.42 (0.17, 1.02 | 0.06 |
| (JUPITER cut-offs) | |||
| LDL ≥1.8 and C-reactive protein ≥1 | 46/126 | 1.08 (0.68, 1.72) | 0.75 |
| LDL ≥1.8 and C-reactive protein <1 | 11/49 | 0.76 (0.36, 1.60) | 0.48 |
| LDL <1.8 and C-reactive protein ≥1 | 5/53 | 0.23 (0.08, 0.61) | 0.003 |
| LDL <1.8 and C-reactive protein <1 | 5/30 | 0.53 (0.19, 1.48) | 0.23 |
LDL-cholesterol in mmol/L and C-reactive protein in mg/L. Adjusted for current smoking status, diabetes mellitus, randomized BP treatment (atenolol/amlodipine), left ventricular hypertrophy, baseline SBP, total cholesterol, HDL-cholesterol, BMI, loge-glucose, family history of CHD, creatinine, educational attainment, baseline LDL or total cholesterol, and loge baseline C-reactive protein.
Discussion
In ASCOT, subjects were recruited on the basis of conventional risk factors and, although atorvastatin lowered C-reactive protein by 27% over 6 months in trial, neither baseline C-reactive protein nor C-reactive protein on-treatment with atorvastatin related to the magnitude of statin efficacy in the prevention of CV events, whereas, as expected, on-treatment LDL-c was strongly predictive. These results are important as they enrich the evidence base on a topical issue relevant to clinical practice.
In ASCOT, the lack of association of baseline C-reactive protein with statin-associated relative CVD risk reduction is not controversial. Indeed, the recently reported large data set from the Heart Protection Study13 and the previously reported PROSPER14 and JUPITER15 studies all concur on this aspect. However, in ASCOT, our observation that on-statin C-reactive protein was not associated with CV outcomes does differ from results reported by JUPITER and PROVE IT-TIMI 22.5,16 Given these discrepancies, it is important to consider underlying differences between the trials. First, PROVE IT-TIMI 22 recruited high-risk patients with proven CHD.17 JUPITER recruited apparently healthy men and women (aged >50 and 60 years, respectively) with low LDL-c < 3.4 mmol/L (<130 mg/dL) [median at randomization, 2.8 mmol/L (107 mg/dL)] but an elevated C-reactive protein ≥2 mg/L (median at randomization 4.3 mg/L).5 Men and women recruited into ASCOT (aged 40–79 years) had no previous history of CHD, but did have three or more prevalent classical CV risk factors and hypertension. Eligibility for ASCOT-LLA required a total cholesterol of ≤6.5 mmol/L (≤250 mg/dL) [median LDL-c at randomization 3.8 mmol/L (145 mg/dL)]. The median C-reactive protein at randomization into ASCOT was 2.7 mg/L (IQR: 1.4, 5.2).This compares with pooled data from 54 observational studies where the median C-reactive protein was 1.72 mg/L (95% CI: 0.25–12.4).18 Second, JUPITER outcomes were a combination of hard and soft outcomes (10.9% of primary endpoints in JUPITER were from hospitalization for unstable angina events), whereas the present study is based on hard outcomes only. Third, the type and equivalent dose of statin used in JUPITER was different and higher than that in ASCOT (rosuvastatin 20 mg vs. atorvastatin 10 mg), although relative reductions in LDL-c (50 vs. 40%, respectively) and C-reactive protein (37 vs. 27%, respectively) were broadly comparable. Fourth, the study designs were different—ASCOT was a nested case–control study, whereas JUPITER and PROVE IT-TIMI 22 were cohort studies.
It has been argued that since statins lower both C-reactive protein and LDL-c, it is difficult to distinguish whether reductions in risk observed are due to LDL-c lowering, C-reactive protein lowering, or some combination.19 This is the case even among people starting with low LDL-c (such as JUPITER) because of the log-linear association of LDL-c with vascular risk over the whole range,20 which suggests that a beneficial effect of LDL-c lowering is likely irrespective of the baseline LDL-c.
Our findings are clinically important, since the ASCOT-LLA patient group is likely to represent a more typical population considered at risk of CV events than JUPITER and thus would more often be candidates for lipid-lowering therapy. Cardiovascular event rates were higher in ASCOT than JUPITER (for myocardial infarction 7.9 vs. 3.7 and stroke 5.4 vs. 3.4 per 1000 patient-years, respectively) and all-cause mortality 15.1 vs. 12.5 per 1000 patient-years, respectively). However, absolute risk reduction from statin use in the two trials was broadly similar (for myocardial infarction 2.7 vs. 2.0 and for stroke 1.5 vs. 1.6 per 1000 patient-years in ASCOT and JUPITER, respectively). JUPITER investigators have also recently reported that the baseline use of C-reactive protein adds clinical utility to risk prediction among those with low LDL-c and high C-reactive protein.21 Our results suggest that any incremental clinical utility of baseline C-reactive protein in identifying those at risk of future CV events, in addition to Framingham, is likely to be at best modest among the relatively typical hypertensive patients recruited into ASCOT. The IDI improved <0.5% when C-reactive protein was added to a Framingham model. These findings are broadly in line with other prospective studies showing statistically significant, but modest absolute improvements with the use of C-reactive protein in clinical risk prediction.22,23
Limitations of our study require consideration. The choice of LDL-c and C-reactive protein cut-offs is somewhat arbitrary. We studied medians and the pre-specified cut-offs reported by JUPITER; overall results by different cut-offs are broadly consistent, reducing the possibility that the selection of cut-offs has biased our results. Some of the comparisons of event incidence in subgroups by LDL-c and C-reactive protein cut-offs have modest numbers of events, although the number of events in the present study are broadly comparable to JUPITER. Although we used a case–control design, case–control analyses result in only very small reduction in power in a study of this size.24 ASCOT-LLA participants were followed for events beyond the duration of this arm of the overall trial, although this would not be expected to affect the efficacy of the statin therapy (risk reductions in the statin arm were the same at 3.3- and 5.5-year follow-up).25 Sensitivity analyses, restricting events to those occurring during the 3.3 years of the LLA arm of the trial, were similar to those reported here. In addition, the association of C-reactive protein with CV risk is broadly as expected from other prospective studies.2
In conclusion, we find that in this hypertensive population selected on the basis of traditional common coexisting risk factors, C-reactive protein did not usefully improve the prediction of CV events and, critically, reduction in C-reactive protein associated with statin therapy was not a predictor of CV outcome.
Contributors
P.S.S. and N.R.P. were co-Chief Investigator and Secretary, respectively, of the ASCOT Executive Committee and members of the ASCOT Steering Committee. P.S.S., N.R.P., C.L.C., P.W., and N.S. constituted the writing committee for the current manuscript, designed the present study, wrote the protocol and the analysis plan, supervised the analyses, interpreted the results, and wrote the report. S.A.M.T., A.D.H., and A.H. reviewed the protocol and analysis plan and commented upon the manuscript.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
The sponsors of the study (Pfizer) had no role in the study design, data collection, data analyses, data interpretation, or writing of the report. The database was held by the ASCOT Executive Committee who had final responsibility for the decision to submit for publication. P.S.S., N.R.P., A.D.H., and S.A.M.T. are supported by the Biomedical Research Centre Award to Imperial NHS Healthcare Trust and the BHF Research Centre Excellence Award to Imperial College. P.W. is supported by BHF fellowship grant FS/10/005/28147. Funding to pay the Open Access publication charges for this article was provided by Pfizer Inc.
Conflict of interest: P.S.S. and N.R.P. have served as consultants to, received travel expenses from and payment for speaking at meetings for, and received research funding from Pfizer to cover administrative staffing and analytical costs of the biomarker analyses. N.S. has received consulting and lecture fees from Merck & Co., Pfizer, and AstraZeneca and has received research grant support from Pfizer. A.D.H. and S.A.M.T. have received research grant support from Pfizer. P.S.S. and N.R.P. are recipients of NIHR Senior Investigator Awards.
Supplementary Material
Acknowledgments
We thank Lynne Cherry, Anne Alexander, and Anne Currie for their excellent technical support.
References
- 1.Danesh J, Kaptoge S, Mann AG, Sarwar N, Wood A, Angleman SB, Wensley F, Higgins JP, Lennon L, Eiriksdottir G, Rumley A, Whincip PH, Lowe GD, Gudnason V. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: two new prospective studies and a systematic review. PLoS Med. 2008;5:e78. doi: 10.1371/journal.pmed.0050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. Emerging Risk Factors Collaboration, C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morrow DA, de Lemos JA, Sabatine MS, Wiviott SD, Blazing MA, Shui A, Rifai N, Califf RM, Braunwald E. Clinical relevance of C-reactive protein during follow-up of patients with acute coronary syndromes in the Aggrastat-to-Zocor Trial. Circulation. 2006;114:281–288. doi: 10.1161/CIRCULATIONAHA.106.628909. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373:1175–1182. doi: 10.1016/S0140-6736(09)60447-5. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM. Moving toward new statin guidelines in a post-JUPITER world: principles to consider. Curr Atheroscler Rep. 2009;11:249–256. doi: 10.1007/s11883-009-0039-1. [DOI] [PubMed] [Google Scholar]
- 7.Robinson JG. Models for describing relations among the various statin drugs, low-density lipoprotein cholesterol lowering, pleiotropic effects, and cardiovascular risk. Am J Cardiol. 2008;101:1009–1015. doi: 10.1016/j.amjcard.2007.11.060. [DOI] [PubMed] [Google Scholar]
- 8.Robinson JG, Smith B, Maheshwari N, Schrott H. Pleiotropic effects of statins: benefit beyond cholesterol reduction? A meta-regression analysis. J Am Coll Cardiol. 2005;46:1855–1862. doi: 10.1016/j.jacc.2005.05.085. [DOI] [PubMed] [Google Scholar]
- 9.Sever PS, Dahlöf B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, McInnes GT, Mehlsen J, Nieminen M, O'Brien E, Ostergren J for the ASCOT Investigators. Rationale, design, methods and baseline demography of participants of the Anglo-Scandinavian Cardiac Outcomes Trial. ASCOT investigators. J Hypertens. 2001;19:1139–1147. doi: 10.1097/00004872-200106000-00020. [DOI] [PubMed] [Google Scholar]
- 10.Dahlöf B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O'Brien E, Ostergren J for the ASCOT Investigators. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 11.Sever PS, Dahlöf B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O'Brien E, Ostergren J for the ASCOT investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–1158. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 12.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. discussion 207–212. [DOI] [PubMed] [Google Scholar]
- 13.Emberson J, Bennett D, Link E, Parish S, Danesh J, Armitage J, Collins R Heart Protection Study Collaborative Group. C-reactive protein concentration and the vascular benefits of statin therapy: an analysis of 20,536 patients in the Heart Protection Study. Lancet. 2011;377:469–476. doi: 10.1016/S0140-6736(10)62174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sattar N, Murray HM, McConnachie A, Blauw GJ, Bollen EL, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, Kamper AM, Macfarlane PW, Murphy MB, Packard CJ, Perry IJ, Stott DJ, Sweeney BJ, Twomey C, Westendorp RG, Shepherd J PROSPER Study Group. C-reactive protein and prediction of coronary heart disease and global vascular events in the Prospective Study of Pravastatin in the Elderly at Risk (PROSPER) Circulation. 2007;115:981–989. doi: 10.1161/CIRCULATIONAHA.106.643114. [DOI] [PubMed] [Google Scholar]
- 15.Ridker PM, MacFadyen J, Libby P, Glynn RJ. Relation of baseline high-sensitivity C-reactive protein level to cardiovascular outcomes with rosuvastatin in the Justification for Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) Am J Cardiol. 2010;106:204–209. doi: 10.1016/j.amjcard.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 16.Ray KK, Cannon CP, Cairns R, Morrow DA, Ridker PM, Braunwald E. Prognostic utility of apoB/AI, total cholesterol/HDL, non-HDL cholesterol, or hs-CRP as predictors of clinical risk in patients receiving statin therapy after acute coronary syndromes: Results from PROVE IT-TIMI 22. Artrioscler Thromb Vasc Biol. 2009;29:424–430. doi: 10.1161/ATVBAHA.108.181735. [DOI] [PubMed] [Google Scholar]
- 17.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 18.Emerging Risk Factors Collaboration. C-reactive protein concentration and risk of coronary heart disease, stroke and mortality; an individual participant meta-analysis. Lancet. 2010;375:132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sattar N, Hingorani AD. C-reactive protein and prognosis in diabetes: getting to the heart of the matter. Diabetes. 2009;58:798–799. doi: 10.2337/db08-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, Qizilbash N, Peto R, Collins R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370:1829–1839. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 21.Ridker PM, MacFadyen JG, Nordestgaard BG, Koenig W, Kastelein JJ, Genest J, Glynn RJ. Rosuvastatin for primary prevention among individuals with elevated high-sensitivity C-reactive protein and 5% to 10% and 10% to 20% 10-year risk: implications of the Justification for Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) Trial for intermediate risk. Circ Cardiovasc Qual Outcomes. 2010;3:447–452. doi: 10.1161/CIRCOUTCOMES.110.938118. [DOI] [PubMed] [Google Scholar]
- 22.Woodward M, Welsh P, Rumley A, Tunstall-Pedoe H, Lowe GD. Do inflammatory biomarkers add to the discrimination of cardiovascular disease after allowing for social deprivation? Results from a 10 year cohort study in Glasgow, Scotland. Eur Heart J. 2010;21:2669–2675. doi: 10.1093/eurheartj/ehp115. [DOI] [PubMed] [Google Scholar]
- 23.Wilson PW, Pencina M, Jacques P, Selhub J, D'Agostino R, Sr, O'Donnell CJ. C-reactive protein and reclassification of cardiovascular risk in the Framingham Heart Study. Circ Cardiovasc Qual Outcomes. 2008;1:92–97. doi: 10.1161/CIRCOUTCOMES.108.831198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woodward M. Epidemiology: Study Design and Data Analysis. FL, USA: Chapman & Hall/CRC; 2005. [Google Scholar]
- 25.Sever PS, Poulter NR, Dahlöf B, Wedel H, Beevers G, Caulfield M, Collins M, Kjeldsen SE, Kristinsson A, McInnes G, Mehlsen J, Nieminen MS, O'Brien ET, Ostergren J. The Anglo-Scandinavian Cardiac Outcomes Trial lipid lowering arm: extended observations 2 years after trial closure. Eur Heart J. 2008;29:499–508. doi: 10.1093/eurheartj/ehm583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.