Abstract
Omega-3 fatty acids, which are found abundantly in fish oil, exert pleiotropic cardiometabolic effects with a diverse range of actions. The results of previous studies raised a lot of interest in the role of fish oil and omega-3 fatty acids in primary and secondary prevention of cardiovascular diseases. The present review will focus on the current clinical uses of omega-3 fatty acids and provide an update on their effects. Since recently published trials in patients with coronary artery diseases or post-myocardial infarction did not show an effect of omega-3 fatty acids on major cardiovascular endpoints, this review will examine the limitations of those data and suggest recommendations for the use of omega-3 fatty acids.
Keywords: Fish oils, Cardiovascular disease
Introduction
In 1929, the essential fatty acids were discovered by the biochemists Evans and Burr.1 They showed that mammals do not possess enzymes able to synthesize double bonds at the n-3 and n-6 positions of the carbon chain of a fatty acid. Therefore, humans must obtain the essential fatty acids linoleic acid (C18:2n-6) and alpha linolenic acid (ALA, C18:3n-3) from dietary sources. Alpha linolenic acid can be extended to eicosapentaenoic acid (EPA C20:5n-3) and docosahexaenoic acid (DHA C22:6n-3) through elongation and desaturation. Fish oil is a rich source of these omega-3 fatty acids.
In 1937, the British physiologist Hugh Sinclair visited Evans and became interested in the possibility that deficiencies in polyunsaturated fatty acids could cause coronary artery diseases (CAD). In 1944, he undertook his first visit to the Inuit and became convinced that their diet protects against atherosclerosis and Western diseases.2 In a letter to the Lancet, he hypothesized in 1956 that omega-3 fatty acids may be responsible for the protective effect of their diet.3 This view was contrary to the dogma of that time that all animal fats are harmful. In the 1970s, he joined the Danish investigators Bang and Dyerberg4,5 during one of their expeditions to Greenland. They found that the Inuit consumed ∼400 g of seafood per day and their average intake of omega-3 fatty acids was 14 g per day compared with 3 g per day among Danes. An epidemiological study showed that the incidence of myocardial infarction (MI) was 10 times lower among the Inuit compared with the Danes.6
The difference between the Inuit and the Danes in the intake of omega-3 fatty acids was reflected in their fatty acid composition of platelets. Differences were also observed in haemostatic factors, bleeding time, serum triglycerides, and high-density lipoprotein (HDL)—cholesterol levels. To show that these associations are causal, Sinclair put himself in 1977 on an Inuit diet for 100 days.7 His bleeding time rose from 3–5 to 50 min and substantial decreases were observed in blood platelets, erythrocytes, packed cell volume, and haemoglobin. The triglyceride-rich very low-density lipoprotein (VLDL) fell and the HDL fraction increased considerably. A substantial increase in the EPA concentration and a marked decrease in the linoleic acid concentration of cholesteryl esters were noted. Sinclair concluded from this experiment that it is necessary to have the right balance of omega-3 and omega-6 fatty acids to prevent thrombotic disorders.
In 1985, Kromhout et al.8 showed in the Zutphen Study, a prospective cohort study in the Netherlands, that eating fish once or twice per week was associated with a lower risk of fatal CAD compared with men who did not eat fish. Four years later in 1989, Burr et al.9 showed in the Diet and Reinfarction Trial (DART) that cardiac patients who received an advice to add two fatty fish meals per week to their diet reduced CAD mortality significantly, compared with those who did not get a fish advice. The results of these studies raised a lot of interest in the role of fish oil and omega-3 fatty acids in primary and secondary prevention of cardiovascular diseases (CVD). In this article, we summarize the mechanisms of the action of omega-3 fatty acids and the results of cohort studies and clinical trials on omega-3 fatty acids and CVD. Finally, we draw conclusions on whether omega-3 fatty acids reduce the incidence of these diseases.
Mechanisms of action of omega-3 fatty acids
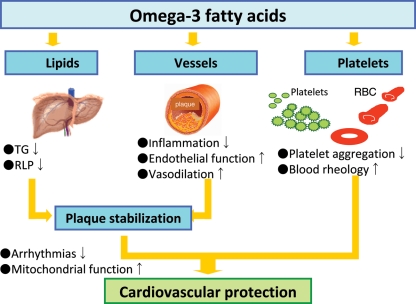
The cardiometabolic effects of omega-3 fatty acids continue to be extensively investigated and remain an active area of research. Omega-3 fatty acids can ultimately increase arrhythmic thresholds, reduce blood pressure, improve arterial and endothelial function, reduce platelet aggregation, and favourably affect autonomic tone (Figure 1). In this section, we briefly review recent studies that extend our knowledge on the cardioprotective effects of omega-3 fatty acids.10
Figure 1.
Beneficial effects of omega-3 fatty acids. TG, triglycerides; RLP, remnant lipoproteins; RBC, red blood cells.
Anti-inflammatory effects
Recently, the anti-inflammatory effects of omega-3 fatty acids have attracted much attention. Omega-3 fatty acids reduce the content of arachidonic acid (AA) in membrane phospholipids in platelets, endothelial cells, and inflammatory cells with a resultant reduced production of AA-derived pro-inflammatory mediators, including prostaglandin (PG)-E2, thromboxane (TX)-B2, leucotriene (LT)-B4, hydroxyeicosatetraenoic acid (5-HETE), and LT-E4. Importantly, EPA also acts as a substrate for cyclo-oxygenase and lipoxygenase enzymes, which could increase a different family of eicosanoids—the three-series PGs and TXs.11 In addition to these anti-inflammatory effects, omega-3 fatty acids have a number of other effects that may occur either downstream of altered eicosanoid production or independent of this activity.12 For example, the effects of omega-3 fatty acids on inflammatory cytokine expression could be at least in part through modulating intra-cellular signalling pathways that inactivates transcriptional factors.12 Recent studies demonstrated that omega-3 fatty acids could down-regulate the activity of the nuclear factor (NF)-κB,12 which plays a key role in the regulation of gene expression in inflammatory responses and has been implicated in the pathogenesis of CVD.13 The inhibition of NF-κB activation can be mediated by the mechanism that is related to the activation of peroxisome proliferator-activated receptor (PPAR) or the inhibition of toll-like receptors.13
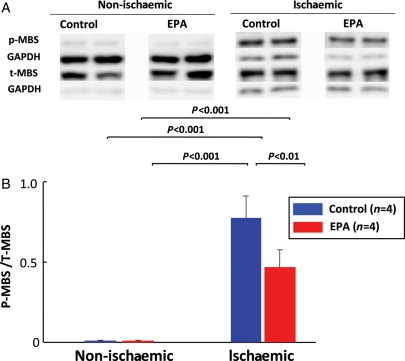
Rho-kinase is a downstream effector of the small GTPase Rho and mediates diverse cellular functions, such as smooth muscle cell contraction, cell migration, and proliferation.14 Rho-kinase also up-regulates pro-inflammatory molecules and down-regulates endothelial nitric oxide (NO) synthase (eNOS).15,16 It has been recently demonstrated that long-term treatment with EPA significantly inhibits Rho-kinase activation in the myocardium subjected to ischaemia–reperfusion in vivo (Figure 2).17
Figure 2.
Long-term eicosapentaenoic acid treatment inhibits myocardial Rho-kinase activation induced by ischaemia/reperfusion. Male pigs were treated with either a control chow or eicosapentaenoic acid (600 mg/kg/day) for 3 weeks and were subjected to myocardial ischaemia by 90 min occlusion of the left circumflex coronary artery and subsequent 60 min reperfusion. The eicosapentaenoic acid group had increased eicosapentaenoic acid level in red blood cells (eicosapentaenoic acid 4.30 ± 0.63 mol%). The eicosapentaenoic acid treatment significantly ameliorated myocardial ischaemia/reperfusion injury and significantly inhibited myocardial Rho-kinase activity, assessed by the extent of myosin-binding subunit (MBS) phosphorylation. Top panel (A) shows representative western blots of myocardial expression of phosphorylated (p)- and total (t)-MBS. Bottom panel (B) shows quantitative results of the ratio of p-MBS to t-MBS, indicating Rho-kinase activity. Results are expressed as mean ± SD. (Reproduced from Gao et al.17 with permission.)
In addition, supplementation with EPA and DHA could exert a protective effect on the heart through improvement in mitochondrial function and the efficiency of ATP generation.18 This effect may be due to changes in mitochondrial membrane phospholipids composition and improved efficiency of ATP generation.18
Inhibition of platelet aggregation
Omega-3 fatty acids decrease the risk of thrombosis by inhibiting platelet aggregation. Importantly, omega-3 fatty acids inhibit platelet TXA2 synthesis and acts as antagonists of the pro-aggregatory TXA2/PG H2 receptor in human platelets in vitro.19 Supplementing a diet with omega-3 fatty acids down-regulate mRNA expression of platelet-derived growth factor-A and -B in mononuclear blood cells in humans.20
Triglyceride-lowering effects
Omega-3 fatty acids play an important role to regulate genes that are critical for controlling lipid homeostasis. Omega-3 fatty acids decrease VLDL assembly and secretion, resulting in diminished triacylglycerol production, through a decreased activity of sterol receptor element-binding protein-1c, which is the key switch in controlling lipogenesis.21 In addition, omega-3 fatty acids could promote β-oxidation simultaneously in mitochondria and/or peroxisomes, possibly through the activation of peroxisome PPAR-α, leading to the reduction of fatty acids substrate for triglyceride synthesis.21,22 The remnant lipoprotein (RLP), produced from the triacylglycerol-rich chylomicrons and VLDL, exerts potent pro-atherogenic effects and is thus regarded as an important risk factor of CVD.22,23 The involvement of RLP has been suggested in the pathogenesis of sudden cardiac death22 and restenosis after coronary angioplasty.23 Although omega-3 fatty acids do not have a major effect on fasting total cholesterol and LDL cholesterol levels, EPA effectively reduces RLP in hyperlipidaemic patients.24
Improvement of endothelial function
Long-term treatment with fish oils augments endothelium-dependent relaxation of normal porcine coronary arteries,25 for which EPA, a major omega-3 fatty acids of fish oils, is responsible for the augmentation.26 This augmenting effect of EPA was also noted in porcine coronary microvessels.27 Long-term treatment with fish oils improves endothelium-dependent relaxation of hypercholesterolaemic and atherosclerotic porcine coronary arteries28 and femoral veins.29 Eicosapentaenoic acid augments endothelium-dependent relaxation by NO as well as that by endothelium-derived hyperpolarizing factor.30 Docosahexaenoic acid alters caveolae microenvironment not only by modifying membrane lipid composition, but also by changing distribution of major structural proteins, eventually increasing eNOS activity in human umbilical vein endothelial cells.31 Nitric oxide also inhibits platelet aggregation and adhesion, leucocytes adhesion, and smooth muscle cell proliferation. In addition, in endothelial cells, co-incubation with DHA following challenge with interleukin (IL)-1, IL-4, tumour necrosis-α, or lipopolysaccharide decreases expression of vascular cell adhesion molecule-1, intercellular adhesion molecule-1 and E-selectin, and secretion of IL-6 and IL-8.32
Plaque stabilization
As mentioned above, through their anti-inflammatory effects, omega-3 fatty acids could not only prevent the plaque development but also contribute to the plaque stabilization.33 The randomized clinical trial demonstrated that omega-3 fatty acids supplementation substantially increases tissue levels of EPA and DHA and decreases macrophage infiltration and thickened fibrous cap in human carotid arteries.34 Exacerbated release of matrix metalloproteinase (MMP) by the activated endothelium and macrophages plays a pathological role in plaque progression and instabilization.35 Eicosapentaenoic acid significantly suppresses the development of atherosclerotic lesions in ApoE−/− and LDL-receptor−/− mice with reduced production of MMPs by macrophages in a PPARα-dependent manner.36
Anti-arrhythmic effects
The omega-3 fatty acids are incorporated into cell membranes and affect the ion-channel function of myocytes. There are several mechanisms by which omega-3 fatty acids could exert anti-arrhythmic effects. Omega-3 fatty acids inhibit voltage-gated Na channels, prolonging relative refractory period and increased voltage that are required for membrane depolarization.37 Omega-3 fatty acids also exhibit a modulatory action on L-type calcium Ca channels, resulting in lowered cytosolic free Ca and Ca influx rate and in preventing cytosolic Ca overload during ischaemic insult.38 Long-term treatment with EPA reduces ischaemia-induced ventricular fibrillation in pigs in vivo, for which attenuation of shortening of monophasic action potential duration through suppression of cardiac KATP channels may be involved.39 Anti-arrhythmic effect of omega-3 fatty acids may be mediated in part by their effects on autonomic control, especially by an increased vagal tone.40 Through these mechanisms, omega-3 fatty acids may prevent ventricular tachyarrhythmias and hence decrease sudden cardiac death.41
Fish, omega-3 fatty acids, and coronary artery disease in cohort studies
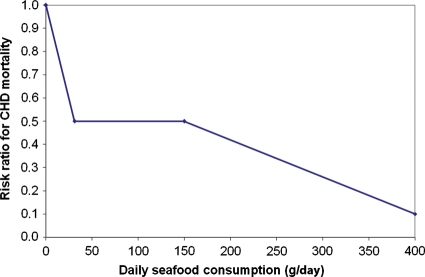
Based on the ecological studies among the Inuit and those comparing farmers and fishermen in Japan, Kromhout et al.8 hypothesized that a low level of fish consumption may reduce CAD mortality. They investigated this association in 852 middle-aged men free from CAD who were followed for 20 years. The average fish consumption in these men was 20 g per day, including those who did not eat fish (20%). About two-thirds of the fish was lean (e.g. cod and plaice) and one-third consisted of fatty fish (e.g. herring and mackerel). An inverse dose–response relationship was observed and CAD mortality was >50% lower among those who consumed at least 30 g of fish per day. Kromhout42 deduced from the studies among the Inuit, Japanese fishermen and farmers, and the Zutphen men that two different mechanisms could be responsible for the association between fish consumption and CAD. He hypothesized an acute effect on fatal CAD in cultures with a low level of fish consumption and a chronic effect in cultures with a high level of fish consumption (Figure 3).
Figure 3.
The association between seafood consumption and fatal coronary heart disease. (Reproduced from Kromhout42 with permission.)
Since 1985, results of many prospective cohort studies on fish consumption and CAD have been published, with several studies showing a protective effect although others did not. The first quantitative review was published in 1999 by Marckmann and Gronbaek43 and included 11 studies with 116 764 individuals. Four studies were judged to be of high quality, of which the two were performed in populations at high risk and the two in populations at low risk. In the high-risk populations, a protective association was found but not in the low-risk populations. The authors drew the conclusion that only in high-risk populations, a fish consumption of 40–60 g per day is associated with a markedly lower CAD mortality.
In 2004, two meta-analyses were published on fish consumption and fatal CAD.44,45 The study of Whelton et al.44 included both prospective cohort studies and case–control studies and the study by He et al.45 only cohort studies. Case–control studies are more prone to selection and information bias and it is particularly difficult to obtain accurate data on fish consumption in patients before the occurrence of a CAD event. Therefore, only the results of the cohort studies are summarized here. The meta-analyses by Whelton et al.44 and He et al.45 were based on 14 and 13 cohort studies, respectively. Both had approximately 220 000 participants who were followed for ∼12 years.
Whelton et al.44 found a 17% lower incidence of fatal CAD (RR = 0.83, 95% CI 0.75–0.92) among those who consumed fish less than twice a week compared with those who ate little or no fish. A similar result was found by He et al.45 for fish consumed once a week (RR = 0.85, 95% CI 0.76–0.96). He et al. observed a dose–response relationship between fish consumption and CAD death and individuals who consumed fish five or more times per week had a 38% lower risk of fatal CAD (RR = 0.62, 95% CI 0.46–0.82). These associations were confirmed in cohort studies in which, besides fish consumption, information about the intake of the omega-3 fatty acids EPA and DHA was also obtained.46–49
There is less evidence for a relationship between fish consumption and non-fatal MI. Based on the results of their meta-analysis, He et al.45 concluded that the evidence for an inverse association between fish consumption and non-fatal MI was weak, even though there was a significant association for those eating fish five times per week or more. This conclusion was confirmed by De Goede et al.,49 who found that consuming fish less than once per month up to once per week was not associated with non-fatal MI in a population-based study in the Netherlands. However, a Japanese cohort study showed that a high level of fish consumption may be protective against non-fatal CAD. In the Japan Public Health Center-based Study, the relative risk (RR) of non-fatal MI was 0.43 (95% CI 0.23–0.81) in participants with a median fish consumption of 180 g per day compared with participants with a daily consumption of 23 g per day.48 These results support the outcome of the meta-analysis of He et al.45 that only a high level of fish consumption may reduce the risk of non-fatal MI.
Fish, omega-3 fatty acids, and sudden death in observational studies
The hypothesis that fish consumption may be protective against sudden cardiac death is derived from the DART trial. This secondary prevention trial showed a significant 33% reduction in CAD mortality in cardiac patients who consumed at least two portions of fatty fish per week and were followed for 2 years. The authors suggested that the protective effect of fatty fish may be due to preventing ventricular fibrillation during acute ischaemia. This hypothesis was tested in two population-based case–control studies.50,51
Siscovick et al.50 identified 334 patients with primary cardiac arrest and 493 population-based controls. An average intake of 185 mg per day of EPA–DHA corresponding to eating fatty fish once a week was associated with a 50% lower risk of primary cardiac arrest (OR = 0.5, 95% CI 0.4–0.8). An even stronger association was observed for the corresponding quartile of red blood cell membrane omega-3 fatty acids (OR = 0.3, 95% CI 0.2–0.6). Similarly, a strong inverse relation was found between baseline blood levels of long-chain omega-3 fatty acids and sudden death in the Physicians' Health Study.51 The RR value was 90% lower in those in the highest compared with the lowest quartile of omega-3 fatty acids (RR = 0.10, 95% CI 0.02–0.48).
The evidence from prospective cohort studies on fish, omega-3 fatty acids, and sudden cardiac death is less convincing than that from population-based case–control studies.52–54 Albert et al.53 showed, using again data from the Physicians' Health Study, that men who consumed one fish meal per week had a 52% lower risk of sudden cardiac death (RR = 0.48, 95% CI 0.24–0.96) compared with those who consumed fish less than once a month. A significant inverse dose–response relationship with sudden cardiac death was not observed for the intake of omega-3 fatty acids, although the data suggested that an intake of ∼200 mg omega-3 fatty acids per day compared with ∼10 mg per day was associated with a lower risk of sudden cardiac death.
In contrast to these findings, sudden cardiac death was not significantly inversely associated with fish consumption in the Western Electric Study.52 In this study, information on causes of death was obtained only from death certificates. Sudden cardiac death was defined as death occurring no more than 12 h after the onset of the terminal acute illness. In the Physicians' Health Study, detailed information was available from next of kin, medical records, and autopsy reports; and sudden death was defined as death within 1h of the onset of symptoms. This definition of sudden cardiac death is superior to the one used in the Western Electric Study.
The association between long-term fish consumption, omega-3 fatty acids, and sudden cardiac death was also investigated in the Zutphen Study.54 Long-term fatty fish consumption was inversely associated with sudden coronary death, and men who consumed fatty fish had a 54% lower risk (RR = 0.46, 95% CI 0.27–0.78) than those who did not eat fatty fish. Lean fish consumption was not associated with sudden coronary death. The intake of omega-3 fatty acids was also inversely related to sudden coronary death but this association was not statistically significant.
In summary, the results of the population-based case–control and prospective cohort studies suggest a protective effect of fish consumption on cardiac arrest and sudden death. The two case–control studies showed the strongest effect for the omega-3 fatty acids measured in blood.
Fish oils and cardiovascular diseases in randomized trials
Several trials tested the hypothesis that omega-3 fatty acids reduce fatal CAD and sudden death. The first meta-analysis of these trials was published in 2002,55 followed by others.56–59 However, several meta-analyses included not only trials in which the effect of omega-3 fatty acids in fish oils was investigated but also trials in which a fish advice or margarines enriched with ALA were given.55,56,58 One meta-analysis on fish oils included besides patients with MI, CAD, and heart failure also patients with peripheral vascular diseases, hypercholesterolaemia, and implanted cardioverter defibrillators (ICDs).58 Only the meta-analysis by León et al.57 evaluated the effect of EPA–DHA in a homogeneous group of patients with CAD or had had an MI. They used fatal CAD, sudden cardiac death, and severe arrhythmias as endpoints.
In three trials, patients with an ICD were included. In these trials, fish oil capsules containing an additional amount of 0.9–2.8 g omega-3 fatty acids per day reduced the risk of severe arrhythmias by 10% (OR = 0.90, 95% CI 0.55–1.46).57 A similar result was found in a meta-analysis by Brouwer et al.60 based on the same studies. Eight trials using fish oil capsules containing 0.9–2.8 g of EPA–DHA showed a significant 20% reduction of cardiac death (OR = 0.80, 95% CI 0.69–0.93).57 In four trials, an additional amount of 0.9–2.4 g of EPA–DHA per day reduced the incidence of sudden cardiac death by 26% (OR = 0.74, 95% CI 0.59–0.92).57 The results for fatal CAD and sudden death were dominated by those of the GISSI-Prevenzione trial41 that contributed >85% to both endpoints.57
In 2010, the results of the Alpha Omega, OMEGA, and SU.FOL.OM3 trials were published.61–63 The results of these trials and those of the large trials published before 2010—the GISSI-Prevenzione trial, the secondary prevention component of the JELIS trial, and the GISSI Heart Failure trial—will be discussed in detail64,65 (Table 1). The GISSI-HF published in 200865 and the three trials published in 201061–63 were not included in the meta-analysis of León et al.57
Table 1.
Effects of fish oil on cardiovascular diseases in trials with patients with heart disease
| GISSI-P 1999 (41) | JELIS 2007 (64) | GISSI-HF 2008 (65) | Alpha Omega 2010 (61) | OMEGA 2010 (62) | SU.FOL.OM3 2010 (63) | |
|---|---|---|---|---|---|---|
| Number | 11 324 | 3664 | 7046 | 4837 | 3851 | 2501 |
| Patients | Post-MI | CAD | HF | Post-MI | Post-MI | CAD |
| Post-event | <3 months | <10 years | 3–14 days | <12 months | ||
| Design | Open label | Open label | Double-blind | Double-blind | Double-blind | Double-blind |
| Inclusion period | 1993–95 | 1996–99 | 2002–05 | 2002–06 | 2003–07 | 2003–07 |
| Follow-up (months) | 42 | 55 | 47 | 41 | 12 | 56 |
| Person-years | 38 505 | 15 531 | 10 656 | |||
| Dose EPA (mg) | 289 | 1800 | 394 | 226 | 460 | 400 |
| Dose DHA (mg) | 577 | 0 | 472 | 150 | 380 | 200 |
| Medication use (%) | ||||||
| Antiplatelets | 88 | 87 | 98 | 95 | 94 | |
| Antihypertensives | 90 | |||||
| Beta-blockers | 41 | 65 | 69 | 94 | 68 | |
| ACE-I/ARBs | 41 | 94 | 56 | 91 | 66 | |
| Statins | 29 | 97 | 23 | 85 | 94 | 87 |
| Number of events | ||||||
| MCE | 1115 | 355 | 4359 | 671 | 331 | |
| Fatal CVD | 639 | 1447 | 162 | 157a | ||
| Fatal CAD | 479 | 39 | 236a | 138 | ||
| Sudden death | 286 | 26 | 632 | 57 | 57 | |
| Relative risk | ||||||
| MCE | 0.80* | 0.81* | 0.92* | 1.01 | 1.21 | |
| Fatal CVD | 0.70* | 0.90* | 0.98 | 1.08a | ||
| Fatal CAD | 0.65* | 0.87a | 0.82a | 0.95 | ||
| Sudden death | 0.55* | 1.02 | 0.93 | 0.90 | 0.95 | |
MI, myocardial infarction; CAD, coronary artery diseases; HF, heart failure; MCE, major cardiovascular event; CVD, cardiovascular diseases.
aFatal and non-fatal events.
*P < 0.05.
The number of patients included in these trials ranged from 2501 to 11 324 with 15–26% females. Three trials included post-MI patients,41,61,62 two trials CAD patients,63,64 and one trial heart failure patients.65 The average age of the patients varied between 59 and 69 years. Two trials recruited patients in the 1990s and used an open-label design.41,64 The remaining trials were initiated between 2002 and 2007 and were double-blind.61–63,65 The OMEGA trial had a 12-month follow-up and in the other trials the average follow-up period varied between 41 and 56 months. In four trials, the patients received fish oil capsules containing 600–900 mg of EPA–DHA per day and in the JELIS trial 1800 mg of EPA per day. In the Alpha Omega Trial, margarine spreads provided an average additional intake of EPA–DHA of ∼400 mg per day.61
The most important commonly used endpoints in these trials were major cardiovascular events, fatal CVD, fatal CAD, and sudden death. The strongest effects were observed in the GISSI-P trial for patients surviving a recent MI. In this trial, an additional amount of EPA–DHA of 900 mg per day reduced significantly fatal CVD by 30%, fatal CAD by 35%, and sudden death by 45%.41 In the GISSI-HF trial, in which heart failure patients were included, fatal CVD was significantly reduced by 10%, sudden death non-significantly by 7%, and first hospital admissions for ventricular arrhythmias significantly by 28%.65 The JELIS trial showed that an additional intake of 1800 mg of EPA per day reduced only major coronary events (fatal and non-fatal CAD, unstable angina, percutaneous coronary intervention, and coronary artery bypass grafting).64 The three trials published in 2010 included either post-MI or CAD patients.61–63 Additional amounts of EPA–DHA varying from 400–800 mg/day did not reduce cardiovascular events.
The strongest reductions in cardiovascular endpoints were obtained in the oldest trials. An explanation could be differences in study design. The GISSI-P and the JELIS trial used an open label design.41,64 This may have confounded the results of these trials, because placebo capsules were lacking. Another explanation could be that the patients in the more recent trials were very well treated not only by antithrombotics but also by antihypertensives and statins. Compared with the recent trials, the treatment level with statins was low in the GISSI-P trial (29%).41 This could be the reason for the high risk of fatal CAD and sudden death in the GISSI-P trial compared with the Alpha Omega Trial. The absolute risk for fatal CAD in the control group was 15.8/1000 person-years in the GISSI-P and 8.9/1000 person-years in the Alpha Omega Trial. For sudden death, the rates were 10.4/1000 person-years in the GISSI-P and 3.7/1000 person-years in the Alpha Omega Trial. A likely explanation is that these differences in absolute risk between the trials were responsible for the absence of an effect of EPA–DHA on fatal CAD and sudden death in the recent trials.
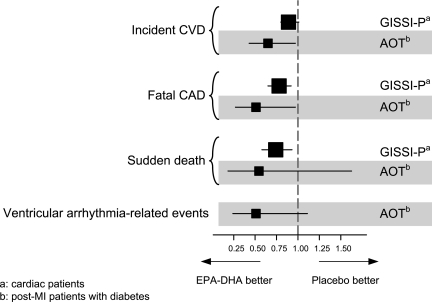
Those differences in absolute risk of fatal CAD and sudden death could play an important role in explaining the different results in the GISSI-P and the Alpha Omega Trial. This is supported by the results of the subgroup analysis of patients in the Alpha Omega Trial who also had diabetes.61 The absolute risk of fatal CAD in the control group of diabetes patients in the Alpha Omega Trial was 17.1/1000 person-years. This is in the same order of magnitude as the absolute risk in the control group of the GISSI-P trial.41 In the diabetes patients who received an additional amount of 400 mg of EPA–DHA per day, a significant reduction in fatal CAD was obtained comparable with the GISSI-P trial (Figure 4). Similar results were found in the Alpha Omega Trial for sudden death and ventricular arrhythmia-related events, although these effects were not statistically significant.
Figure 4.
Effect of eicosapentaenoic acid–docosahexaenoic acid on cardiovascular diseases in the Alpha Omega Trial (AOT) and the GISSI-Prevenzione trial (GISSI-P). CVD, cardiovascular diseases; CAD, coronary artery diseases.
Emerging issues on the effects of fish oils/omega-3 fatty acids
Results of observational prospective cohort studies and randomized trials in subjects with or without CVD published before 2000 demonstrated that diets with higher amounts of omega-3 fatty acids or supplements with omega-3 fatty acids reduced cardiovascular mortality. These results formed the basis for recommendations, including the American Heart Association Guidelines, that patients with documented CAD should be advised to take 900–1000 mg of omega-3 fatty acids (EPA–DHA combined) per day.66 However, this recommendation was challenged in a review and meta-analysis published by Hooper et al.56 in 2006. They concluded that there was no clear benefit of additional amount of omega-3 fatty acids on cardiovascular events. In addition, the three recently published double-blind trials—the Alpha Omega, the OMEGA, and the SU.FOL.OM3—did not show an effect of an additional amount of EPA–DHA on major cardiovascular endpoints.61–63 These negative results with omega-3 fatty acids supplementation were disappointing but were obtained in the current practice where other optimal conventional drug therapy was performed. It should be pointed out, however, that the OMEGA and the SU.FOL.OM3 trial were also underpowered.62,63
In addition, there is some evidence for possible pro-arrhythmic effects of omega-3 fatty acids in certain subgroups with CVD. In the three randomized controlled trials of patients with an implantable cardioverter defibrillator (ICD) and a history of ventricular tachyarrhythmias, fish oil of omega-3 fatty acids did not show a significant benefit on the risk of appropriate ICD shocks.57,60 In a trial of patients with stable angina pectoris without previous MI, a detrimental effect of omega-3 fatty acids on sudden death was observed.67 Thus, further studies are needed to determine which patient population may or may not benefit from omega-3 fatty acids supplementation. Evidence is also insufficient regarding the optimal dose, source (oily fish or fish-oil supplements), and formulation of EPA and/or DHA in order to reduce cardiovascular events.68
Recently, a potential new indication of omega-3 fatty acids has been demonstrated, that is heart failure.36 In the GISSI-HF trial,65 a placebo-controlled trial of approximately 7000 patients with class II to IV heart failure, the patients were randomized to 1 g of omega-3 fatty acids (containing 850–882 mg of EPA plus DHA), rosuvastatin (10 mg), both of them, or dual placebo. This study was performed in addition to well-established current therapies, and the results showed a significant benefit of omega-3 fatty acids.65 However, the optimal dose of omega-3 fatty acids remains to be determined depending on different stages and/or aetiology of heart failure and underlying mechanisms.68 Growing evidence demonstrates anti-inflammatory effects of omega-3 fatty acids, including reduced circulating levels of inflammatory cytokines and AA-derived eicosanoids, and elevated plasma adiponectin.18 In animal studies, fish oil favourably alters cardiac mitochondrial function.18 All of these effects may work together to prevent the development and progression of heart failure.
Several issues remain to be elucidated. First, no evidence has been found for the optimal dosage, ratios of DHA to EPA, and ratios of omega-3 to omega-6. Second, whether dietary intake or therapeutic supplements are the best source of omega-3 fatty acids is yet to be determined. These issues remain to be clarified in future studies.
Conclusions
Omega-3 fatty acids exert pleiotropic, cardiometabolic effects with a diverse range of actions, most of which are beneficial for the cardiovascular system. Supplementation up to 1 g of omega-3 fatty acids per day is well tolerated except dysgeusia and does not increase the risk of bleeding. Recently published trials in patients with CAD or after MI did not show an effect of omega-3 fatty acids on major cardiovascular endpoints, probably due to state of the art drug treatment. However, as suggested by the current guidelines, the potential value of omega-3 fatty acids supplementation in patients with CAD or after MI and possibly in those with heart failure remains to be encouraged.
Funding
The work by the authors in this review article was supported in part by the Netherlands Heart Foundation, the Netherlands Prevention Foundation, the National Institutes of Health, USA, and an unrestricted grant of Unilever R&D (to D.K.), and the grants from the Japanese Ministry of Education, Sports, and Culture, Tokyo, Japan, and those from the Japanese Ministry of Health, Labour and Welfare, Tokyo, Japan (to H.S.).
Conflict of interest: none declared.
References
- 1.Burr ML. Lessons from the story of n-3 fatty acids. Am J Clin Nutr. 2000;71(Suppl. 1):397S–398S. doi: 10.1093/ajcn/71.1.397s. [DOI] [PubMed] [Google Scholar]
- 2.Sinclair HM. The diet of Canadian Eskimos. Proc Nutr Soc. 1953;12:69–82. doi:10.1079/PNS19530016. [Google Scholar]
- 3.Kromann N, Green A. Epidemiological studies in the Upernavik district, Greenland. Incidence of some chronic diseases 1950–1974. Acta Med Scand. 1980;208:401–406. doi:10.1111/j.0954-6820.1980.tb01221.x. [PubMed] [Google Scholar]
- 4.Bang HO, Dyerberg J. Plasma lipids and lipoproteins in Greenlandic west coast Eskimos. Acta Med Scand. 1972;192:85–94. doi: 10.1111/j.0954-6820.1972.tb04782.x. doi:10.1111/j.0954-6820.1972.tb04782.x. [DOI] [PubMed] [Google Scholar]
- 5.Bang HO, Dyerberg J, Sinclair HM. The composition of the Eskimo food in north western Greenland. Am J Clin Nutr. 1980;33:2657–2661. doi: 10.1093/ajcn/33.12.2657. [DOI] [PubMed] [Google Scholar]
- 6.Sinclair HM. Deficiency of essential fatty acids and atherosclerosis, etcetera. Lancet. 1956;270:381–383. [PubMed] [Google Scholar]
- 7.Sinclair HM. Advantages and disadvantages of an Eskimo diet. In: Fumagalli R, Kritchevsky D, Peoletti R, editors. Drugs Affecting Lipid Metabolism. Amsterdam: Elsevier/North-Holland Biomedical Press; 1980. pp. 363–370. [Google Scholar]
- 8.Kromhout D, Bosschieter EB, de Lezenne Coulander C. The inverse relation between fish consumption and 20-year mortality from coronary heart disease. N Engl J Med. 1985;312:1205–1209. doi: 10.1056/NEJM198505093121901. doi:10.1056/NEJM198505093121901. [DOI] [PubMed] [Google Scholar]
- 9.Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, Elwood PC, Deadman NM. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: Diet and Reinfarction Trial (DART) Lancet. 1989;2:757–761. doi: 10.1016/s0140-6736(89)90828-3. doi:10.1016/S0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- 10.Shimokawa H. Beneficial effects of eicosapentaenoic acid on endothelial vasodilator functions in animals and humans. In: Hamazaki T, Okuyama H, editors. Fatty Acids and Lipids - New Findings, World Review of Nutrition and Dietics. Vol. 88. Basel, Switzerland: Karger; 2001. pp. 100–108. [DOI] [PubMed] [Google Scholar]
- 11.Calder PC. N-3 polyunsaturated fatty acids and inflammation: from molecular biology to the clinic. Lipids. 2003;38:343–352. doi: 10.1007/s11745-003-1068-y. doi:10.1007/s11745-003-1068-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adkins Y, Kelley DS. Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. J Nutr Biochem. 2010;21:781–792. doi: 10.1016/j.jnutbio.2009.12.004. doi:10.1016/j.jnutbio.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 13.de Winther MP, Kanters E, Kraal G, Hofker MH. NF-κB signaling in atherogenesis. Arterioscler Thromb Vasc Biol. 2005;25:904–914. doi: 10.1161/01.ATV.0000160340.72641.87. doi:10.1161/01.ATV.0000160340.72641.87. [DOI] [PubMed] [Google Scholar]
- 14.Shimokawa H, Takeshita A. Rho-kinase is an important therapeutic target in cardiovascular medicine. Arterioscler Thromb Vasc Biol. 2005;25:1767–1775. doi: 10.1161/01.ATV.0000176193.83629.c8. doi:10.1161/01.ATV.0000176193.83629.c8. [DOI] [PubMed] [Google Scholar]
- 15.Takemoto M, Sun J, Hiroki J, Shimokawa H, Liao JK. Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation. 2002;106:57–62. doi: 10.1161/01.cir.0000020682.73694.ab. doi:10.1161/01.CIR.0000020682.73694.AB. [DOI] [PubMed] [Google Scholar]
- 16.Yada T, Shimokawa H, Hiramatsu O, Kajita T, Shigeto F, Tanaka E, Shinozaki Y, Mori H, Kiyooka T, Katsura M, Ohkuma S, Goto M, Ogasawara Y, Kajiya F. Beneficial effect of hydroxyfasudil, a specific Rho-kinase inhibitor, on ischemia/reperfusion injury in canine coronary microcirculation in vivo. J Am Coll Cardiol. 2005;45:599–607. doi: 10.1016/j.jacc.2004.10.053. doi:10.1016/j.jacc.2004.10.053. [DOI] [PubMed] [Google Scholar]
- 17.Gao JY, Yasuda S, Tsuburaya R, Ito Y, Shiroto T, Hao K, Aizawa K, Kikuchi Y, Ito K, Shimokawa H. Long-term treatment with eicosapentaenoic acid ameliorates myocardial ischemia-reperfusion injury in pigs in vivo. Involvement of Rho-kinase pathway inhibition. Circ J. 2011;75:1843–1851. doi: 10.1253/circj.cj-11-0209. [DOI] [PubMed] [Google Scholar]
- 18.Duda MK, O'Shea KM, Stanley WC. Omega-3 polyunsaturated fatty acid supplementation for the treatment of heart failure: mechanisms and clinical potential. Cardiovasc Res. 2009;84:33–41. doi: 10.1093/cvr/cvp169. doi:10.1093/cvr/cvp169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swann PG, Venton DL, Le Breton GC. Eicosapentaenoic acid and docosahexaenoic acid are antagonists at the thromboxane A2/prostaglandin H2 receptor in human platelets. FEBS Lett. 1989;243:244–246. doi: 10.1016/0014-5793(89)80137-1. doi:10.1016/0014-5793(89)80137-1. [DOI] [PubMed] [Google Scholar]
- 20.Kaminski WE, Jendraschak E, Kiefl R, von Schacky C. Dietary omega-3 fatty acids lower levels of platelet-derived growth factor mRNA in human mononuclear cells. Blood. 1993;81:1871–1879. [PubMed] [Google Scholar]
- 21.Sampath H, Ntambi JM. Polyunsaturated fatty acid regulation of genes of lipid metabolism. Annu Rev Nutr. 2005;25:317–340. doi: 10.1146/annurev.nutr.25.051804.101917. doi:10.1146/annurev.nutr.25.051804.101917. [DOI] [PubMed] [Google Scholar]
- 22.Oi K, Shimokawa H, Hiroki J, Uwatoku T, Abe K, Matsumoto Y, Nakajima Y, Nakajima K, Takeichi S, Takeshita A. Remnant lipoproteins from patients with sudden cardiac death enhance coronary vasospastic activity through upregulation of Rho-kinase. Arterioscler Thromb Vasc Biol. 2004;24:918–922. doi: 10.1161/01.ATV.0000126678.93747.80. doi:10.1161/01.ATV.0000126678.93747.80. [DOI] [PubMed] [Google Scholar]
- 23.Oi K, Shimokawa H, Hirakawa Y, Tashiro H, Nakaike R, Kozai T, Ohzono K, Yamamoto K, Koyanagi S, Okamatsu S, Tajimi T, Kikuchi Y, Takeshita A. Postprandial increase in plasma concentrations of remnant-like particles: an independent risk factor for restenosis after percutaneous coronary intervention. J Cardiovasc Pharmacol. 2004;44:66–73. doi: 10.1097/00005344-200407000-00009. doi:10.1097/00005344-200407000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura N, Hamazaki T, Ohta M, Okuda K, Urakaze M, Sawazaki S, Yamazaki K, Satoh A, Temaru R, Ishikura Y, Takata M, Kishida M, Kobayashi M. Joint effects of HMG-CoA reductase inhibitors and eicosapentaenoic acids on serum lipid profile and plasma fatty acid concentrations in patients with hyperlipidemia. Int J Clin Lab Res. 1999;29:22–25. doi: 10.1007/s005990050057. doi:10.1007/s005990050057. [DOI] [PubMed] [Google Scholar]
- 25.Shimokawa H, Lam JYT, Chesebro JH, Bowie EJW, Vanhoutte PM. Effects of dietary supplementation with cod-liver oil on endothelium-dependent responses in porcine coronary arteries. Circulation. 1987;76:898–905. doi: 10.1161/01.cir.76.4.898. doi:10.1161/01.CIR.76.4.898. [DOI] [PubMed] [Google Scholar]
- 26.Shimokawa H, Vanhoutte PM. Dietary ω-3 fatty acids and endothelium-dependent relaxations in porcine coronary arteries. Am J Physiol. 1989;256:H968–H973. doi: 10.1152/ajpheart.1989.256.4.H968. [DOI] [PubMed] [Google Scholar]
- 27.Shimokawa H, Aarhus LL, Vanhoutte PM. Dietary ω-3 polyunsaturated fatty acids augment endothelium-dependent relaxation to bradykinin in coronary microvessels of the pig. Br J Pharmacol. 1988;95:1197–1203. doi: 10.1111/j.1476-5381.1988.tb11755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimokawa H, Vanhoutte PM. Dietary cod-liver oil improves endothelium-dependent responses in hypercholesterolemic and atherosclerotic porcine coronary arteries. Circulation. 1988;78:1421–1430. doi: 10.1161/01.cir.78.6.1421. doi:10.1161/01.CIR.78.6.1421. [DOI] [PubMed] [Google Scholar]
- 29.Komori K, Shimokawa H, Vanhoutte PM. Endothelium-dependent relaxation to aggregating platelets in porcine femoral veins and its modulation by diets. Circulation. 1989;80:401–409. doi: 10.1161/01.cir.80.2.401. doi:10.1161/01.CIR.80.2.401. [DOI] [PubMed] [Google Scholar]
- 30.Tagawa T, Hirooka Y, Shimokawa H, Hironaga K, Sakai K, Oyama J, Takeshita A. Long-term treatment with eicosapentaenoic acid improves exercise-induced vasodilation in patients with coronary artery disease. Hypertens Res. 2002;25:823–829. doi: 10.1291/hypres.25.823. doi:10.1291/hypres.25.823. [DOI] [PubMed] [Google Scholar]
- 31.Li Q, Zhang Q, Wang M, Liu F, Zhao S, Ma J, Luo N, Li N, Li Y, Xu G, Li J. Docosahexaenoic acid affects endothelial nitric oxide synthase in caveolae. Arch Biochem Biophys. 2007;466:250–259. doi: 10.1016/j.abb.2007.06.023. doi:10.1016/j.abb.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 32.De Caterina R, Cybulsky MI, Clinton SK, Gimbrone MA, Jr, Libby P. The omega-3 fatty acid docosahexaenoate reduces cytokine-induced expression of proatherogenic and proinflammatory proteins in human endothelial cells. Arterioscler Thromb. 1994;14:1829–1836. doi: 10.1161/01.atv.14.11.1829. doi:10.1161/01.ATV.14.11.1829. [DOI] [PubMed] [Google Scholar]
- 33.Yasuda S, Shimokawa H. Potential usefulness of fish oil in the primary prevention of acute coronary syndrome. Eur Heart J. 2010;31:15–16. doi: 10.1093/eurheartj/ehp478. doi:10.1093/eurheartj/ehp478. [DOI] [PubMed] [Google Scholar]
- 34.Thies F, Garry JM, Yaqoob P, Rerkasem K, Williams J, Shearman CP, Gallagher PJ, Calder PC, Grimble RF. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. Lancet. 2003;361:477–485. doi: 10.1016/S0140-6736(03)12468-3. doi:10.1016/S0140-6736(03)12468-3. [DOI] [PubMed] [Google Scholar]
- 35.Morishige K, Shimokawa H, Matsumoto Y, Eto Y, Uwatoku T, Abe K, Sueishi K, Takeshita A. Overexpression of matrix metalloproteinase-9 promotes intravascular thrombus formation in porcine coronary arteries in vivo. Cardiovasc Res. 2003;57:572–585. doi: 10.1016/s0008-6363(02)00710-1. doi:10.1016/S0008-6363(02)00710-1. [DOI] [PubMed] [Google Scholar]
- 36.Matsumoto M, Sata M, Fukuda D, Tanaka K, Soma M, Hirata Y, Nagai R. Orally administered eicosapentaenoic acid reduces and stabilizes atherosclerotic lesions in ApoE-deficient mice. Atherosclerosis. 2008;197:524–533. doi: 10.1016/j.atherosclerosis.2007.07.023. doi:10.1016/j.atherosclerosis.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 37.Billman GE, Kang JX, Leaf A. Prevention of sudden cardiac death by dietary pure omega-3 polyunsaturated fatty acids in dogs. Circulation. 1999;99:2452–2457. doi: 10.1161/01.cir.99.18.2452. [DOI] [PubMed] [Google Scholar]
- 38.Hallaq H, Smith TW, Leaf A. Modulation of dihydropyridine-sensitive calcium channels in heart cells by fish oil fatty acids. Proc Natl Acad Sci USA. 1992;89:1760–1764. doi: 10.1073/pnas.89.5.1760. doi:10.1073/pnas.89.5.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsuburaya R, Yasuda S, Ito Y, Shiroto T, Gao JY, Ito K, Shimokawa H. Eicosapentaenoic acid reduces ischemic ventricular fibrillation via altering monophasic action potential in pigs. J Mol Cell Cardiol. 2011;51:329–336. doi: 10.1016/j.yjmcc.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 40.Christensen JH, Schmidt EB. Autonomic nervous system, heart rate variability and n-3 fatty acids. J Cardiovasc Med (Hagerstown) 2007;8(Suppl. 1):S19–S22. doi: 10.2459/01.JCM.0000289276.10675.a1. doi:10.2459/01.JCM.0000289276.10675.a1. [DOI] [PubMed] [Google Scholar]
- 41.GISSI Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet. 1999;354:447–455. doi:10.1016/S0140-6736(99)07072-5. [PubMed] [Google Scholar]
- 42.Kromhout D. N-3 fatty acids and coronary heart disease: epidemiology from Eskimos to Western populations. J Intern Med Suppl. 1989;731:47–51. doi: 10.1111/j.1365-2796.1989.tb01435.x. [DOI] [PubMed] [Google Scholar]
- 43.Marckmann P, Gronbaek M. Fish consumption and coronary heart disease mortality. A systematic review of prospective cohort studies. Eur J Clin Nutr. 1999;53:585–590. doi: 10.1038/sj.ejcn.1600832. doi:10.1038/sj.ejcn.1600832. [DOI] [PubMed] [Google Scholar]
- 44.Whelton SP, He J, Whelton PK, Muntner P. Meta-analysis of observational studies on fish intake and coronary heart disease. Am J Cardiol. 2004;93:1119–1123. doi: 10.1016/j.amjcard.2004.01.038. doi:10.1016/j.amjcard.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 45.He K, Song Y, Daviglus ML, Liu K, Van Horn L, Dyer AR, Greenland P. Accumulated evidence on fish consumption and coronary heart disease mortality: a meta-analysis of cohort studies. Circulation. 2004;109:2705–2711. doi: 10.1161/01.CIR.0000132503.19410.6B. doi:10.1161/01.CIR.0000132503.19410.6B. [DOI] [PubMed] [Google Scholar]
- 46.Ascherio A, Rimm EB, Stampfer MJ, Giovannucci EL, Willett WC. Dietary intake of marine n-3 fatty acids, fish intake, and the risk of coronary disease among men. N Engl J Med. 1995;332:977–982. doi: 10.1056/NEJM199504133321501. doi:10.1056/NEJM199504133321501. [DOI] [PubMed] [Google Scholar]
- 47.Hu FB, Bronner L, Willett WC, Stampfer MJ, Rexrode KM, Albert CM, Hunter D, Manson JE. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA. 2002;287:1815–1821. doi: 10.1001/jama.287.14.1815. doi:10.1001/jama.287.14.1815. [DOI] [PubMed] [Google Scholar]
- 48.Iso H, Kobayashi M, Ishihara J, Sasaki S, Okada K, Kita Y, Kokubo Y, Tsugane S. Intake of fish and n3 fatty acids and risk of coronary heart disease among Japanese: the Japan Public Health Center-Based (JPHC) Study Cohort I. Circulation. 2006;113:195–202. doi: 10.1161/CIRCULATIONAHA.105.581355. doi:10.1161/CIRCULATIONAHA.105.581355. [DOI] [PubMed] [Google Scholar]
- 49.de Goede J, Geleijnse JM, Boer JM, Kromhout D, Verschuren WM. Marine (n-3) fatty acids, fish consumption, and the 10-year risk of fatal and nonfatal coronary heart disease in a large population of Dutch adults with low fish intake. J Nutr. 2010;140:1023–1028. doi: 10.3945/jn.109.119271. doi:10.3945/jn.109.119271. [DOI] [PubMed] [Google Scholar]
- 50.Siscovick DS, Raghunathan TE, King I, Weinmann S, Wicklund KG, Albright J, Bovbjerg V, Arbogast P, Smith H, Kushi LH, Cobb LA, Copass MK, Psaty BM, Lemaitre R, Retzlaff B, Childs M, Knopp RH. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA. 1995;274:1363–1367. doi: 10.1001/jama.1995.03530170043030. doi:10.1001/jama.274.17.1363. [DOI] [PubMed] [Google Scholar]
- 51.Albert CM, Campos H, Stampfer MJ, Ridker PM, Manson JE, Willett WC, Ma J. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med. 2002;346:1113–1118. doi: 10.1056/NEJMoa012918. doi:10.1056/NEJMoa012918. [DOI] [PubMed] [Google Scholar]
- 52.Daviglus ML, Stamler J, Orencia AJ, Dyer AR, Liu K, Greenland P, Walsh MK, Morris D, Shekelle RB. Fish consumption and the 30-year risk of fatal myocardial infarction. N Engl J Med. 1997;336:1046–1053. doi: 10.1056/NEJM199704103361502. doi:10.1056/NEJM199704103361502. [DOI] [PubMed] [Google Scholar]
- 53.Albert CM, Hennekens CH, O'Donnell CJ, Ajani UA, Carey VJ, Willett WC, Ruskin JN, Manson JE. Fish consumption and risk of sudden cardiac death. JAMA. 1998;279:23–28. doi: 10.1001/jama.279.1.23. doi:10.1001/jama.279.1.23. [DOI] [PubMed] [Google Scholar]
- 54.Streppel MT, Ocké MC, Boshuizen HC, Kok FJ, Kromhout D. Long-term fish consumption and n-3 fatty acid intake in relation to (sudden) coronary heart disease death: the Zutphen study. Eur Heart J. 2008;29:2024–2030. doi: 10.1093/eurheartj/ehn294. doi:10.1093/eurheartj/ehn294. [DOI] [PubMed] [Google Scholar]
- 55.Bucher HC, Hengstler P, Schindler C, Meier G. N-3 polyunsaturated fatty acids in coronary heart disease: a meta-analysis of randomized controlled trials. Am J Med. 2002;112:298–304. doi: 10.1016/s0002-9343(01)01114-7. doi:10.1016/S0002-9343(01)01114-7. [DOI] [PubMed] [Google Scholar]
- 56.Hooper L, Thompson RL, Harrison RA, Summerbell CD, Ness AR, Moore HJ, Worthington HV, Durrington PN, Higgins JP, Capps NE, Riemersma RA, Ebrahim SB, Davey Smith G. Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: systematic review. BMJ. 2006;332:752–760. doi: 10.1136/bmj.38755.366331.2F. doi:10.1136/bmj.38755.366331.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.León H, Shibata MC, Sivakumaran S, Dorgan M, Chatterley T, Tsuyuki RT. Effect of fish oil on arrhythmias and mortality: systematic review. BMJ. 2008;337:a2931. doi: 10.1136/bmj.a2931. doi:10.1136/bmj.a2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marik PE, Varon J. Omega-3 dietary supplements and the risk of cardiovascular events: a systematic review. Clin Cardiol. 2009;32:365–372. doi: 10.1002/clc.20604. doi:10.1002/clc.20604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao YT, Chen Q, Sun YX, Li XB, Zhang P, Xu Y, Guo JH. Prevention of sudden cardiac death with omega-3 fatty acids in patients with coronary heart disease: a meta-analysis of randomized controlled trials. Ann Med. 2009;41:301–310. doi: 10.1080/07853890802698834. doi:10.1080/07853890802698834. [DOI] [PubMed] [Google Scholar]
- 60.Brouwer IA, Raitt MH, Dullemeijer C, Kraemer DF, Zock PL, Morris C, Katan MB, Connor WE, Camm JA, Schouten EG, McAnulty J. Effect of fish oil on ventricular tachyarrhythmia in three studies in patients with implantable cardioverter defibrillators. Eur Heart J. 2009;30:820–826. doi: 10.1093/eurheartj/ehp003. doi:10.1093/eurheartj/ehp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kromhout D, Giltay EJ, Geleijnse JM. n-3 Fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363:2015–2026. doi: 10.1056/NEJMoa1003603. doi:10.1056/NEJMoa1003603. [DOI] [PubMed] [Google Scholar]
- 62.Rauch B, Schiele R, Schneider S, Diller F, Victor N, Gohlke H, Gottwik M, Steinbeck G, Del Castillo U, Sack R, Worth H, Katus H, Spitzer W, Sabin G, Senges J. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation. 2010;122:2152–2159. doi: 10.1161/CIRCULATIONAHA.110.948562. [DOI] [PubMed] [Google Scholar]
- 63.Galan P, Kesse-Guyot E, Czernichow S, Briancon S, Blacher J, Hercberg S. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo controlled trial. BMJ. 2010;341:c6273. doi: 10.1136/bmj.c6273. doi:10.1136/bmj.c6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. doi:10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 65.Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–1230. doi: 10.1016/S0140-6736(08)61239-8. doi:10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 66.Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckelbaum RJ, Erdman JW, Jr, Kris-Etherton P, Goldberg IJ, Kotchen TA, Lichtenstein AH, Mitch WE, Mullis R, Robinson K, Wylie-Rosett J, St Jeor S, Suttie J, Tribble DL, Bazzarre TL. AHA Dietary Guidelines: revision 2000: a statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation. 2000;102:2284–2299. doi: 10.1161/01.cir.102.18.2284. [DOI] [PubMed] [Google Scholar]
- 67.Burr ML, Ashfield-Watt PA, Dunstan FD, Fehily AM, Breay P, Ashton T, Zotos PC, Haboubi NA, Elwood PC. Lack of benefit of dietary advice to men with angina: results of a controlled trial. Eur J Clin Nutr. 2003;57:193–200. doi: 10.1038/sj.ejcn.1601539. doi:10.1038/sj.ejcn.1601539. [DOI] [PubMed] [Google Scholar]
- 68.Lavie CJ, Milani RV, Mehra MR, Ventura HO. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J Am Coll Cardiol. 2009;54:585–594. doi: 10.1016/j.jacc.2009.02.084. doi:10.1016/j.jacc.2009.02.084. [DOI] [PubMed] [Google Scholar]