Abstract
Aims
Increasing evidence supports a role for inflammation in promoting atrial fibrillation (AF) and statins have anti-inflammatory effects that may be relevant for the prevention of AF. However, studies of statin therapy and incident AF have yielded mixed results and not focused on individuals with an underlying pro-inflammatory response. We studied whether high-sensitivity C-reactive protein is associated with incident AF and whether treatment with rosuvastatin is associated with a lower incidence of AF compared with placebo.
Methods and results
We randomized men and women with LDL cholesterol <130 mg/dL and high-sensitivity C-reactive protein ≥2 mg/L to receive either rosuvastatin 20 mg daily or placebo. Atrial fibrillation was determined from treatment-blind adverse event reports. Among 17 120 participants without prior history of arrhythmia, each increasing tertile of baseline high-sensitivity C-reactive protein was associated with a 36% increase in the risk of developing AF (95% CI: 1.16–1.60; P-trend < 0.01). Allocation to rosuvastatin when compared with placebo was associated with a 27% reduction in the relative risk of developing AF during the trial period; specifically, AF was reported among 138 participants in the placebo group and 100 in the rosuvastatin group (incidence rate 0.78 vs. 0.56/100 person-years, HR: 0.73, 95% CI: 0.56–0.94, P = 0.01). The exclusion of participants who developed a major cardiovascular event prior to the report of AF yielded similar results.
Conclusion
Within the JUPITER trial cohort of individuals selected for underlying inflammation, increasing levels of high-sensitivity C-reactive protein were associated with an increased risk of incident AF and random allocation to rosuvastatin significantly reduced that risk.
Keywords: C-reactive protein, Atrial fibrillation, Statins
Introduction
Atrial fibrillation (AF) is the most frequent arrhythmia clinicians encounter, with a prevalence of 9.0% in adults over the age of 80.1 It is a significant risk factor for stroke, heart failure, dementia, and overall mortality.2 There has been increasing recognition of the role of inflammation in both the initiation and maintenance of AF.3 Elevation of the inflammatory biomarker, high-sensitivity C-reactive protein, has been associated with paroxysmal AF,4 recurrent AF after successful cardioversion,5 and post-operative AF after cardiac surgery.6–8
Statins possess anti-inflammatory properties and have been demonstrated to reduce high-sensitivity C-reactive protein.9 However, prior studies of statin therapy for the prevention of AF have been inconsistent,8,10–14 and some have included populations with known cardiovascular disease, where ischaemia may have played a role in the development of AF, and none focused on a population at risk due to elevated high-sensitivity C-reactive protein.
We had the opportunity to address this question among participants in the JUPITER (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin) trial. Specifically, we sought to determine whether (i) elevated high-sensitivity C-reactive protein is associated with incident AF among participants in the JUPITER trial and (ii) whether treatment with rosuvastatin is associated with a lower incidence of AF compared with placebo in a primary prevention setting.
Methods
Study population
The design and main results of the JUPITER study have been described in detail previously.15 Briefly, 17 802 men over the age of 50 and women over the age of 60 without prior cardiovascular disease or diabetes and a high-sensitivity C-reactive protein ≥2.0 mg/L and LDL-C <130 mg/dL were randomized to receive either rosuvastatin 20 mg daily or placebo. Subjects were followed for up to 5 years (median follow-up 1.9 years).15 The primary endpoint of the trial was the first occurrence of a major cardiovascular event defined as the composite of non-fatal myocardial infarction, non-fatal stroke, hospitalization for unstable angina, arterial revascularization, or cardiovascular death.
Participants underwent a screening physical exam and had a detailed medical history taken prior to randomization. After randomization, all participants received follow-up at 3-month intervals. In-person encounters alternated with telephone encounters. At each encounter, adverse events and new medications were assessed.
For this analysis, participants with baseline AF, atrial flutter, supraventricular arrhythmia, or non-specified arrhythmia were excluded. This resulted in a final study population of 17 120 participants.
Endpoints
Cases of incident AF were identified by investigator report of an adverse event during follow-up visits. These were then coded using MedDRA-10 criteria. The MedDRA (Medical Dictionary for Regulatory Activities) is a standard medical terminology used to classify adverse event information in clinical trials. At each follow-up encounter, participants were asked about new medication usage, and the reasons for initiating new drugs were recorded. Additional cases of AF were identified by examining the reasons for commencement of certain drugs during follow-up such as antiplatelet agents, vitamin K antagonists, digoxin and other cardiac glycosides, anti-arrhythmic drugs, beta-blocking agents, and non-dihydropyridine calcium channel blockers. If these drugs were initiated for a diagnosis of AF, the participant was considered to have incident AF.
The JUPITER trial was terminated early on 30 March 2008 after the data and safety monitoring committee determined that the accumulated evidence on safety and efficacy provided proof beyond a reasonable doubt that prolonged use of rosuvastatin was clearly indicated for some specific types of patients. Although follow-up for the primary endpoint ended on that day, subjects remained blinded and follow-up for adverse-events, including incident AF, continued until a study participant's final study visit. The last study visit occurred on 30 August 2008.
Statistical analysis
Baseline characteristics between participants who developed AF during follow-up and those who did not were compared using the χ2 test for categorical variables and the Wilcoxon rank sum test for continuous variables. We first sought to determine whether baseline high-sensitivity C-reactive protein predicts incident AF in the JUPITER cohort. Cox proportional hazard models were used to calculate the hazard ratio and associated 95% confidence interval for the risk of incident AF according to increasing tertiles of high-sensitivity C-reactive protein, as well as continuous log transformed high-sensitivity C-reactive protein at study entry, controlling for randomized treatment assignment. To evaluate the consistency of high-sensitivity C-reactive protein effects across treatment groups, we included an interaction term between high-sensitivity C-reactive protein and drug assignment. Covariates were selected for inclusion in a multivariate model based on a priori clinical knowledge. The final multivariable model included age (continuous), sex, blood pressure ≥140/90 mmHg or taking antihypertensive medications (yes/no), body mass index (categories: <22, 22–25, 25–30, ≥30 kg/m2), HbA1c (quartiles: <5.5, 5.5–5.7, 5.7–5.9, ≥5.9%), metabolic syndrome (yes/no), race, exercise (less than once/week vs. ≥1/week), high-sensitivity C-reactive protein (tertiles used in high-sensitivity C-reactive protein analysis: <3.2, 3.2–5.8, ≥5.8 mg/L; continuous log transformed high-sensitivity C-reactive protein used in drug analysis), current smoking (yes/no) and alcohol use (categories:<1–3/month, 1–6/week, daily).
We also constructed Cox proportional hazard models to calculate the hazard ratio and 95% confidence intervals for the comparison of rates of incident AF in the placebo and rosuvastatin groups. To enhance parsimony, the same Cox proportional hazard models were used to evaluate the effect of rosuvastatin on incident AF as the high-sensitivity C-reactive protein analysis. Because high-sensitivity C-reactive protein is skewed, it was log transformed in these models. Our primary analysis included AF events that occurred prior to study termination on 30 March 2008. However, we also performed secondary analyses of AF events that occurred during safety monitoring; a period that continued until 30 August 2008. Only the first occurrence of AF is included in this analysis.
Multiplicative interaction terms between drug assignment and various baseline characteristics were inserted into the unadjusted model to assess possible effect modification. Rates of incident AF were compared between the placebo and rosuvastatin groups using the Kaplan–Meier method, with differences between the two groups compared using the likelihood ratio test.
Because cardiovascular ischaemia can precipitate AF, we performed an additional analysis to evaluate whether the effect of rosuvastatin on AF was secondary to its primary beneficial effect on cardiovascular events. To accomplish this, we refit the Cox models censoring those participants with a cardiovascular event prior to the development of AF. The proportional hazards assumption was tested using interaction terms between each covariate and mean centred logarithm of study time. No violation of the proportional hazards assumption was detected. All analyses were performed according to the intent-to-treat principle. A P-value < 0.05 was considered statistically significant and all P-values are two-sided. All analyses were performed using SAS software version 9.1 (SAS Institute, Inc., Cary, NC, USA).
Results
Study participants
Baseline characteristics of the 17 120 participants from the JUPITER trial free of arrhythmia at study entry are illustrated in Table 1. Overall, the median age was 66 years, 38% of participants were women, and 57% had a blood pressure >140/90 mmHg or were taking antihypertensive medications. The median BMI was in the overweight category (28.4 kg/m2), and the median high-sensitivity C-reactive protein was 4.3 mg/L. Overall, the median systolic and diastolic blood pressure was 134 and 80 mmHg, respectively.
Table 1.
Baseline characteristics
| Characteristics | Placebo (n = 8566) | Rosuvastatin (n = 8554) | Atrial fibrillation (n = 238) | No Atrial fibrillation (n = 16 882) | P-value |
|---|---|---|---|---|---|
| Age, years | 66.0 (60–71) | 66.0 (60–71) | 70.0 (64–76) | 66.0 (60–71) | <0.0001 |
| Female | 3237 (37.8) | 3274 (38.3) | 71 (29.8) | 6440 (38.2) | 0.01 |
| Race | |||||
| White | 6034 (70.4) | 6055 (70.8) | 215 (90.3) | 11 874 (70.3) | <0.0001 |
| Black | 1111 (13.0) | 1086 (12.7) | 6 (2.5) | 2191 (13.0) | |
| Asian | 136 (1.6) | 146 (1.7) | 1 (0.4) | 281 (1.7) | |
| Hispanic | 1114 (13.0) | 1093 (12.8) | 13 (5.5) | 2194 (13.0) | |
| Other | 171 (2.0) | 172 (2.0) | 3 (1.3) | 340 (2.0) | |
| Current smoking | 1384 (16.2) | 1369 (16.0) | 33 (13.9) | 2720 (16.1) | 0.35 |
| Hypertension | 4921 (57.5) | 4864 (56.9) | 159 (66.8) | 9626 (57.1) | 0.003 |
| Systolic blood pressure, mmHg | 134 (124–145) | 134 (124–145) | 134 (126–145) | 134 (124–145) | 0.61 |
| Diastolic blood pressure, mmHg | 80 (75–87) | 80 (75–87) | 80 (72–85) | 80 (75–87) | 0.002 |
| Body mass index, kg/m2 | 28.3 (25.2–32.0) | 28.3 (25.3–32.0) | 29.6 (25.5–33.5) | 28.4 (25.3–32.0) | 0.02 |
| Baseline lipids, mg/dL | |||||
| Total cholesterol | 185 (169–199) | 186 (168–200) | 181 (162–198) | 185 (169–200) | 0.01 |
| LDL-C | 108 (94–119) | 108 (94–119) | 106 (91–117) | 108 (94–119) | 0.08 |
| HDL-C | 49 (40–60) | 49 (40–59) | 48 (40–58) | 49 (40–60) | 0.17 |
| Triglycerides | 118 (86–170) | 118 (85–169) | 111 (83–166) | 118 (86–170) | 0.22 |
| High-sensitivity C-reactive protein, mg/L | 4.3 (2.9–7.2) | 4.2 (2.8–7.1) | 5.0 (3.3–7.8) | 4.3 (2.8–7.1) | 0.0009 |
| Metabolic syndrome | 3584 (42.1) | 3519 (41.5) | 112 (47.9) | 6991 (41.2) | 0.06 |
| Alcohol intake | |||||
| <1–3 month | 4953 (57.8) | 5026 (58.8) | 146 (61.3) | 9833 (58.3) | 0.41 |
| 1–6 week | 1969 (23.0) | 1950 (22.8) | 46 (19.3) | 3873 (23.0) | |
| Daily | 1641 (19.2) | 1574 (18.4) | 46 (19.3) | 3169 (18.8) | |
| Exercise | |||||
| <1/week | 4876 (57.0) | 4750 (56.0) | 150 (63.0) | 9476 (56.2) | 0.04 |
| ≥1/week | 3687 (43.0) | 3799 (44.4) | 88 (37.0) | 7398 (43.8) | |
Values are median (inter-quartile range) or n (%). P-values refer to comparisons between participants who developed AF and those who did not develop AF during study follow-up. Race is self-reported. Hypertension was defined as blood pressure ≥140/90 mmHg or taking anti-hypertensive agents. The metabolic syndrome was defined according to consensus criteria of the National Heart, Lung, Blood Institute and American Heart Association. Values of high-sensitivity C-reactive protein are the average of the values obtained at the first two study visits. HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
As expected, participants who developed AF were older, had a higher body mass index, and were more likely to have hypertension.
Baseline high-sensitivity C-reactive protein and Incident atrial fibrillation
From the time of randomization to trial termination on 30th March 2008, AF was reported as an adverse event in 238 participants. Of these 238 cases of AF, 16 were identified by examining reasons for initiation of concomitant drugs during follow-up. Consistent with prior literature, increasing high-sensitivity C-reactive protein was a significant predictor of incident AF. In the continuous high-sensitivity C-reactive protein analysis, each 1 unit increase in log-high-sensitivity C-reactive protein was associated with a 28% increase in the risk of incident AF (95% CI: 1.08–1.52, P = 0.005) after adjustment for confounders. In the tertile analysis, each increasing tertile of high-sensitivity C-reactive protein was associated with a 36% increase in the risk of incident AF (95% CI: 1.16–1.60, P-trend < 0.01), an estimate that remained significant in multivariate analysis (HR: 1.37, 95% CI: 1.16–1.61, P-trend < 0.01). In this analysis, the HR for the highest high-sensitivity C-reactive protein tertile compared with the lowest was 1.96, 95% CI: 1.40–2.75, P < 0.01. The relationship between high-sensitivity C-reactive protein and incident AF remained significant after adjustment in a multivariate model (adjusted HR for the highest high-sensitivity C-reactive protein tertile compared with the lowest tertile 1.96, 95% CI: 1.38–2.78, P < 0.01) (Table 2).
Table 2.
Relationship between baseline high-sensitivity C-reactive protein in tertiles and risk of atrial fibrillation
| Tertile | High-sensitivity C-reactive protein (mg/L) | Patients (n) | Incidence rate (per 100 person-years) | HRa | 95% CI | P-value |
|---|---|---|---|---|---|---|
| Total cohort | ||||||
| Highest | ≥5.8 | 5862 | 0.83 | 1.96 | 1.38–2.78 | 0.0002 |
| Middle | 3.2–5.8 | 5696 | 0.75 | 1.70 | 1.20–2.41 | 0.003 |
| Lowest | <3.2 | 5562 | 0.43 | Ref. | Ref. | Ref. |
| P-trend | 0.0002 | |||||
| Placebo | ||||||
| Highest | ≥5.8 | 2719 | 1.00 | 2.26 | 1.42–3.60 | 0.0005 |
| Middle | 3.2–5.8 | 2878 | 0.85 | 1.74 | 1.08–2.80 | 0.02 |
| Lowest | <3.2 | 2969 | 0.47 | Ref. | Ref. | Ref. |
| P-trend | 0.0005 | |||||
| Rosuvastatin | ||||||
| Highest | ≥5.8 | 2843 | 0.66 | 1.62 | 0.95–2.76 | 0.81 |
| Middle | 3.2–5.8 | 2818 | 0.65 | 1.63 | 0.96–2.74 | 0.08 |
| Lowest | <3.2 | 2893 | 0.38 | Ref. | Ref. | Ref. |
| P-trend | 0.08 | |||||
aHazard ratio adjusted for age (continuous), sex, blood pressure ≥140/90 mmHg or taking antihypertensive medications (yes/no), body mass index (categories: <22, 22–25, 25–30, ≥30 kg/m2), HbA1c (quartiles: <5.5, 5.5–5.7, 5.7–5.9, ≥5.9%), metabolic syndrome (yes/no), race, exercise (less than once/week vs. ≥1/week), drug assignment (in total cohort), current smoking (yes/no), and alcohol use (categories: <1–3/month, 1–6/week, daily).
An analysis of the placebo group alone yielded similar results (adjusted HR for the highest high-sensitivity C-reactive protein tertile compared with the lowest tertile was 2.26, 95% CI: 1.42–3.60, P < 0.01) (Table 2). In the corresponding analysis in the rosuvastatin group, the magnitude of effect was attenuated (adjusted HR for the highest high-sensitivity C-reactive protein tertile compared with the lowest tertile, 1.62, 95% CI: 0.95–2.76, P = 0.81) (Table 2). However, there was no evidence of an interaction between drug assignment and high-sensitivity C-reactive protein modelled in categorical tertiles (P = 0.50). We also performed sensitivity analyses modelling high-sensitivity C-reactive protein in quartiles as well as gender-specific tertiles and obtained similar results (data not shown). The 3-year Kaplan–Meier estimates for incident AF in the highest, middle, and lowest tertile were 0.03, 0.02, 0.01, respectively (P = 0.0002).
Effect of rosuvastatin on incident atrial fibrillation
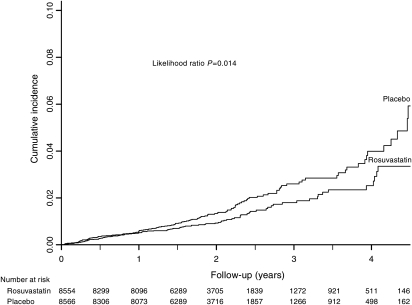
Prior to trial termination on 30 March 2008, AF was reported as an adverse event in 138 participants in the placebo group and 100 participants in the rosuvastatin group (Table 3). This yielded incidence rates of 0.78 and 0.56 per 100 person-years in the placebo and rosuvastatin groups, respectively (HR for the rosuvastatin group 0.73, 95% CI: 0.56–0.94, P = 0.01). Inclusion of the additional 14 cases of AF that occurred during safety follow-up through 30 August 2008 resulted in a total of 142 cases of AF in the placebo group and 110 in the rosuvastatin group and a similar effect estimate (HR for the rosuvastatin group 0.77, 95% CI: 0.60–0.99, P = 0.04) (Table 3). The cumulative incidence curves for AF in the placebo and rosuvastatin groups are shown in Figure 1. While the curves visually appear to diverge at 1 year, an interaction term between drug assignment and follow-up time was not statistically significant (P = 0.54).
Table 3.
Effect of rosuvastatin on incident atrial fibrillation
| Placebo |
Rosuvastatin |
Hazard ratio (95% CI) | P-value | |||
|---|---|---|---|---|---|---|
| n (%) | Incidence rate (per 100 person-years) | n (%) | Incidence rate (per 100 person-years) | |||
| All participants | ||||||
| Primary analysisa | ||||||
| Crude | 138 (1.6%) | 0.78 | 100 (1.2%) | 0.56 | 0.73 (0.56–0.94) | 0.01 |
| Adjustedb | — | — | — | — | 0.72 (0.55–0.93) | 0.01 |
| Safety follow-upc | ||||||
| Crude | 142 (1.7%) | 0.77 | 110 (1.3%) | 0.60 | 0.77 (0.60–0.99) | 0.04 |
| Adjustedb | — | — | — | — | 0.77 (0.60–0.98) | 0.04 |
| Participants censored after first cardiovascular eventd | ||||||
| Primary analysisa | ||||||
| Crude | 124 (1.4%) | 0.71 | 93 (1.1%) | 0.53 | 0.75 (0.57–0.98) | 0.04 |
| Adjustedb | — | — | — | — | 0.74 (0.57–0.97) | 0.03 |
aThe primary analysis was performed on cases occurring before trial termination on 30 March 2008.
bAdjusted for age (continuous), sex, blood pressure ≥140/90 mmHg or taking antihypertensive medications (yes/no), body mass index (categories: <22, 22–25, 25–30, ≥30 kg/m2), HbA1c (quartiles: <5.5, 5.5–5.7, 5.7–5.9, ≥5.9%), metabolic syndrome (yes/no), race, exercise (less than once/week vs. ≥1/week), log high-sensitivity C-reactive protein (mg/L), current smoking (yes/no), and alcohol use (categories:<1–3/month, 1–6/week, daily).
cThe safety follow-up analysis includes additional cases occurring between 30 March 2008 and 30 August 2008.
dA cardiovascular event refers to the combined endpoint of myocardial infarction, arterial revascularization, stroke, hospitalization for unstable angina, or cardiovascular death.
Figure 1.
Cumulative incidence of atrial fibrillation according to study group. Shown is the incidence of atrial fibrillation in the placebo and rosuvastatin groups. The P-value was calculated using a likelihood ratio test of the effect of rosuvastatin, using a proportional hazards model.
After multivariate adjustment, treatment with rosuvastatin 20 mg daily remained significantly associated with a decreased risk of AF reported as an adverse event (HR for rosuvastatin 0.72, 95% CI: 0.55–0.93, P = 0.01) (Table 3). In multivariate analyses including AF events that occurred during safety monitoring, this relationship remained significant (HR for rosuvastatin 0.77, 95% CI: 0.60–0.98, P = 0.04). Because cardiovascular ischaemia can precipitate AF and the overall JUPITER trial demonstrated a 44% relative risk reduction in first major cardiovascular events, we attempted to disentangle the effect of statins on AF from their primary effect on major cardiovascular events. Censoring 21 participants who experienced a major cardiovascular event prior to the development of AF yielded findings similar to our primary analysis (adjusted HR for rosuvastatin 0.74, 95% CI: 0.57–0.97, P = 0.03) (Table 3).
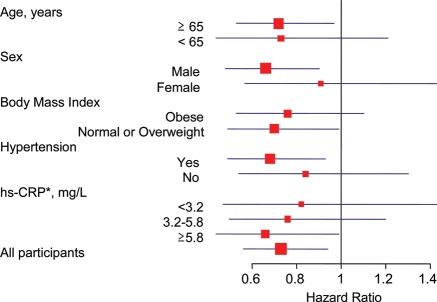
The effect of rosuvastatin on incident AF appeared comparable across major subgroups (Figure 2). Specifically, treatment with rosuvastatin was associated with a lower risk of AF in women as well as men, among those with and without hypertension, and across body mass index, age, and high-sensitivity C-reactive protein categories. Although we had limited power to detect differences between subgroups, we did not observe any evidence of effect modification (P-interaction for all subgroup comparisons >0.1).
Figure 2.
Effect of rosuvastatin on incident atrial fibrillation according to various baseline characteristics. Shown are hazard ratios (square boxes) for the rosuvastatin group as compared with the placebo group. The size of each square box is inversely proportional to the confidence interval (horizontal lines). Tests of interaction between rosuvastatin and each subgroup variable were non-significant (Peach>0.10). *Hs-CRP, high-sensitivity C-reactive protein.
Discussion
In this exploratory analysis of apparently healthy men and women without prior cardiovascular disease and with elevated high-sensitivity C-reactive protein, treatment with rosuvastatin 20 mg daily was associated with a 23–28% reduction in blinded adverse event reports of AF. The benefit afforded by treatment with rosuvastatin remained significant after adjustment for potential confounders as well as in analyses excluding participants with a prior cardiovascular event. The latter observation suggests that randomization to rosuvastatin therapy in the JUPITER trial was associated with a reduction in AF events independent of the effect of rosuvastatin on major cardiovascular events. In addition, consistent with prior studies5,6,16,17 we noted that increasing baseline high-sensitivity C-reactive protein was associated with a significant increase in the risk of an adverse event report of AF.
Our findings are congruent with some,8,10,13,18 but not all11,12,14 prior studies examining the relationship between statin use and the risk of AF. In a post hoc analysis of the GISSI-HF trial, administration of rosuvastatin 10 mg daily was associated with a 12% reduction in the risk of incident AF compared with placebo in a chronic heart failure population after multivariate adjustment.13 Similarly, treatment with atorvastatin has been associated with a reduction in high-sensitivity C-reactive protein levels as well as number of episodes of paroxysmal AF,19 and a decreased risk of post-operative AF in the setting of cardiac surgery.8 However, in a recent analysis of a large trial including patients with prior stroke or transient ischaemic attack, high dose atorvastatin therapy was not associated with a decreased risk of AF.14
Our findings with regard to high-sensitivity C-reactive protein are also consistent with prior prospective data. In the Cardiovascular Health Study, a population-based cohort of over 5000 participants, baseline high-sensitivity C-reactive protein predicted the development of AF (HR for 1 standard deviation increase in high-sensitivity C-reactive protein, 1.24, 95% CI: 1.11–1.40, P < 0.001).16 However, in a recent analysis of the GISSI-AF trial, baseline high-sensitivity C-reactive protein level was not associated with a higher risk of recurrent AF among patients with a history of AF.20 Our analysis of healthy persons without prior cardiovascular disease or history of AF extends this prior work. To our knowledge, the analysis we describe in this report is the first large, randomized trial analysis of statins and incident AF in a primary prevention setting.
Several biological mechanisms have been proposed for the anti-arrhythmic effect of statins in AF. Anti-inflammatory properties, enhanced nitric oxide-dependent endothelial function and reduction in neurohormonal activation have all been postulated as potential mechanisms.21 Supporting the role of inflammation, an early histologic study of patients with lone AF demonstrated inflammatory lymphomononuclear infiltrates consistent with myocarditis in a greater number of patients with lone AF compared with controls.22 In a canine sterile pericarditis model designed to mimic the post-operative state, atorvastatin therapy was associated with a reduction in high-sensitivity C-reactive protein levels, shorter duration of AF, and longer atrial effective refractory period.23 Atorvastatin and simvastatin have also been shown to modify atrial plateau currents in mouse models.24
Our study design has numerous strengths. In contrast to some of the previous observational and randomized studies of statin therapy and AF, our analysis involved a large, ethnically diverse trial population. The randomized design and double-blinding of the study drug also reduce the chance of bias in our study. Furthermore, the inclusion of relatively healthy men and women without prior cardiovascular disease enhances generalizability and extends previous work done in patients with established coronary disease or heart failure.
However, our findings must be interpreted within the context of our study design and its limitations. First, AF was not a prespecified endpoint and thus we relied on adverse event reports to ascertain this outcome. We were not able to confirm cases of AF with electrocardiographic monitoring, and silent cases of AF may not have been detected. Our method of assessment of AF could have lead to under-reporting of the outcome or misclassification. However, under-reporting would be expected to occur with equal frequency in both the placebo and drug groups and is unlikely have affected our results. Last, the JUPITER trial population is limited to those with high baseline high-sensitivity C-reactive protein and thus these findings may not apply to a broader population without evidence of chronic inflammation.
Conclusions
In conclusion, in this randomized trial of healthy men and women without established cardiovascular disease and elevated high-sensitivity C-reactive protein, elevated baseline high-sensitivity C-reactive protein was associated with a greater risk of AF, and randomization to rosuvastatin 20 mg daily was associated with a reduction in adverse event reports of AF compared with placebo.
Funding
This work was supported by the National Heart, Lung, And Blood Institute at the National Institutes of Health (T32HL007575 to J.P.). The content in this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute or the National Institutes of Health. The JUPITER trial was supported by Astra Zeneca.
Conflict of interest: P.M.R. reports having received research funding support from multiple not-for-profit entities including the National Heart, Lung, and Blood Institute, the National Cancer Institute, the American Heart Association, the Doris Duke Charitable Foundation, the Leducq Foundation, the Donald W. Reynolds Foundation, and the James and Polly Annenberg La Vea Charitable Trusts. P.M.R. also reports having received investigator-initiated research support from Astra-Zeneca and Novartis, as well as non-financial research support from Amgen. P.M.R. is listed as a co-inventor on patents held by the Brigham and Women's Hospital that relate to the use of inflammatory biomarkers in cardiovascular disease that have been licenced to Siemens and Astra-Zeneca, and has served as a research consultant to Merck, Isis, Vascular Biogenics, Amylin, and Genzyme. R.J.G. reports having received investigator-initiated research support from Astra-Zeneca and Novartis, and has served as a research consultant to Merck.
References
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. doi:10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Chen PS, Bild DE, Mascette AM, Albert CM, Alonso A, Calkins H, Connolly SJ, Curtis AB, Darbar D, Ellinor PT, Go AS, Goldschlager NF, Heckbert SR, Jalife J, Kerr CR, Levy D, Lloyd-Jones DM, Massie BM, Nattel S, Olgin JE, Packer DL, Po SS, Tsang TS, Van Wagoner DR, Waldo AL, Wyse DG. Prevention of atrial fibrillation: report from a National Heart, Lung, and Blood Institute workshop. Circulation. 2009;119:606–18. doi: 10.1161/CIRCULATIONAHA.108.825380. doi:10.1161/CIRCULATIONAHA.108.825380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engelmann MDM, Svendsen JH. Inflammation in the genesis and perpetuation of atrial fibrillation. Eur Heart J. 2005;26:2083–2092. doi: 10.1093/eurheartj/ehi350. doi:10.1093/eurheartj/ehi350. [DOI] [PubMed] [Google Scholar]
- 4.Dernellis J, Panaretou M. C-reactive protein and paroxysmal atrial fibrillation: evidence of the implication of an inflammatory process in paroxysmal atrial fibrillation. Acta Cardiol. 2001;56:375–80. doi: 10.2143/AC.56.6.2005701. doi:10.2143/AC.56.6.2005701. [DOI] [PubMed] [Google Scholar]
- 5.Liu T, Li G, Li L, Korantzopoulos P. Association between c-reactive protein and recurrence of atrial fibrillation after successful electrical cardioversion: a meta-analysis. J Am Coll Cardiol. 2007;49:1642–1648. doi: 10.1016/j.jacc.2006.12.042. doi:10.1016/j.jacc.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 6.Bruins P, te Velthuis H, Yazdanbakhsh AP, Jansen PG, van Hardevelt FW, de Beaumont EM, Wildevuur CR, Eijsman L, Trouwborst A, Hack CE. Activation of the complement system during and after cardiopulmonary bypass surgery: postsurgery activation involves c-reactive protein and is associated with postoperative arrhythmia. Circulation. 1997;96:3542–3548. doi: 10.1161/01.cir.96.10.3542. [DOI] [PubMed] [Google Scholar]
- 7.Lo B, Fijnheer R, Nierich AP, Bruins P, Kalkman CJ. C-reactive protein is a risk indicator for atrial fibrillation after myocardial revascularization. Ann of Thorac Surg. 2005;79:1530–1535. doi: 10.1016/j.athoracsur.2004.10.004. doi:10.1016/j.athoracsur.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Patti G, Chello M, Candura D, Pasceri V, D'Ambrosio A, Covino E, Di Sciascio G. Randomized trial of atorvastatin for reduction of postoperative atrial fibrillation in patients undergoing cardiac surgery: results of the ARMYDA-3 (Atorvastatin for Reduction of Myocardial Dysrhythmia after Cardiac Surgery) study. Circulation. 2006;114:1455–1461. doi: 10.1161/CIRCULATIONAHA.106.621763. doi:10.1161/CIRCULATIONAHA.106.621763. [DOI] [PubMed] [Google Scholar]
- 9.Albert MA, Danielson E, Rifai N, Ridker PM for the PRINCE Investigators. Effect of statin therapy on c-reactive protein levels. JAMA. 2001;286:64–70. doi: 10.1001/jama.286.1.64. doi:10.1001/jama.286.1.64. [DOI] [PubMed] [Google Scholar]
- 10.Fauchier L, Pierre B, de Labriolle A, Grimard C, Zannad N, Babuty D. Antiarrhythmic effect of statin therapy and atrial fibrillation: a meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2008;51:828–835. doi: 10.1016/j.jacc.2007.09.063. doi:10.1016/j.jacc.2007.09.063. [DOI] [PubMed] [Google Scholar]
- 11.Liu T, Li L, Korantzopoulos P, Liu E, Li G. Statin use and development of atrial fibrillation: a systematic review and meta-analysis of randomized clinical trials and observational studies. Int J Cardiol. 2008;126:160–170. doi: 10.1016/j.ijcard.2007.07.137. doi:10.1016/j.ijcard.2007.07.137. [DOI] [PubMed] [Google Scholar]
- 12.Negi S, Shukrullah I, Veledar E, Bloom HL, Jones DP, Dudley SC. Statin therapy for the prevention of atrial fibrillation trial (STOP AF trial) J Cardiovasc Electrophysiol. 2011;22:414–419. doi: 10.1111/j.1540-8167.2010.01925.x. doi:10.1111/j.1540-8167.2010.01925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maggioni AP, Fabbri G, Lucci D, Marchioli R, Franzosi MG, Latini R, Nicolosi GL, Porcu M, Cosmi F, Stefanelli S, Tognoni G, Tavazzi L on behalf of the GISSI-HF Investigators. Effects of rosuvastatin on atrial fibrillation occurrence: ancillary results of the GISSI-HF trial. Eur Heart J. 2009;30:2327–2336. doi: 10.1093/eurheartj/ehp357. doi:10.1093/eurheartj/ehp357. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz GG, Chaitman BR, Goldberger JJ, Messig M. High-dose atorvastatin and risk of atrial fibrillation in patients with prior stroke or transient ischemic attack: analysis of the stroke prevention by aggressive reduction in cholesterol levels (SPARCL) trial. Am Heart J. 2011;161:993–999. doi: 10.1016/j.ahj.2011.02.002. doi:10.1016/j.ahj.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated c-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. doi:10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 16.Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS, Chung MK. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010. doi: 10.1161/01.CIR.0000103131.70301.4F. doi:10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 17.Chung MK, Martin DO, Sprecher D, Wazni O, Kanderian A, Carnes CA, Bauer JA, Tchou PJ, Niebauer MJ, Natale A, Van Wagoner DR. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–2891. doi: 10.1161/hc4901.101760. doi:10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- 18.Young-Xu Y, Jabbour S, Goldberg R, Blatt CM, Graboys T, Bilchik B, Ravid S. Usefulness of statin drugs in protecting against atrial fibrillation in patients with coronary artery disease. Am J Cardiol. 2003;92:1379–1383. doi: 10.1016/j.amjcard.2003.08.040. doi:10.1016/j.amjcard.2003.08.040. [DOI] [PubMed] [Google Scholar]
- 19.Dernellis J, Panaretou M. Effect of c-reactive protein reduction on paroxysmal atrial fibrillation. Am Heart J. 2005;150:1064.e7–1064.e12. doi: 10.1016/j.ahj.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 20.Masson S, Aleksova A, Favero C, Staszewsky L, Bernardinangeli M, Belvito C, Cioffi G, Sinagra G, Mazzone C, Bertocchi F, Vago T, Peri G, Cuccovillo I, Masuda N, Barlera S, Mantovani A, Maggioni AP, Franzosi MG, Disertori M, Latini R. Predicting atrial fibrillation recurrence with circulating inflammatory markers in patients in sinus rhythm at high risk for atrial fibrillation: data from the GISSI atrial fibrillation trial. Heart. 2010;96:1909–1914. doi: 10.1136/hrt.2009.191460. doi:10.1136/hrt.2009.191460. [DOI] [PubMed] [Google Scholar]
- 21.Adam O, Neuberger H-R, Bohm M, Laufs U. Prevention of atrial fibrillation with 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Circulation. 2008;118:1285–1293. doi: 10.1161/CIRCULATIONAHA.107.760892. doi:10.1161/CIRCULATIONAHA.107.760892. [DOI] [PubMed] [Google Scholar]
- 22.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–1184. doi: 10.1161/01.cir.96.4.1180. [DOI] [PubMed] [Google Scholar]
- 23.Kumagai K, Nakashima H, Saku K. The HMG-coA reductase inhibitor atorvastatin prevents atrial fibrillation by inhibiting inflammation in a canine sterile pericarditis model. Cardiovasc Res. 2004;62:105–111. doi: 10.1016/j.cardiores.2004.01.018. doi:10.1016/j.cardiores.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 24.Vaquero M, Caballero R, Gomez R, Nunez L, Tamargo J, Delpon E. Effects of atorvastatin and simvastatin on atrial plateau currents. J Mol Cell Cardiol. 2007;42:931–945. doi: 10.1016/j.yjmcc.2007.03.807. doi:10.1016/j.yjmcc.2007.03.807. [DOI] [PubMed] [Google Scholar]