Abstract
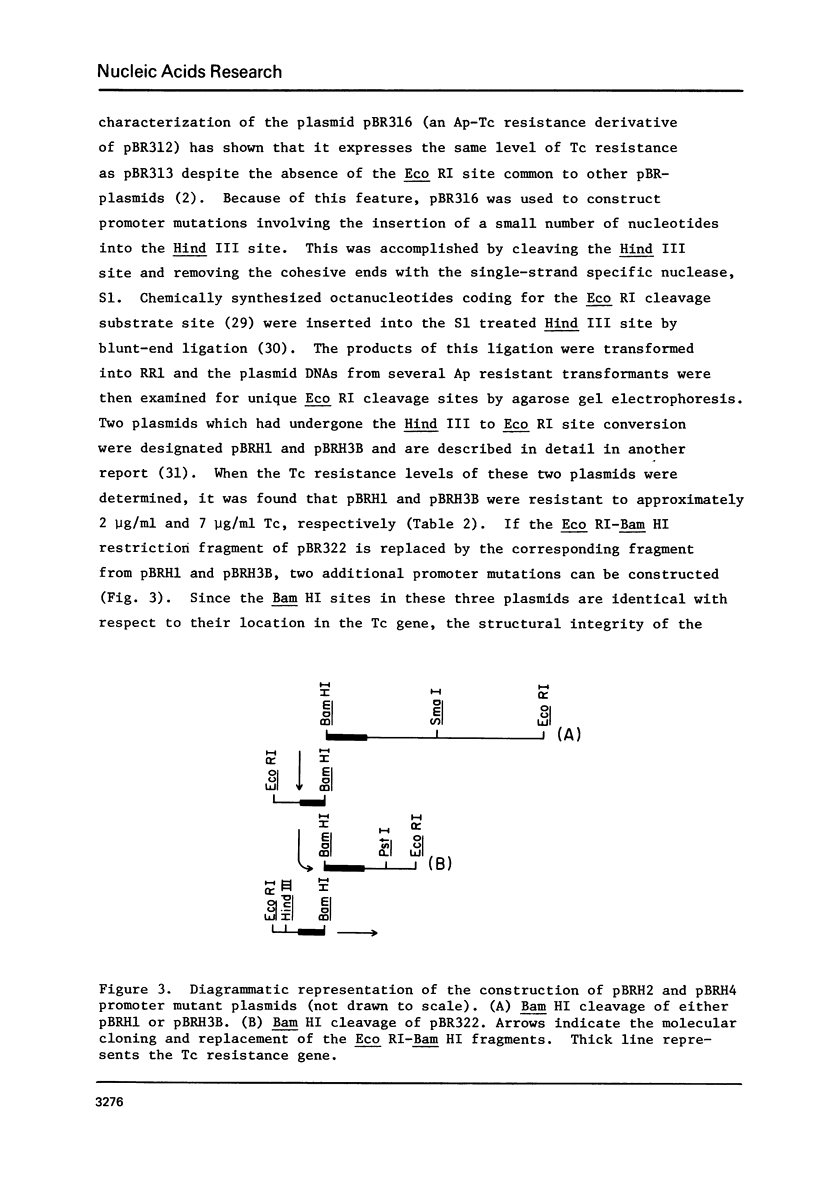
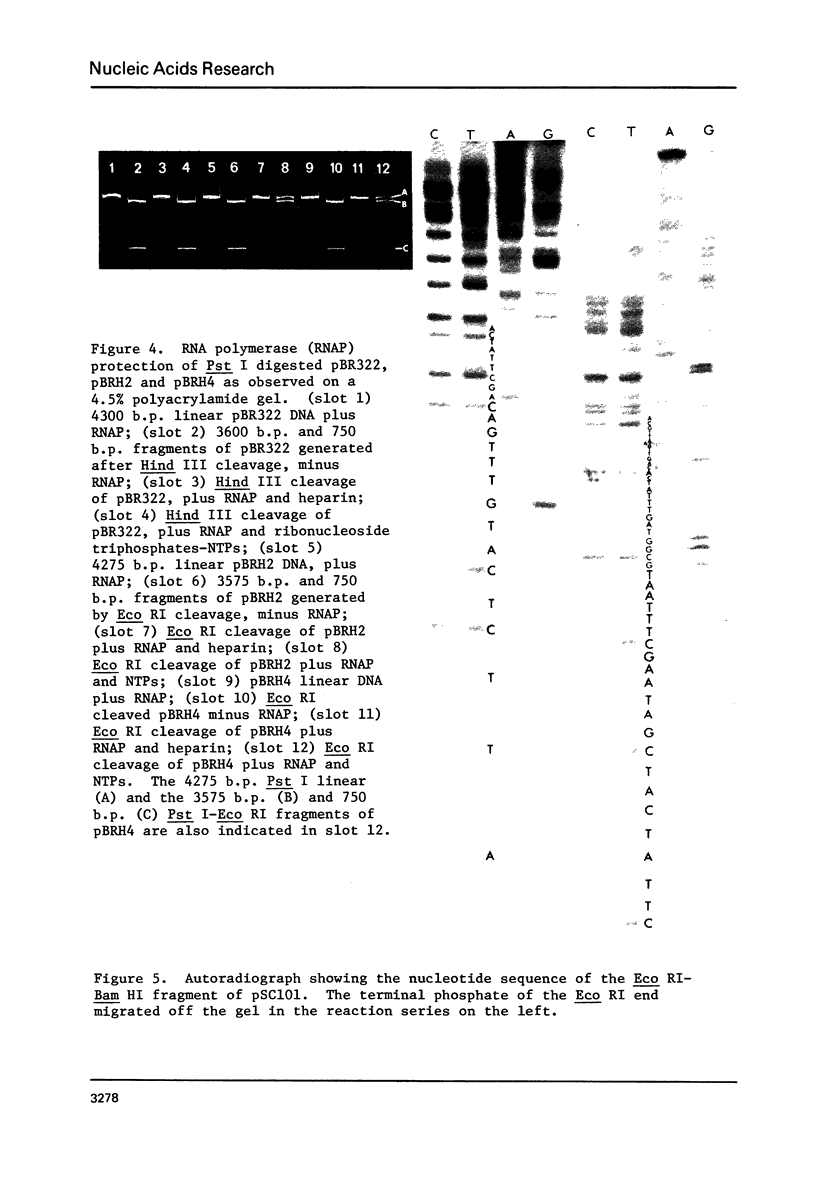
By employing a system of RNA polymerase binding and restriction endonuclease digestion, we demonstrate that the region in and around the Hind III site of pBR313 and pBR322 is the promoter for the tetracycline (Tc) resistance gene(s). Furthermore, it is shown that this region was transferred intact from pSC101 during the construction of the latter plasmids. The in vitro insertion of a few base pairs at the Hind III site produces a series of "down" promoter mutations in which the level of in vivo Tc resistance is reduced. Sequence analysis of the various promoter mutations revealed significant base pair rearrangements in the region between -40 and -12 of the promoter. While these base alterations do not appear to affect the firm binding of RNA polymerase, they do affect the ability of mutant promoters to initiate transcription. These observations suggest that the region from -40 to -12, previously designated as the "recognition region", is actually involved in the process of initiation of transcription.
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allet B., Roberts R. J., Gesteland R. F., Solem R. Class of promotor sites for Escherichia coli DNA-dependent RNA polymerase. Nature. 1974 May 17;249(454):217–221. doi: 10.1038/249217a0. [DOI] [PubMed] [Google Scholar]
- Backman K., Ptashne M., Gilbert W. Construction of plasmids carrying the cI gene of bacteriophage lambda. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4174–4178. doi: 10.1073/pnas.73.11.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Circular DNA forms of colicinogenic factors E1, E2 and E3 from Escherichia coli. J Mol Biol. 1968 Sep 14;36(2):185–194. doi: 10.1016/0022-2836(68)90374-4. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Betlach M. C., Heyneker H. L., Shine J., Rodriguez R. L., Boyer H. W. Origin of replication of pBR345 plasmid DNA. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5265–5269. doi: 10.1073/pnas.74.12.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Betlach M. C., Boyer H. W. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene. 1977;2(2):75–93. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- Calos M. P. DNA sequence for a low-level promoter of the lac repressor gene and an 'up' promoter mutation. Nature. 1978 Aug 24;274(5673):762–765. doi: 10.1038/274762a0. [DOI] [PubMed] [Google Scholar]
- Chamberlin M. J. The selectivity of transcription. Annu Rev Biochem. 1974;43(0):721–775. doi: 10.1146/annurev.bi.43.070174.003445. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C. Revised interpretation of the origin of the pSC101 plasmid. J Bacteriol. 1977 Nov;132(2):734–737. doi: 10.1128/jb.132.2.734-737.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R. C., Abelson J., Barnes W. M., Reznikoff W. S. Genetic regulation: the Lac control region. Science. 1975 Jan 10;187(4171):27–35. doi: 10.1126/science.1088926. [DOI] [PubMed] [Google Scholar]
- Goulian M., Lucas Z. J., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXV. Purification and properties of deoxyribonucleic acid polymerase induced by infection with phage T4. J Biol Chem. 1968 Feb 10;243(3):627–638. [PubMed] [Google Scholar]
- Greene P. H., Poonian M. S., Nussbaum A. L., Tobias L., Garfin D. E., Boyer H. W., Goodman H. M. Restriction and modification of a self-complementary octanucleotide containing the EcoRI substrate. J Mol Biol. 1975 Dec 5;99(2):237–261. doi: 10.1016/s0022-2836(75)80143-4. [DOI] [PubMed] [Google Scholar]
- Greene P. J., Heyneker H. L., Bolivar F., Rodriguez R. L., Betlach M. C., Covarrubias A. A., Backman K., Russel D. J., Tait R., Boyer H. W. A general method for the purification of restriction enzymes. Nucleic Acids Res. 1978 Jul;5(7):2373–2380. doi: 10.1093/nar/5.7.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer D. H., Thomas C. A., Jr Molecular cloning of DNA fragments produced by restriction endonucleases Sa1I and BamI. Proc Natl Acad Sci U S A. 1976 May;73(5):1537–1541. doi: 10.1073/pnas.73.5.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershfield V., Boyer H. W., Chow L., Helinski D. R. Characterization of a mini-ColC1 plasmid. J Bacteriol. 1976 Apr;126(1):447–453. doi: 10.1128/jb.126.1.447-453.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyneker H. L., Shine J., Goodman H. M., Boyer H. W., Rosenberg J., Dickerson R. E., Narang S. A., Itakura K., Lin S., Riggs A. D. Synthetic lac operator DNA is functional in vivo. Nature. 1976 Oct 28;263(5580):748–752. doi: 10.1038/263748a0. [DOI] [PubMed] [Google Scholar]
- Johnsrud L. Contacts between Escherichia coli RNA polymerase and a lac operon promoter. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5314–5318. doi: 10.1073/pnas.75.11.5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleid D., Humayun Z., Jeffrey A., Ptashne M. Novel properties of a restriction endonuclease isolated from Haemophilus parahaemolyticus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):293–297. doi: 10.1073/pnas.73.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangel W. F., Chamberlin M. J. Studies of ribonucleic acid chain initiation by Escherichia coli ribonucleic acid polymerase bound to T7 deoxyribonucleic acid. I. An assay for the rate and extent of ribonucleic acid chain initiation. J Biol Chem. 1974 May 25;249(10):2995–3001. [PubMed] [Google Scholar]
- Maquat L. E., Reznikoff W. S. In vitro analysis of the Escherichia coli RNA polymerase interaction with wild-type and mutant lactose promoters. J Mol Biol. 1978 Nov 15;125(4):467–490. doi: 10.1016/0022-2836(78)90311-x. [DOI] [PubMed] [Google Scholar]
- Maurer R., Maniatis T., Ptashne M. Promoters are in the operators in phage lambda. Nature. 1974 May 17;249(454):221–223. doi: 10.1038/249221a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miozzari G., Yanofsky C. Naturally occurring promoter down mutation: nucleotide sequence of the trp promoter/operator/leader region of Shigella dysenteriae 16. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5580–5584. doi: 10.1073/pnas.75.11.5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Yura T. Hyperproduction of the sigma subunit of RNA polymerase in a mutant of Escherichia coli. Mol Gen Genet. 1975 Nov 24;141(2):97–111. doi: 10.1007/BF00267677. [DOI] [PubMed] [Google Scholar]
- Neve R. L., West R. W., Rodriguez R. L. Eukaryotic DNA fragments which act as promoters for a plasmid gene. Nature. 1979 Jan 25;277(5694):324–325. doi: 10.1038/277324a0. [DOI] [PubMed] [Google Scholar]
- Okamoto T., Sugimoto K., Sugisaki H., Takanami M. DNA regions essential for the function of a bacteriophage fd promoter. Nucleic Acids Res. 1977 Jul;4(7):2213–2222. doi: 10.1093/nar/4.7.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panet A., van de Sande J. H., Loewen P. C., Khorana H. G., Raae A. J., Lillehaug J. R., Kleppe K. Physical characterization and simultaneous purification of bacteriophage T4 induced polynucleotide kinase, polynucleotide ligase, and deoxyribonucleic acid polymerase. Biochemistry. 1973 Dec 4;12(25):5045–5050. doi: 10.1021/bi00749a003. [DOI] [PubMed] [Google Scholar]
- Pribnow D. Nucleotide sequence of an RNA polymerase binding site at an early T7 promoter. Proc Natl Acad Sci U S A. 1975 Mar;72(3):784–788. doi: 10.1073/pnas.72.3.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer G. E., Walkinshaw M. D., Arnott S. A computer aided oligonucleotide analysis provides a model sequence for RNA polymerase-promoter recognition in E.coli. Nucleic Acids Res. 1978 Oct;5(10):3759–3773. doi: 10.1093/nar/5.10.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg P. H., Schaller H. Mapping and characterization of promoters in bacteriophages fd, f1 and m13. J Mol Biol. 1975 Feb 25;92(2):261–277. doi: 10.1016/0022-2836(75)90226-0. [DOI] [PubMed] [Google Scholar]
- Selzer G., Bolle A., Krisch B., Epstein R. Construction and properties of recombinant plasmids containing the rII genes of bacteriophage T4. Mol Gen Genet. 1978 Feb 27;159(3):301–309. doi: 10.1007/BF00268267. [DOI] [PubMed] [Google Scholar]
- Tait R. C., Boyer H. W. On the nature of tetracycline resistance controlled by the plasmid pSC101. Cell. 1978 Jan;13(1):73–81. doi: 10.1016/0092-8674(78)90139-3. [DOI] [PubMed] [Google Scholar]
- Tait R. C., Rodriguez R. L., Boyer H. W. Altered tetracycline resistance in pSC101 recombinant plasmids. Mol Gen Genet. 1977 Mar 16;151(3):327–331. doi: 10.1007/BF00268797. [DOI] [PubMed] [Google Scholar]
- Timmis K., Cabello F., Cohen S. N. Utilization of two distinct modes of replication by a hybrid plasmid constructed in vitro from separate replicons. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4556–4560. doi: 10.1073/pnas.71.11.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widera G., Gautier F., Lindenmaier W., Collins J. The expression of tetracycline resistance after insertion of foreign DNA fragments between the EcoRI and HindIII sites of the plasmid cloning vector pBR 322. Mol Gen Genet. 1978 Jul 25;163(3):301–305. doi: 10.1007/BF00271959. [DOI] [PubMed] [Google Scholar]
- Wiggs J. L., Bush J. W., Chamberlin M. J. Utilization of promoter and terminator sites on bacteriophage T7 DNA by RNA polymerases from a variety of bacterial orders. Cell. 1979 Jan;16(1):97–109. doi: 10.1016/0092-8674(79)90191-0. [DOI] [PubMed] [Google Scholar]