Abstract
In an effort to understand the morphogenetic forces that shape the bones of the skull, we inactivated Msx1 and Msx2 conditionally in neural crest. We show that Wnt1-Cre inactivation of up to three Msx1/2 alleles results in a progressively larger defect in the neural crest-derived frontal bone. Unexpectedly, in embryos lacking all four Msx1/2 alleles, the large defect is filled in with mispatterned bone consisting of ectopic islands of bone between the reduced frontal bones, just anterior to the parietal bones. The bone is derived from neural crest, not mesoderm, and, from DiI cell marking experiments, originates in a normally non-osteogenic layer of cells through which the rudiment elongates apically. Associated with the heterotopic osteogeneis is an upregulation of Bmp signaling in this cell layer. Prevention of this upregulation by implantation of noggin-soaked beads in head explants also prevented heterotopic bone formation. These results suggest that Msx genes have a dual role in calvarial development: They are required for the differentiation and proliferation of osteogenic cells within rudiments, and they are also required to suppress an osteogenic program in a cell layer within which the rudiments grow. We suggest that the inactivation of this repressive activity may be one cause of Wormian bones, ectopic bones that are a feature of a variety of pathological conditions in which calvarial bone development is compromised.
Keywords: Msx1, Msx2 neural crest, heterotopic bone, skull vault, Bmp
Introduction
The vertebrate skull vault consists of several component bones of mixed lineage origin. In the mouse, these are the paired frontal and parietal bones and a single interparietal bone. The frontal bones are derived from neural crest, the parietal bones from mesoderm (Jiang et al., 2002). The interparietal bone is a composite, its central portion derived from neural crest and its lateral portions from mesoderm. The patterned growth of the vertebrate skull is a complex and as yet poorly understood process that involves an exquisitely controlled series of migratory and proliferative events, as well as the specification and differentiation of osteogenic and chondrogenic cells among others (Chai and Maxson, 2006; Morriss-Kay and Wilkie, 2005). During the first stages of skull vault development, precursor neural crest and mesodermal cells migrate to positions on the lateral aspects of the cerebral hemispheres. The frontal and parietal bone rudiments become evident at E12.5 as condensations within these mesenchymal cell populations. They express early osteoblast markers, and then expand apically through the remainder of prenatal development and the first several days of postnatal development, ultimately coming into apposition with their paired counterparts at the midline (Ishii et al., 2003; Jiang et al., 2002).
Two models have been put forward to explain the apical expansion of the frontal and parietal bones (Lana-Elola et al., 2007; Ting et al., 2009; Yoshida et al., 2008);. One posits proliferation and migration of precursor cells located at base, the second, differentiation of preexisting precursors. In favor of the first model, DiI injections into the rudiment at E13.5, followed by exo utero culture of embryos revealed that during apical expansion cells migrate from the base of the rudiments to the leading edge of the growing bone. Cells within the rudiment are also proliferating during this period of expansion. Thus migration and proliferation are probably important morphogenetic forces contributing to the growth of the calvarial bones. Arguing against the second model are results of diI injections into mesenchymal cells located apical to the rudiment. Labeled cells do not have an ostoegenic fate, but are located in a non-osteogenic layer flanking the bone (Yoshida et al., 2008). We note, however, that when diI injections are performed at late stages in the presumptive sagittal suture, a small number of labeled cells are found in bone (Lana-Elola et al., 2007). Thus at least some sutural cells are capable of differentiating into osteoblasts. The emerging view of at least the early stages of calvarial bone growth is that the bone rudiments grow by end-addition of migratory cells through a layer of non-osteogenic mesenchyme.
Twist1, Msx1, and Msx2 have been shown to have major influences on calvarial growth and patterning (Chai and Maxson, 2006). Twist1, a basic helix-loop-helix transcription factor, is mutated in Saethre-Chozen Syndrome, characterized by craniosynostosis as well as other craniofacial and limb defects (el Ghouzzi et al., 1997). Twist1 is required for proper targeting of migratory osteogenic cells to the leading edges of growing bones. This activity requires EphA4, which functions in a layer of cells in which osteogenic precursors migrate, flanking the prospective bone (Merrill et al., 2006; Ting et al., 2009). Twist1, EphA4 and Twist1-EphA4 mutants exhibit inappropriate migration of osteogenic cells into the coronal suture and consequent differentiation of normally non osteogenic suture cells. The result is synostosis of the frontal and parietal bones (Ting et al., 2009).
Msx genes function in the apical expansion of the frontal and parietal bone rudiments (Han et al., 2007; Ishii et al., 2003). In Msx2 conventional mutants, the growth of the rudiments is retarded and cells within the rudiments proliferate at a reduced rate (Ishii et al., 2003). In combination Msx1/2 mutants, the frontal and parietal bones do not form and many embryos exhibit exencephaly (Han et al., 2007). The severity of this set of phenotypes precluded a detailed analysis of the role of Msx genes in calvarial bone growth. This limitation, together with the critical role of Msx genes in the apical expansion of the rudiments, prompted us to undertake a more detailed analysis of the activities of Msx1/2.
We produced floxed alleles of Msx1 and Msx2 (Fu et al., 2007). In the present study, as part of an effort to understand more fully the morphogenetic forces shaping calvarial bones, we inactivated Msx1 and Msx2 conditionally in neural crest. We show that Wnt1-Cre-mediated inactivation of up to three Msx1/2 alleles results in a progressively larger frontal bone defect. Unexpectedly, in embryos lacking all four Msx alleles, the large defect is largely filled with bone, which is mispatterned and present in sutures. This bone is derived from neural crest, not mesoderm, and, from diI cell marking experiments, originates in the normally non-osteogenic layer of cells through with the rudiment grows. Associated with the heterotopic osteogeneis is an upregulation of Bmp signaling in this cell layer. Inactivation of such signaling by implantation of noggin-containing beads in calvarial explants prevents heterotopic osteogenesis. These results, together with previous studies, suggest that Msx genes have a dual role in calvarial development: They are required for the differentiation and proliferation of osteogenic cells within rudiments (Han et al., 2007; Ishii et al., 2003), and they are also required to suppress an osteogenic program in a normally non-osteogenic cell layer within which the rudiments grow.
Results
An unexpected regulative response in the frontal bones of Wnt1-Cre; Msx1/2cko/cko mutant embryos
We used Wnt1-Cre to produce a neural crest-specific knockout of Msx1 and Msx2. We showed previously that Wnt1-Cre caused an efficient knockout of each gene (Fu et al., 2007). Wnt1-Cre; Msx1cko/cko ;Msx2cko/cko embryos survived to the newborn stage and died shortly thereafter, with cleft palate, which was fully penetrant (n=10). All embryos examined (n>50) also exhibited a foreshortened mandible and maxilla, making them easily recognizable.
We produced an allelic series of floxed Msx1 and Msx2 alleles together with Wnt1-Cre, and examined the morphology of skulls at the newborn stage (Figure 1). The skulls exhibited a defect in the frontal bone that became progressively larger as the number of functional Msx alleles decreased. Analysis of different allelic combinations of Msx1 and Msx2 revealed that the two genes were equivalent in their effects on the frontal bone defect (Figure 1; data not shown).
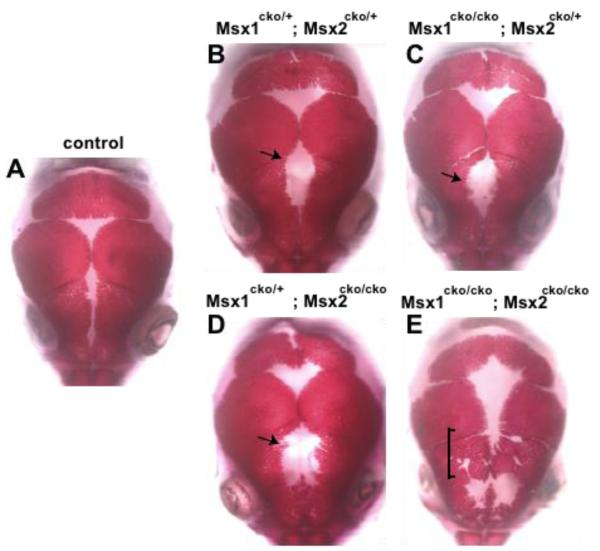
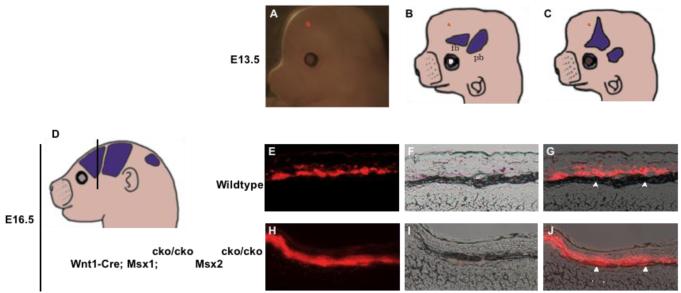
Figure 1. Dual functions of Msx1/2 in promoting frontal bone development and suppressing heterotopic bone formation in early migrating cranial mesenchyme.
Skulls of Wnt1-Cre; Msx1cko/cko; Msx2cko/cko mutants at the newborn stage were stained with Alizarin Red to reveal bone. A, wild type; B, Msx1cko/+;Msx2cko/+; C, Msx1cko/+;Msx2cko/cko; D, Msx1cko/cko; Msx2cko/+; E, Msx1cko/cko; Msx2cko/cko. At least five skulls of each genotype were examined. We show representative images. Note increasing size of frontal foramen with decreasing Msx gene dosage up to homozygote-heterozytgote combination (arrows). Note unpatterned, heterotopic bone in area of posterior frontal bone in E (bracket). Images are representative of at least four skulls with similar morphology.
Since the calvarial bones do not develop in conventional Msx1/2 knockouts (Han et al., 2007), we expected that complete Wnt-Cre-mediated inactivation of Msx1/2 would result in a more severe foramen than in homozygous-heterozgyous combinations. Strikingly, however, upon inactivation of the fourth Msx allele, a new phenotype became evident: Alizarin-stained bone was present over much of the area where we expected to see an unossified persistent foramen. This phenotype was fully penetrant (10/10 skulls examined). Thus Msx1/2 mutant embryos “regulated” and partially repaired the frontal bone defect. This regulative bone obliterated part of the frontal suture and was irregular in shape, suggesting that it was not subject to normal patterning mechanisms. In addition, the extent of apical growth of the parietal bones was reduced in Msx1/2 homozygous mutant embryos. This effect was non-autonomous since the parietal bones are derived from mesoderm. Finally, double heterozygous Msx1/2 mutants had a cleft in the posterior portion of the interparietal bone. In double homozygous mutants, an additional defect was evident in the anterior of the interparietal bone. Both defects occurred in the central portion of the bone, which is derived from neural crest (Jiang et al., 2002).
We examined a developmental series of embryos to determine when frontal bone regulation was first detectible. We stained embryos in whole mount for the early osteoblast marker, alkaline phosphatase (ALP) (Figure 2). In control embryos at E12.5, the frontal bone rudiment was evident as a crescent of ALP-stained cells in the supraorbital ridge. Immediately posterior to the rudiment is an ALP-free area corresponding to the presumptive coronal suture; posterior to the suture is the parietal bone rudiment. In Msx1/2cko/cko mutants, no staining was apparent in the area of the frontal bone rudiment, nor was there staining on the apical portion of the head in the area where the regulative bone would later form.
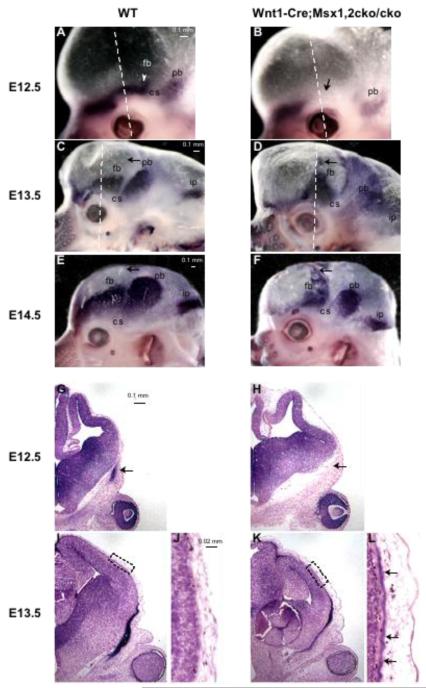
Figure 2. Prospective heterotopic bone is detectible at E13.5.
Embryonic heads were stained in whole mount (A-F) or in cross sections (G-L) for alkaline phosphatase (ALP activity at E12.5 (A, B, G, H) and E13.5 (C, D, I-L) and E14.5 (E, F). At least three mutant and three control embryos were examined at each stage. Shown are representative images. Dotted lines in whole mount figures indicate planes of section in Figs G-L. Note that in the Wnt1-Cre; Msx1/2 cko/cko mutant at E12.5, the frontal bone rudiment is not detectible (arrows, B, H). At E13.5, an area of ALP stain is evident apical to the eye, extending approximately 2/3 of the distance to the dorsum of the head (D, arrows). Sections show an ALP positive layer in the apical region of the head (K, L, arrows). Fb, frontal bone; pb, parietal bone; ip, interparietal bone, cs, coronal suture.
At E13.5, as revealed by ALP staining, the frontal and parietal bone rudiments of control embryos were significantly larger than at E12.5 (Figure 2). No ALP activity was present apical to the rudiments. In contrast, in Msx1/2cko/cko ; Wnt1-Cre mutants, the ALP-positive area was irregular in shape, and extended apically to a much greater degree than controls. By E14.5, there was extensive regulative bone in the Msx1/2 mutant. The parietal bone was substantially smaller than in the wild type embryo. Thus the inactivation of Msx1/2 in the frontal bone territory influenced the growth of the parietal bone during embryogenesis.
Regulative frontal bone in Wnt1-Cre; Msx1/2 mutants is derived from a population of neural crest whose normal fate is non-osteogenic
To understand the processes that gave rise to the regulative bone in Msx1/2 mutants, we sought to determine its tissue of origin. We considered two possibilities. The first was that the bone arose from mesoderm-derived cells that normally form the parietal bone. The close proximity of the regulative bone to the parietal bone made this an attractive possibility. Also consistent with this possibility is the finding that regulative bone does not occur in the frontal bone territory of conventional Msx1/2 knockouts (Han et al., 2007), suggesting that its development may depend on Msx gene function in a non-neural crest cell type.
We crossed the R26R marker allele into Wnt1-Cre;Msx1/2 mutants and examined the distribution of lacZ positive cells in double homozygous conditional mutant and control embryos at E13.5 (Figure 3). In control embryos, a lacZ positive layer of loose mesenchyme was evident between the epidermis and the meninges. This layer is composed of neural crest cells that migrate apically between E9.5 and E10.5, prior to the growth of the frontal bone rudiment (Ishii et al., 2003); we refer to these cells as “early migrating” neural crest cells. This layer is continuous with the ALP-positive frontal bone rudiment. At E12.5, Msx1 and Msx2 are coexpressed in this layer (Figure 4) (Ishii et al., 2003; unpublished observations). At E13.5, Msx1 is expressed predominantly in the meninges and Msx2 in the overlying mesenchyme (Figure 4).
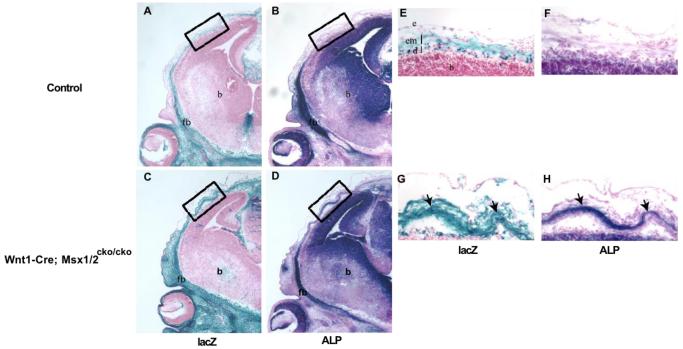
Figure 3. Neural crest origin of heterotopic bone.
To visualize neural crest-derived cells, we produced mice carrying the R26R marker allele along with Wnt1-Cre; Msx1cko/cko; Msx2cko/cko. Embryos were taken at E13.5, and heads were sectioned in the coronal plane. Adjacent sections were stained either for lacZ (A, C, E, G) or ALP (B, D, F, H). Boxed areas in A-D correspond to areas of heterotopic ALP activity (see Figure 2). Arrows in G and H point to heterotopic prospective bone. Images shown are representative of three control and three mutant embryos examined. Note that the ALP-positive cells are in a lacZ positive cell layer and are therefore derived from neural crest. fb, frontal bone; b, brain; em, early-migrating neural crest; d, dura; e, epidermis.
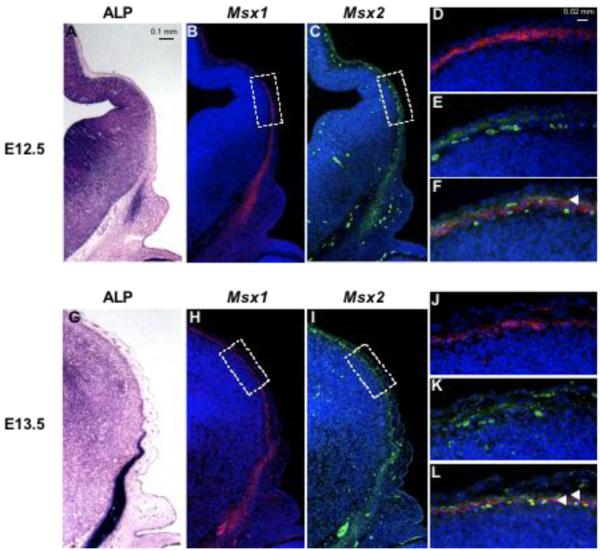
Figure 4. Expression of Msx1/2 in early migrating cranial mesenchyme.
Coronal sections of embryos at E12.5 and E13.5 were stained for ALP activity (A, G) and incubated with Msx1 (B, D, H, J) and Msx2 (C, E, I, K) probes simultaneously. Hybridization signals were visualized by immunofluorescence. Msx1 is in red, Msx2 in green. D, E, J and K show boxed areas in B, C, H and I. F and L are merges of images in D, E, J and K respectively. Note partial overlap of Msx1 and Msx2 signals in the neural crest-derived mesenchyme layer at E12.5 (yellow color, arrowhead, F). At E13.5, Msx1 is expressed in the meninges, internal to Msx2 (L, arrowheads). Msx2 is expressed in the mesenchymal layer (K).
In wild type embryos, the apical portion of the early migrating neural crest layer did not stain appreciably for ALP (Figure 3B). In contrast, in mutant embryos, the regulative prospective bone was detectible as a patchy ALP stained layer apical to the frontal bone rudiment (Figure 3D). Cells of this layer appeared more tightly condensed than their wild type counterparts. Staining for lacZ revealed that these ALP-positive cells were entirely or almost entirely of neural crest origin (Figure 3E-H)). Thus, although the regulative response may require Msx gene function in a non-neural crest cell type, it does not entail recruitment of mesoderm-derived cells to the frontal bone defect.
BrdU incorporation experiments revealed that the proportion of BrdU positive cells in the apical portion of the early migrating neural crest was not significantly different from that of their counterparts in control embryos at E12.5 (Supplementary Figure 1) or E13.5 (data not shown), although there was a 17±5% increase in total cell number. Cells of the early migrating mesenchyme do not undergo apoptosis at an appreciable rate in wild type embryos (Ishii et al., 2003; our unpublished observations); therefore the increase in total cell number is likely due to an earlier event in neural crest development.
We next addressed the source of the ALP-positive, neural crest-derived cells that form the regulative bone of Msx1/2 mutants (Figure 5). The simplest possibility was that the cells originate from the early migrating neural crest. It was also possible that they were the result of an aberrant recruitment of the neural crest-derived osteogenic precursor cells that normally migrate from the supraorbital ridge to the leading edge of the growing frontal bone (Yoshida et al., 2008; Ting et al., 2009). To test these possibilities, we carried out a series of diI cell marking experiments.
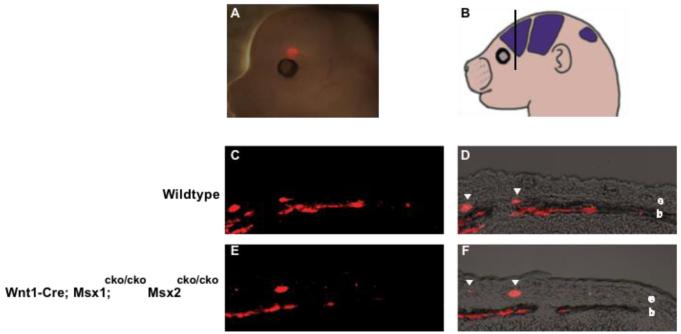
Figure 5. Inactivation of Msx1/2 in neural crest causes a change in the fate of early migrating cranial mesenchyme.
We injected DiI into heads of E13.5 control and Msx1/2 cko/cko; Wnt1-Cre and control embryos and assessed the distribution of dye after exo utero development until E16.5. Dye was placed near the apex, in the area in which the frontal bone will develop in control embryos and heterotopic bone will develop in Msx1/2cko/cko; Wnt1-Cre mutants. The placement of dye is shown in a representative embryo in A, and schematically in B and C. Embryos were allowed to develop to E16.5, and were then sectioned in the coronal plane (D, see also Fig 2) and photographed (E-J). E and H are epifluourescence images, F, I, brightfield images, G, J, merged images. In control embryos, dye was distributed in a layer of cells flanking the prospective bone. Few if any labeled cells were found in the prospective bone (arrowheads). In mutant embryos, dye was located largely in the developing bone. We obtained substantially similar results in several repetitions of this experiment (Table 1).
We labeled cells of the early migrating neural crest layer and asked whether they become incorporated into heterotopic bone (Figure 5). DiI was injected into mutant or control embryonic heads at E13.5, during the early stages of the formation of heterotopic bone. The dye was placed apically, beyond the dorsal margin of bone at this stage (see Figure 2). Embryos were allowed to develop exo utero and then examined for the distribution of the dye (Figure 5). In mutant embryos at E16.5, the dye was located almost exclusively in ALP-expressing cells of heterotopic bone. In control embryos, in contrast, the dye became localized in a cell layer flanking the bone (Figure 5; Table 1). These results suggest that in Wnt1-Cre; Msx1/2cko/cko mutants at E13.5, cells of the early migrating layer of neural crest are allocated to form heterotopic bone.
Table 1.
Location of labeled cells following diI injection into calvarial rudiments or apical mesenchyme
| Experiment | Genotype | Site of injection1 | Location of labeled cells2 |
|---|---|---|---|
| 1-10 | Control3 | r | e, b |
| 11 | Msx1cko/cko; Msx2cko/cko | r | e, b |
| 11 | Control3 | r | e, b |
| 11 | Control | r | e, b |
| 12 | Msx1cko/cko; Msx2cko/cko | r | e, b |
| 12 | Msx1cko/cko; Msx2cko/cko | r | e, b |
| 12 | Control3 | r | e, b |
| 12 | Control3 | r | e, b |
| 13 | Msx1cko/cko; Msx2cko/cko | r | e, b |
| 13 | Control3 | r | e, b |
| 13 | Control3 | r | e, b |
| 14 | Control3 | a | e |
| 14 | Control3 | a | e |
| 14 | Msx1cko/cko; Msx2cko/cko | a | b |
| 15 | Msx1cko/cko; Msx2cko/cko | a | b |
| 15 | Control | a | e |
| 16 | Control | a | e |
| 16 | Msx1cko/cko; Msx2cko/cko | a | e |
diI injection is described in Fig 5; r: frontal bone rudiment, a, apical mesenchyme.
Embryos were analyzed after 48 hours of exo utero development; e, ectocranial mesenchyme, b, prospective frontal bone.
Genotypes of control embryos were either wild type, or Wnt1-Cre; Msx1/2 double heterozygotes, which had no phenotype.
To determine whether neural crest-specific inactivation of Msx1/2 caused changes in the apical migration of osteogenic precursor cells from the area of the frontal bone rudiment, we carried out DiI labeling of the frontal bone rudiment at E13.5 and assessed the distribution of dye at E16.5 (Figure 6). This migration has been documented in detail (Takahashi et al., 2008; Ting et al., 2009). We used the same diI injection protocol as in our previous work (Ting et al., 2009) to assess migration in Msx1/2 mutants. In control embryos, labeled cells were found in both the ectocranial layer in which the cells migrate, and in the developing bone, which is their ultimate fate. We did not detect a significant change in the number of labeled migratory cells in mutant embryos. These results suggest that the apical migration of osteogenic precursor cells from the supraorbital ridge does take place in Msx1/2 mutants. However, our finding that placement of dye in the early migrating mesenchyme in the apical portion of the head at E13.5 results in virtually 100% of the label in bone suggests that the principal source of the heterotopic bone is the early migrating mesenchyme, not the migratory cells of the frontal bone rudiment.
Figure 6. Osteogenic precursor cells can migrate apically from the supraorbital ridge in Msx1/2 cko/cko; Wnt1-Cre mutant embryos.
To assess the apical migration of osteogenic precursor cells, we injected DiI in the supraorbital ridge of control and mutant embryos at E13.5 as shown in A. Embryos were allowed to develop exo utero until E16.5, and were then sectioned in the indicated plane (B) and photographed. Note labeled precursor cells in the ectocranial layer (e, arrowheads) as previously described (Ting et al., 2009; Yoshida et al., 2008). These cells add to the leading edge of the growing bone (b) in both control and mutant embryos, suggesting that this morphogenetic mechanism is functional in Msx1/2cko/cko; Wnt1-Cre mutants. We obtained substantially similar results in several repetitions of this experiment (Table 1).
Timing of action of Msx genes
DiI labeling suggested that in Wnt1-Cre/Msx1/2 mutants, the early migrating neural crest cells are mis-allocated to form bone as early as E13.5. Thus Msx genes must be required for the proper allocation of this cell layer at or before E13.5. To determine when Msx genes are required to suppress heterotopic bone formation, we used a Tamoxifen-inducible Cre mouse line (Hayashi and McMahon, 2002). We produced mice carrying the Cagg-ER transgene together with floxed alleles of Msx1 and Msx2. We used a dosage of tamoxifen that we determined to be sufficient to cause efficient recombination of the R26R marker allele within 24 hours of injection at each of the time points (Hayashi and McMahon, 2002; our unpublished observations).
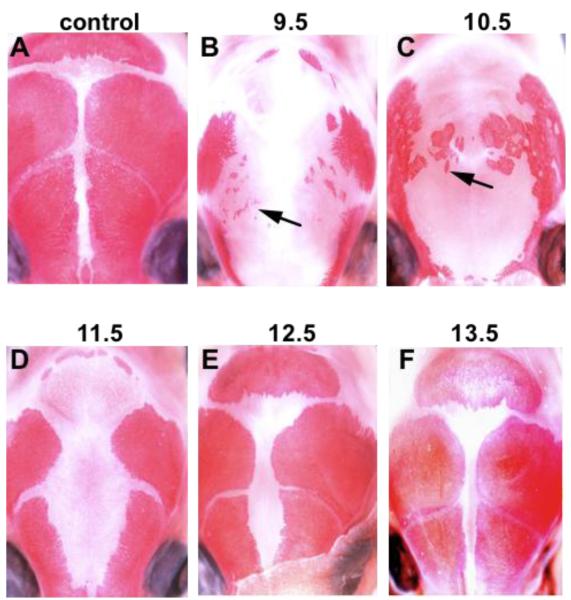
We injected tamoxifen into pregnant females at 24 hour intervals from 9.5 days pc to 12.5 days pc and examined newborns for heterotopic bone in the area that the frontal bone would normally occupy. We found that Cagg-ER-Cre; Msx1cko/cko; Msx2cko/cko embryos injected at E9.5 had small areas of heterotopic bone; embryos injected at E10.5 had substantially larger areas (Figure 7). Embryos injected at E11.5 or E12.5 did not have heterotopic bone. Given that complete Cre-mediated recombination occurs approximately 24 hours after injection of tamoxifen (Hayashi and McMahon, 2002), the interval during which Msx1/2 function to suppress heterotopic bone formation is between E10.5 and E11.5. This is the interval during which the early migrating neural crest migrates apically (Ishii et al., 2003).
Figure 7. Timing of Msx1/2 gene action in heterotopic bone formation.
We used a Tamoxifen-inducible (Cagg-Er-Cre) to inactivate Msx1/2 at different times during development. Tamoxifen was injected IP into pregnant females at the indicated times post coitum. Pups were taken at the newborn stage and bones of the skull vault visualized by staining with Alizarin Red S. Note that Tamoxifen injected at E10.5 caused heterotopic bone (arrows). Tamoxifen injected at E11.5 and E12.5 resulted in retarded growth of the frontal and parietal bones, but not heterotopic bone. Thus Msx1/2 are required between E10.5 and E11.5 to suppress heterotopic bone formation. At least three embryos at each time point were examined, with substantially similar results.
Bmp signaling is necessary for heterotopic ossification in Msx1/2 neural crest-specific mutants
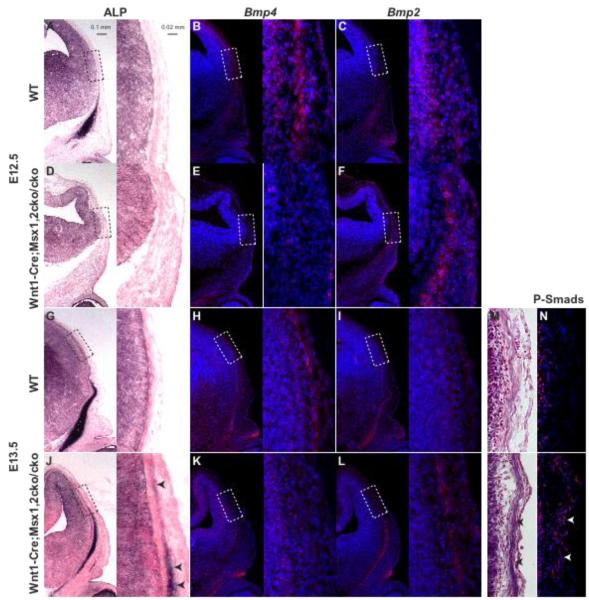
The well-documented role of Bmp signaling in osteogenesis and the known relationship between Msx genes and the Bmp pathway (Brugger et al., 2004; Chai and Maxson, 2006; Maxson and Ishii, 2008) prompted us to examine the status of Bmp signaling during heterotopic bone formation in Wnt1-Cre/Msx1/2 conditional mutants (Figure 8). We assessed the expression of Bmp2 and Bmp4, both known to be expressed in calvarial tissues (Kim et al., 1998). In addition we examined the distribution of P-Smad1/5/8 as an indicator of the net activity of canonical Bmp signaling. In wild type embryos at E12.5, Bmp2 was expressed in the meningeal layer and at a low level in the overlying mesenchyme. Bmp4 was also expressed in the meningeal layer and at a much higher level in the overlying mesenchyme. In Wnt1-Cre; Msx1/2 cko/cko mutants Bmp2 expression in the early migrating mesenchyme increased and Bmp4 expression decreased. A similar result was evident at E13.5. Immunostaining for P-Smad1/5/8 at E13.5 showed elevated levels in the early-migrating mesenchyme layer, indicating that the net change in Bmp signaling was positive. The upregulation of Bmp signaling in Msx mutants in association with the development of heterotopic bone suggests that the Bmp pathway has a role in this process.
Figure 8. Dysregulated Bmp signaling is correlated with heterotopic bone in Wnt1-Cre; Msx1/2 mutants.
We examined the expression of ALP (A, D, G, J), Bmp4 (B, E, H, K), Bmp2 (C, F, I, L), and P-Smad1/5/8 (N) in the apical cranial mesenchyme at E12.5 and E13.5. ALP was detected by histochemistry, Bmp2 and Bmp4 by in situ hybridization, P-smad1/5/8 by immunostaining. Boxed areas are shown in higher magnification in the image to the right. Note reduced expression of Bmp2 and increased expression of Bmp4 in the mesenchymal (ALP-expressing) layer in Wnt1-Cre; Msx1/2cko/cko mutants. Also note increase in P-Smad1.5.8-positive nuclei (N).
To test directly whether the increase in Bmp signaling is necessary for heterotopic bone development in Msx mutants, we carried out a series of bead implantation experiments in cultured calvarial explants (Figure 9). We implanted beads containing either the Bmp inhibitor, noggin, or BSA, in explants of Msx1/2 mutant and control heads taken at E13.5. Beads were placed in several locations along the apical-basal axis in order to ensure that at least one bead would be located in the area of heterotopic bone (Figure 9A). Explants were cultured for 48 hours, then sectioned and stained for ALP activity. Results representative of three repetitions of this experiment are shown.
Figure 9. Development of heterotopic bone in Wnt1-Cre; Msx1/2 mutants is dependent on Bmp signaling.
Embryonic heads were harvested at E12.5 and beads soaked in noggin or BSA were placed as shown in the whole mount photograph in A. After 24 hours in culture, heads were sectioned in the coronal plane and stained for ALP. We show results representative of three repetitions of this experiment. Note that in wild type (B), ALP stain is confined to the frontal bone rudiment in the supraorbital ridge. In the Wnt1-Cre; Msx1/2cko/cko mutant heterotopic bone is visible as a layer of ALP stain extending apically from the rudiment (E). Noggin beads, but not BSA beads, inhibit ALP staining in this layer. Neither noggin nor BSA beads had any effect on the apical mesenchyme of control mice (B, C, D).
In mutant heads that did not receive beads, heterotopic ALP stain was apparent in the apical region, in the mesenchymal layer beneath the surface ectoderm (Figure 9E). No such stain was evident in control heads (Figure 9B). Apical placement of noggin beads in mutants resulted in reduced ALP staining in the mesenchyme surrounding the bead (Figure 9G). BSA beads did not produce this effect (Figure 9F). These data suggest that Bmp signaling is required for the development of heterotopic calvarial bone in Wnt1-Cre; Msx1/2 mutants.
Discussion
Here we demonstrate that Msx1 and Msx2, shown previously to be required for calvarial bone development (Han et al., 2007; Ishii et al., 2003), also suppress bone formation in a head tissue that is normally non-osteogenic. Whereas inactivation of up to three Msx1/2 alleles by means of Wnt1-Cre results in a progressive increase in size of a defect in the frontal bone, inactivation of the final Msx1/2 allele results in the filling in of the defect with disorganized bone. Thus the suppression of such regulative bone formation requires a single allele of Msx1 or Msx2. This bone is derived from neural crest, not mesoderm, and develops largely if not entirely from the early migrating neural crest, a population of neural crest cells that migrates prior to those that normally compose the frontal bone. Finally, we demonstrate that Wnt1-Cre-Msx1/2 mutant embryos exhibit dysregulation of Bmp2 and Bmp4 expression, resulting in increased P-Smad1/5/8 activity in the early migrating neural crest. Moreover, we show that noggin-soaked beads can abrogate the formation of heterotopic bone in explanted heads of mutant embryos. Thus Bmp signaling is required for heterotopic bone development.
In conventional Msx1/2 homozygous knockout embryos, neural crest cells migrate to the supraorbital ridge (site of the calvarial rudiments) in near-normal numbers as judged by Wnt1-Cre/R26R lineage tracing (Han et al., 2007; Ishii et al., 2003). Subsequently neural crest cells of the frontal bone rudiments exhibit impaired proliferation and differentiation, as well as an increase in apoptosis (Han et al., 2007). The end result is that the frontal bones do not develop. Given this profound deficiency, it is perhaps surprising that the growth of the frontal bones is not more compromised in Wnt1-Cre; Msx1/2cko/cko mutants in which rudiments do develop after a delay in the initial appearance of ALP-positive cells. Wnt1-Cre produces an efficient inactivation of the Msx1 and Msx2 alleles used in this study (Fu et al., 2007); therefore an incomplete knockout is not a likely explanation. Instead, the discrepancy suggests that non-neural crest tissues are likely to have a role in the development of the neural crest-derived portion of the skull vault. A candidate tissue is a population of mesoderm that flanks the frontal bone rudiment ectocranially. Our results also provide evidence of an influence of neural crest on mesoderm: the growth of the parietal bone rudiment (mesoderm-derived) is impaired in Wnt1-Cre; Msx1/2cko/cko mutants (Figure 2). It is noteworthy that the dura underlying the anterior portion of the parietal bone is derived from neural crest (Jiang et al., 2000). The dura is known to influence cranial suture development (Opperman et al., 1995) and could thus be part of a mechanism that controls parietal bone growth.
Given the requirement for Msx1 and Msx2 for the growth of the calvarial bones (Chai and Maxson, 2006; Maxson and Ishii, 2008), the bone-suppressive activity in the Wnt1-Cre-mediated Msx1/2 knockout was a surprise. The appearance of the heterotopic bone in the area normally occupied by the posterior frontal bone led us to predict that mesodermal cells normally allocated to the parietal bone were migrating into the defect and forming bone. However, Wnt1-Cre mapping showed that the bone is entirely of neural crest origin. Thus the heterotopic bone appears to depend on the autonomous function of Msx genes in the neural crest. Since the heterotopic bone does not occur in conventional Msx1/2 knockouts, its development may also depend on Msx gene function in a non-neural crest cell type.
From what subpopulation of neural crest does the heterotopic bone arise? Clues came from recent work on the mechanism of growth of the skull vault. The frontal and parietal bones grow by end-addition of migratory precursors (Ting et al., 2009; Yoshida et al., 2008). The bone rudiments grow through a pre-existing layer of mesenchyme. In the case of the frontal bone, cells of this layer are derived from neural crest, and are designated “early migrating neural crest.” DiI labeling of cells in this mesenchyme layer showed that they are not allocated to become bone (Yoshida et al., 2008). Instead, after the calvarial bone rudiments have elongated to the apex of the head, DiI-labeled cells are found in a layer that flanks the bone ectocranially. Intriguingly, DiI labeling of Wnt1-Cre; Msx1/2 mutants showed that cells of this layer are incorporated into bone. Thus the early migrating neural crest forms heterotopic bone. To what extent migratory osteogenic precursor cells originating in the supraorbital ridge contribute to heterotopic bone is not clear. DiI labeling experiments suggest that the rudiments produce migratory cells normally. However, the fact that virtually 100% of the labeled cells in the early migrating mesenchyme are ultimately found in bone suggests the early migrating mesenchyme is the principal source of the heterotopic bone. These results imply a change in the fate map of the early migrating mesenchyme in Wnt1-Cre; Msx1/2 mutants, although we stress that we have not shown directly that individual cells of the early migrating layer convert to an osteogenic fate. BrdU labeling experiments showed not change in the proliferation index of the early migrating mesenchyme of Wnt1-Cre; Msx1/2 mutants, but did reveal a overall increase in cell number of approximately 20% at E12.5. It is possible that this increase promotes condensation of the early migrating cells and thus leads to osteogenic differentiation.
Our data strongly suggest that the action of the Bmp pathway is at least part of the cause of the ectopic bone. Analysis of Bmp2 and Bmp4 expression showed a loss of Bmp4 expression and an upregulation of Bmp2 expression in the early migrating neural crest at E12.5, prior to the detection of ectopic ALP. No change was detected in the expression of noggin (data not shown). Thus the observed increase in the number of P-Smad 1/5/8 positive cells at E13.5 is likely a result of the changes in Bmp2 and Bmp4 expression. It is interesting that Bmp2 and Bmp4 appear to have subtly different spatial patterns of expression: Bmp4 is expressed in the meningeal layer as well as in the overlying mesenchyme; Bmp2 is expressed predominantly in the mesenchyme layer. Thus a critical change in the Msx1/2 neural crest-specific knockout may be the loss of Bmp signaling in the meningeal layer.
A definitive link between Bmp signaling and heterotopic bone came from experiments in which we implanted noggin-soaked beads under the skin of explanted Msx1/2 mutant and control heads. This approach showed that noggin inhibited the appearance of ALP-positive cells in the early migrating neural crest layer. Thus Bmp signaling, downstream of Msx1/2, is required for heterotopic bone formation in the head. It is perhaps surprising that previous work on the conventional Msx1/2 knockout did not demonstrate a change in Bmp 2 or Bmp4 expression at E12.5 in mutant embryos (Han et al., 2007). We do not know the reason for this discrepancy, although it is reasonable to expect that in the germline knockout, compensatory mechanisms operating over all of embryogenesis might damp effects of Msx1/2 inactivation on Bmp gene expression in neural crest-derived tissues.
Heterotopic bone formation driven by Bmp signaling is also observed in fibrodysplasia ossificans progressiva, a disorder characterized by progressive heterotopic ossification in which skeletal muscles and connective tissues are transformed into bone (Billings et al., 2008). This disorder is associated with a specific mutation in ACVR1, which encodes a bone morphogenetic protein type I receptor. The ectopic skeleton is derived largely from cells of vascular origin (Billings et al., 2008).Whether Msx genes have a role in fibrodysplasia ossificans progressiva has not been reported.
The ectopic bone in Msx1/2 mutants bears some resemblance to Wormian bones, defined as cranial bones that have no relationship to the normal ossification centers driving skull growth (Parker, 1905; Sanchez-Lara et al., 2007). Wormian bones often occur in sutures; however, they can be more extensive, as in osteogenesis imperfecta in which the calvarial bones are sometimes largely replaced by a mosaic of Wormian bones (Baljet, 2002). Wormian bones can also be the result of an unusually large head, which increases the time required for the frontal and parietal bones to grow from rudiments in the supraorbital ridge to the skull apex (Sanchez-Lara et al., 2007. Wormian bones are thus, in the most general sense, a regulative response to a deficiency in calvarial bone growth. We suggest that Msx-dependent heterotopic bone can viewed similarly. Whether such heterotopic bone is truly Wormian bone is not clear: The tissue of origin of Wormian bone has not been investigated. However, it is suggestive that the two are not only similar morphologically but also likely result from a regulative response to compromised calvarial bone growth.
Finally, we are intrigued by the potential evolutionary implications of the Msx1/2-dependent program we have uncovered. Our results, together with recent DiI cell marking experiments; (Ting et al., 2009; Yoshida et al., 2008), document two distinct osteogenic programs in skull vault development. One consists of the patterned growth of the calvarial rudiments by end addition of migratory osteogenic precursor cells. The other consists of the differentiation of the early migrating mesenchyme along an osteogenic pathway. The former appears to be the primary mechanism by which the frontal and parietal bones grow. The latter mechanism, we suggest, may be an evolutionary remnant of a program present in basal vertebrates with a mode of calvarial bone growth distinct from that of mammals. Skulls of fish typically have many loosely connected elements; those of mammals are more tightly connected and have fewer elements (de Beer, 1985; Romer, 1997). These changes in skull structure must be a result of changes in the developmental program underlying skull growth. One scenario is that the osteogenic program in the early migrating mesenchyme was the primitive condition, accounting for the multiplicity of loosely patterned skull bones in early vertebrates. The migratory program then arose secondarily. The loss of bones would then be a result of suppression of the primitive program—e.g., by acquisition of Msx1/2-dependent repression of osteogenesis in specific regions—accompanied by an expansion of the growth by end addition. Analysis of cranial development in extant agnathans may shed light on this scenario.
MATERIALS AND METHODS
Mouse mutants and genotyping
Mutant lines were maintained in a C57Bl/6 mixed background. The R26R (Soriano, 1999), Wnt1-Cre (Danielian et al., 1998) and Msx1 and 2 Floxed (Fu et al., 2007) alleles have been described. We genotyped Msx1 and 2 Floxed, R26R, and Wnt1-Cre alleles by PCR as described (Fu et al., 2007; Jiang et al., 2002).
Histology, immunostaining and in situ hybridization
Heads of embryos were embedded in OCT medium (Histoprep, Fisher Scientific) before sectioning. Frozen sections were cut at 10 μm. Analysis of β-galactosidase activity of Wnt1-Cre/R26R reporter gene expression was carried out as described (Ishii et al., 2003). Immunostaining of frozen sections was largely carried out as previously reported (Ishii et al., 2003). Immunohistochemistry was performed using rabbit anti-P-Smad1/5/8 (pSmad1/5/8) (Cell Signaling) or rabbit anti-phosphorylated Histone3 (pH3) (Cell Signaling) diluted in 1%BSA/PBS and incubated overnight at 4°C. Detection of anti-p-Smad1/5/8 or anit-pH3 was performed by incubating rhodamine-labeled goat anti-rabbit IgG (1:100 for anti-pSmad1/5/8) or (1:50 for anti-pH3) for1 hour at room temperature followed by DAPI counterstaining and examination by epifluorescence microscopy. Non-radioactive section in situ hybridization using the tyramide signal amplification (TSA) method was performed as described (Adams, 1992; Paratore et al., 1999; Yang et al., 1999). Briefly, to analyze mRNA expression by TSA, DIG-labeled or F-labeled riboprobes were allowed to hybridize with the section and were detected with anti-DIG or anti-FL antibodies conjugated to horseradish peroxidase (POD). Indirect TSA fluorescence system (TSA-biotin/avidin-FITC) was used to detect the POD-conjugated antibody (Perkin Elmer). RNA probes were generated as described (Yang et al., 1999): the Bmp4 probe was DIG-labeled and Bmp2 FL-labeled (courtesy of Dr. Malcolm Snead).
Whole-mount skull Alizarin Red S and ALP staining
Skulls from P0-day-old postnatal mice were stained for bone with 2% Alizarin Red S in 1% KOH for 1 to 2 days. The specimens were then cleared and stored in 100% glycerol. Whole-mount staining for alkaline phosphatase was carried out as described (Ishii et al., 2003). E12.5-E14.5 embryonic heads were fixed in 4% paraformaldehyde in PBS, and were bisected midsagitally after fixation. Presumptive calvarial bones were stained with NBT and BCIP (Roche).
Exo utero DiI labeling
Details of the exo utero manipulation have been described (Muneoka et al., 1986; Serbedzija et al., 1992). Briefly, E13.5 embryos with embryonic membranes were carefully exposed by incising the uterine wall. Two embryos from each side of the uterine horns were designated as the experimental group, and all others were removed. DiI (Molecular Probes, 1:10 dilution from 0.5% stock solution) was injected into the area of the frontal bone rudiments under a dissecting microscope with a microelectrode (tip diameter, 20 μm) attached to a mouth pipette (Yoshida, 2005). After injection, the embryos were returned to the peritoneal cavity of dams and allowed to continue development exo utero. After 2-3 days of additional development, the embryos were removed and examined by epifluorescence microscopy. The survival rate of the embryos after DiI injection was greater than 70%.
BrdU labeling
Pregnant mice injected intraperitoneally with BrdU (200 μg/gram of body weight) were sacrificed 2 hours after the injection. Embryonic day 12.5 (E12.5) embryo heads were fixed in 4% paraformaldehyde and were embedded in OCT medium (Histoprep, Fisher Scientific). Immunodetection of BrdU was performed according to the manufacturer’s instructions (Zymed). BrdU-positive cells in the area apical to the frontal bone rudiment were counted in three mutant and three wildtype littermate embryos. At least three sections were counted for each embryo examined.
Organ culture and bead implantation
Organ culture of embryonic heads was carried out essentially as described by Mohammed et al. (2008) and Hu et al. (2001). Briefly, control and Wnt1-Cre; Msx1/2cko/cko embryos were harvested at E13.5. Embryos were decapitated and the tongue and lower jaw were removed. Affigel agarose beads were washed with sterile PBS and allowed to dry before incubating them at 37 0C for 30 minutes in either 0.1% BSA or 100 ng/ml noggin (R&D). Beads were implanted under the skin, and skulls were placed on stainless steel grids which were then inserted into the well of an organ culture dish (Falcon) filled with BGJb medium supplemented with 0.1% BSA and 1U/ml penicillin and streptomycin. The skulls were incubated at 37 0 for 48 hours.
Supplementary Material
Acknowledgements
We dedicate this paper to the memory of Dr. Paul G. Roybal, a young scientist with enormous talent, heart, and courage. Paul battled brain cancer throughout earning his PhD and passed away on July 25, 2009, shortly after obtaining his degree. Paul’s family wishes to thank Dr. Jing-jing Sun, Dr. Nancy Wu, Dr. Mandy Ting, Dr. Mamoru Ishii, and particularly Dr. Robert Maxson and Dr. Deborah Johnson for supporting and mentoring Paul as he finished this work. The authors also thank Dr. Randall Widelitz, Damon De La Cruz, and Cathleen Chiu for help with fluorescence microscopy. This work was supported by NIH Grants DE19650 and DE16320 from the NIDCR to REM. PR was supported by NIH Training Grant 5 T32 GM067587.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adams JC. Biotin amplification of biotin and horseradish peroxidase signals in histochemical stains. J Histochem Cytochem. 1992;40:1457–63. doi: 10.1177/40.10.1527370. [DOI] [PubMed] [Google Scholar]
- Baljet B. Aspects of the history of osteogenesis imperfecta (Vrolik’s syndrome) Ann Anat. 2002;184:1–7. doi: 10.1016/S0940-9602(02)80023-1. [DOI] [PubMed] [Google Scholar]
- Billings PC, Fiori JL, Bentwood JL, O’Connell MP, Jiao X, Nussbaum B, Caron RJ, Shore EM, Kaplan FS. Dysregulated BMP signaling and enhanced osteogenic differentiation of connective tissue progenitor cells from patients with fibrodysplasia ossificans progressiva (FOP) J Bone Miner Res. 2008;23:305–13. doi: 10.1359/JBMR.071030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugger SM, Merrill AE, Torres-Vazquez J, Wu N, Ting MC, Cho JY, Dobias SL, Yi SE, Lyons K, Bell JR, et al. A phylogenetically conserved cis-regulatory module in the Msx2 promoter is sufficient for BMP-dependent transcription in murine and Drosophila embryos. Development. 2004;131:5153–65. doi: 10.1242/dev.01390. [DOI] [PubMed] [Google Scholar]
- Chai Y, Maxson RE., Jr. Recent advances in craniofacial morphogenesis. Dev Dyn. 2006;235:2353–75. doi: 10.1002/dvdy.20833. [DOI] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–6. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- de Beer G. The Development of the Vertebrate Skull. University of Chicago Press; 1985. [Google Scholar]
- el Ghouzzi V, Le Merrer M, Perrin-Schmitt F, Lajeunie E, Benit P, Renier D, Bourgeois P, Bolcato-Bellemin AL, Munnich A, Bonaventure J. Mutations of the TWIST gene in the Saethre-Chotzen syndrome. Nat Genet. 1997;15:42–6. doi: 10.1038/ng0197-42. [DOI] [PubMed] [Google Scholar]
- Fu H, Ishii M, Gu Y, Maxson R. Conditional alleles of Msx1 and Msx2. Genesis. 2007;45:477–81. doi: 10.1002/dvg.20316. [DOI] [PubMed] [Google Scholar]
- Han J, Ishii M, Bringas P, Jr., Maas RL, Maxson RE, Jr., Chai Y. Concerted action of Msx1 and Msx2 in regulating cranial neural crest cell differentiation during frontal bone development. Mech Dev. 2007;124:729–45. doi: 10.1016/j.mod.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii M, Merrill AE, Chan YS, Gitelman I, Rice DP, Sucov HM, Maxson RE., Jr. Msx2 and Twist cooperatively control the development of the neural crest-derived skeletogenic mesenchyme of the murine skull vault. Development. 2003;130:6131–42. doi: 10.1242/dev.00793. [DOI] [PubMed] [Google Scholar]
- Jiang X, Iseki S, Maxson RE, Sucov HM, Morriss-Kay GM. Tissue origins and interactions in the mammalian skull vault. Dev Biol. 2002;241:106–16. doi: 10.1006/dbio.2001.0487. [DOI] [PubMed] [Google Scholar]
- Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–16. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Rice DP, Kettunen PJ, Thesleff I. FGF-, BMP- and Shh-mediated signalling pathways in the regulation of cranial suture morphogenesis and calvarial bone development. Development. 1998;125:1241–51. doi: 10.1242/dev.125.7.1241. [DOI] [PubMed] [Google Scholar]
- Lana-Elola E, Rice R, Grigoriadis AE, Rice DP. Cell fate specification during calvarial bone and suture development. Dev Biol. 2007;311:335–46. doi: 10.1016/j.ydbio.2007.08.028. [DOI] [PubMed] [Google Scholar]
- Maxson R, Ishii M. The Bmp pathway in skull vault development. Front Oral Biol. 2008;12:197–208. doi: 10.1159/000115042. [DOI] [PubMed] [Google Scholar]
- Merrill AE, Bochukova EG, Brugger SM, Ishii M, Pilz DT, Wall SA, Lyons KM, Wilkie AO, Maxson RE., Jr. Cell mixing at a neural crest-mesoderm boundary and deficient ephrin-Eph signaling in the pathogenesis of craniosynostosis. Human molecular genetics. 2006;15:1319–28. doi: 10.1093/hmg/ddl052. [DOI] [PubMed] [Google Scholar]
- Morriss-Kay GM, Wilkie AO. Growth of the normal skull vault and its alteration in craniosynostosis: insights from human genetics and experimental studies. J Anat. 2005;207:637–53. doi: 10.1111/j.1469-7580.2005.00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muneoka K, Wanek N, Bryant SV. Mouse embryos develop normally exo utero. J Exp Zool. 1986;239:289–93. doi: 10.1002/jez.1402390216. [DOI] [PubMed] [Google Scholar]
- Opperman LA, Passarelli RW, Morgan EP, Reintjes M, Ogle RC. Cranial sutures require tissue interactions with dura mater to resist osseous obliteration in vitro. J Bone Miner Res. 1995;10:1978–87. doi: 10.1002/jbmr.5650101218. [DOI] [PubMed] [Google Scholar]
- Paratore C, Suter U, Sommer L. Embryonic gene expression resolved at the cellular level by fluorescence in situ hybridization. Histochem Cell Biol. 1999;111:435–43. doi: 10.1007/s004180050379. [DOI] [PubMed] [Google Scholar]
- Parker C. Wormian Bones. Robert Press; Chicago: 1905. [Google Scholar]
- Romer A. The Osteology of the Reptiles. Krieger Publishing Company; 1997. [Google Scholar]
- Sanchez-Lara PA, Graham JM, Jr., Hing AV, Lee J, Cunningham M. The morphogenesis of wormian bones: a study of craniosynostosis and purposeful cranial deformation. Am J Med Genet A. 2007;143A:3243–51. doi: 10.1002/ajmg.a.32073. [DOI] [PubMed] [Google Scholar]
- Serbedzija GN, Bronner-Fraser M, Fraser SE. Vital dye analysis of cranial neural crest cell migration in the mouse embryo. Development. 1992;116:297–307. doi: 10.1242/dev.116.2.297. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature Genetics. 1999;21:70–1. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Ting MC, Wu NL, Roybal PG, Sun J, Liu L, Yen Y, Maxson RE., Jr. EphA4 as an effector of Twist1 in the guidance of osteogenic precursor cells during calvarial bone growth and in craniosynostosis. Development. 2009;136:855–64. doi: 10.1242/dev.028605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Castilla LH, Xu X, Li C, Gotay J, Weinstein M, Liu PP, Deng CX. Angiogenesis defects and mesenchymal apoptosis in mice lacking SMAD5. Development. 1999;126:1571–80. doi: 10.1242/dev.126.8.1571. [DOI] [PubMed] [Google Scholar]
- Yoshida T. [Growth pattern of the frontal bone primordium and involvement of Bmps in this process] Kokubyo Gakkai Zasshi. 2005;72:19–27. doi: 10.5357/koubyou.71and72.19. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Vivatbutsiri P, Morriss-Kay G, Saga Y, Iseki S. Cell lineage in mammalian craniofacial mesenchyme. Mech Dev. 2008;125:797–808. doi: 10.1016/j.mod.2008.06.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.