Abstract
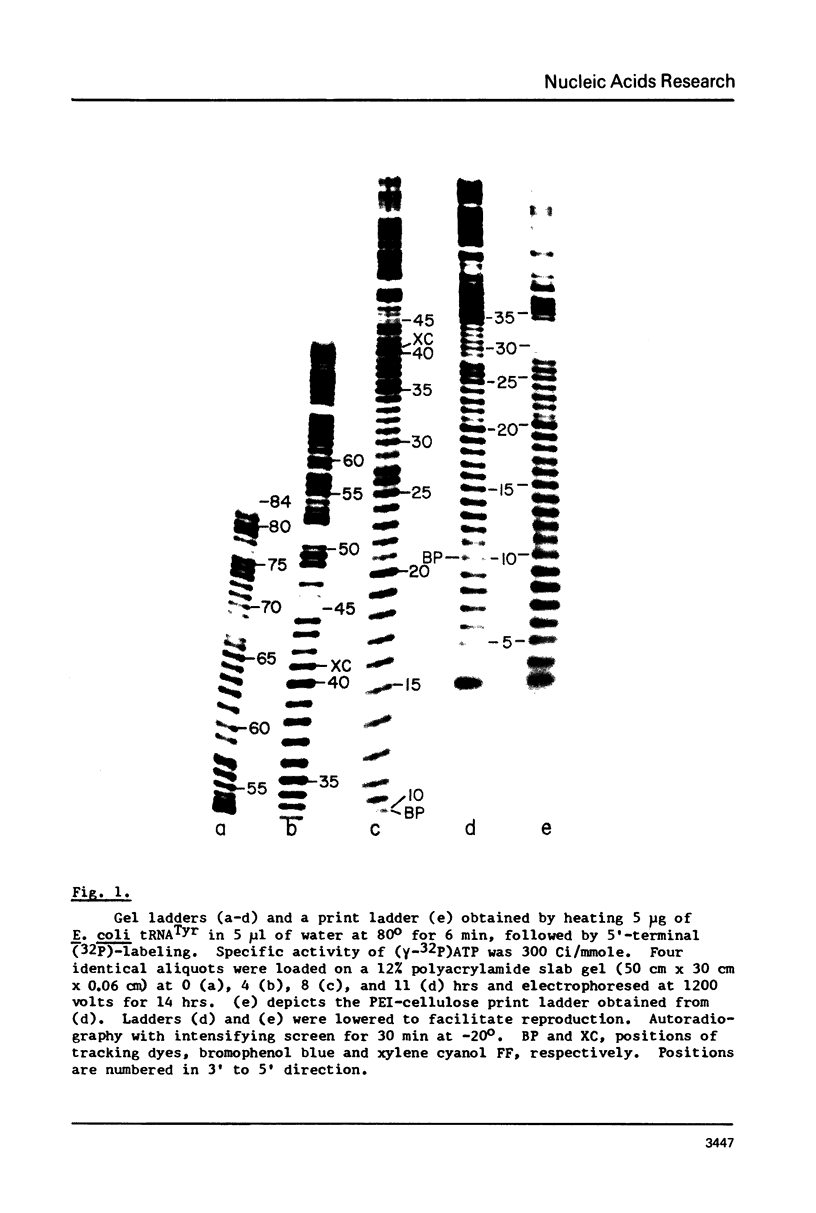
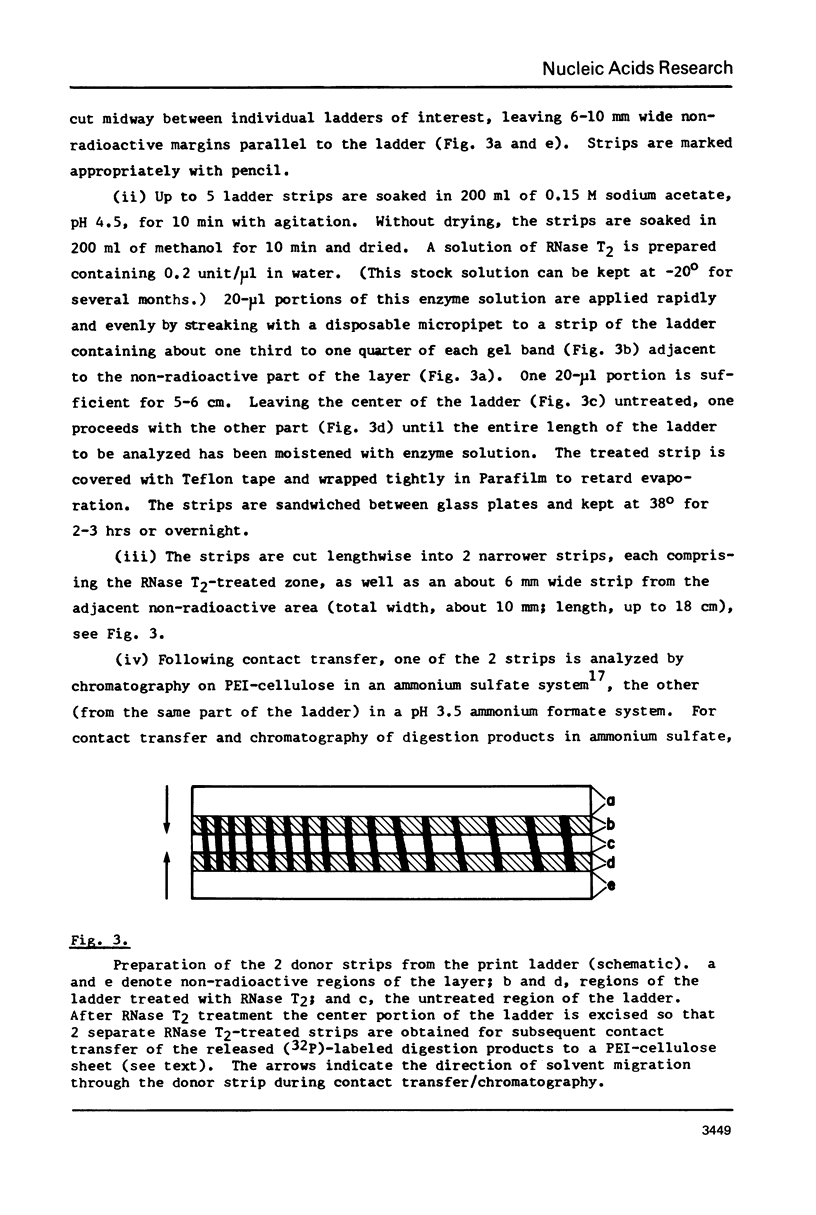
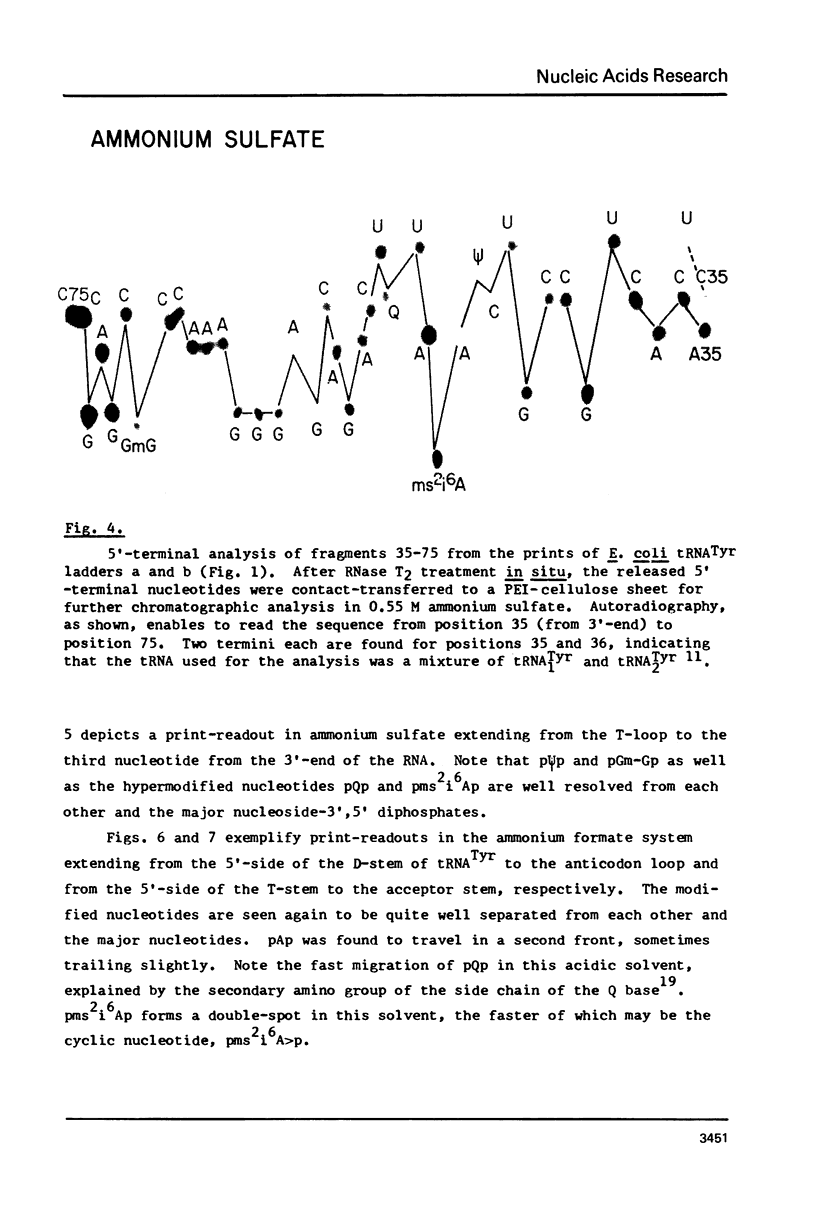
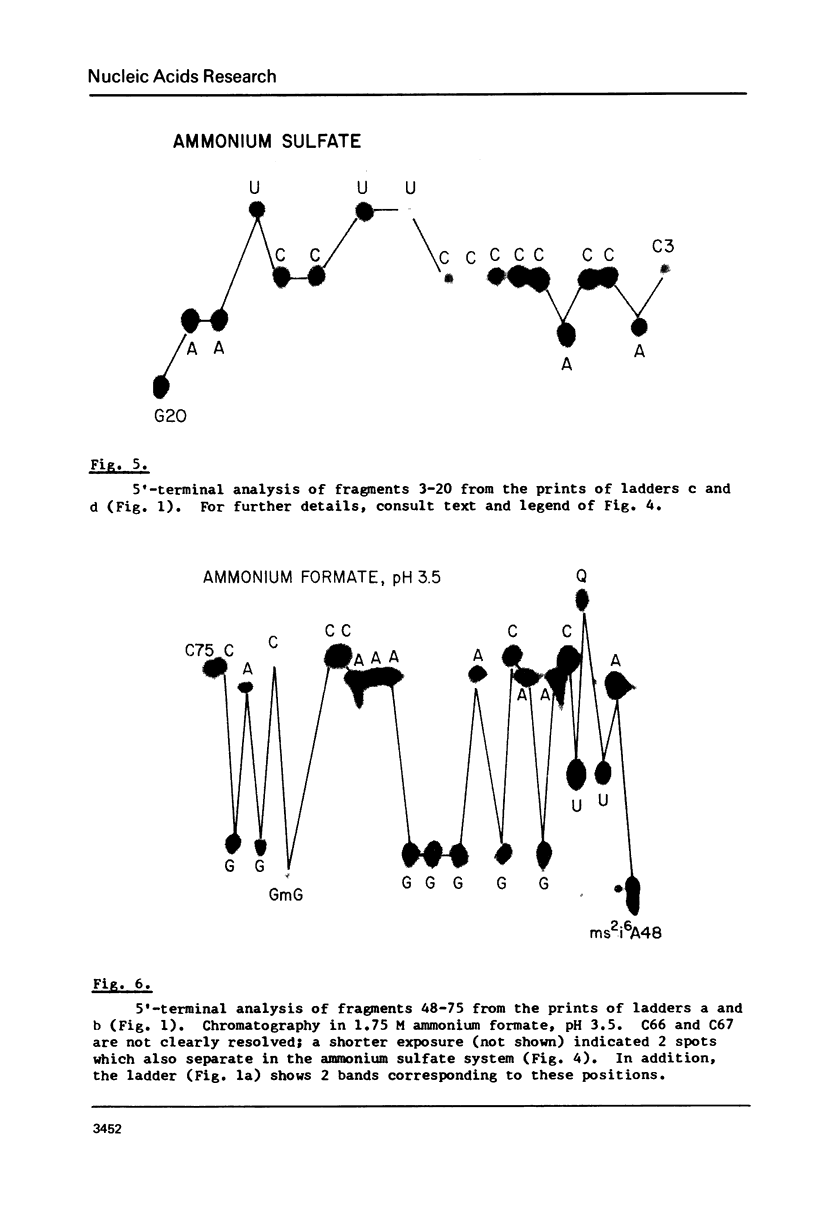
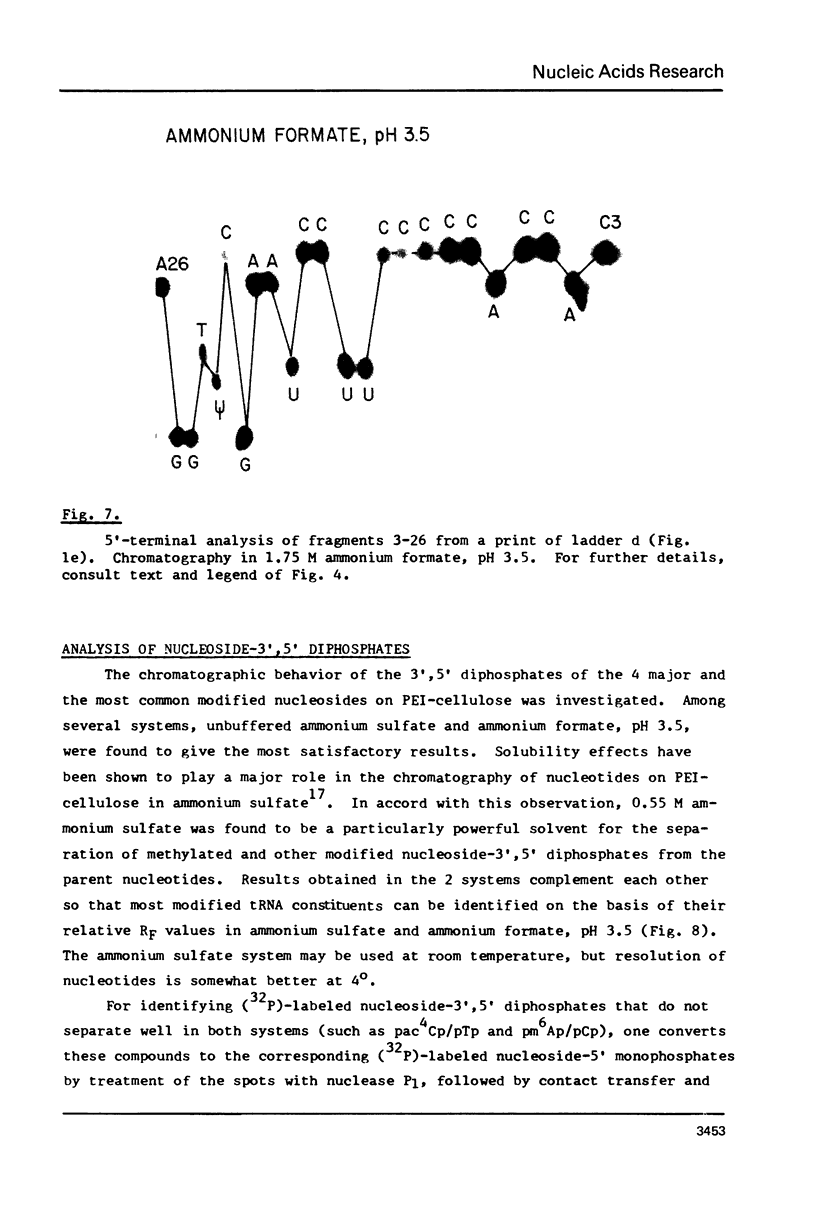
A rapid, simple, and highly sensitive method for sequence analysis of RNA was developed, which consists of the following steps: (i) controlled hydrolysis of the RNA by brief heating in water; (ii) (32P)-labeling of 5'-hydroxyl groups of the fragments produced in (i); (iii) resolution of labeled fragments by size on polyacrylamide gels giving the familiar "ladder"; (iv) contact transfer ("print") of the ladder from the gel to a PEI-cellulose thin layer; (v) in situ treatment of the ladder with RNase T2 resulting in the release of 5'-(32P)-labeled nucleoside-3',5' diphosphates; (vi) contact transfer and thin-layer separation of (32P)-labeled nucleotides on PEI-cellulose in ammonium sulfate and ammonium formate solvents; (vii) autoradiography. The chromatographic behavior of the 4 major and 18 modified nucleotides was determined. The positions of major and modified nucleotides in the sequence can be read directly from the separation patterns displayed on X-ray film. As this is the only sequencing method presently available that allows one to display and identify directly the positions in the RNA chain of major and modified nucleotides, no additional procedures are required to analyze the latter.
Full text
PDF





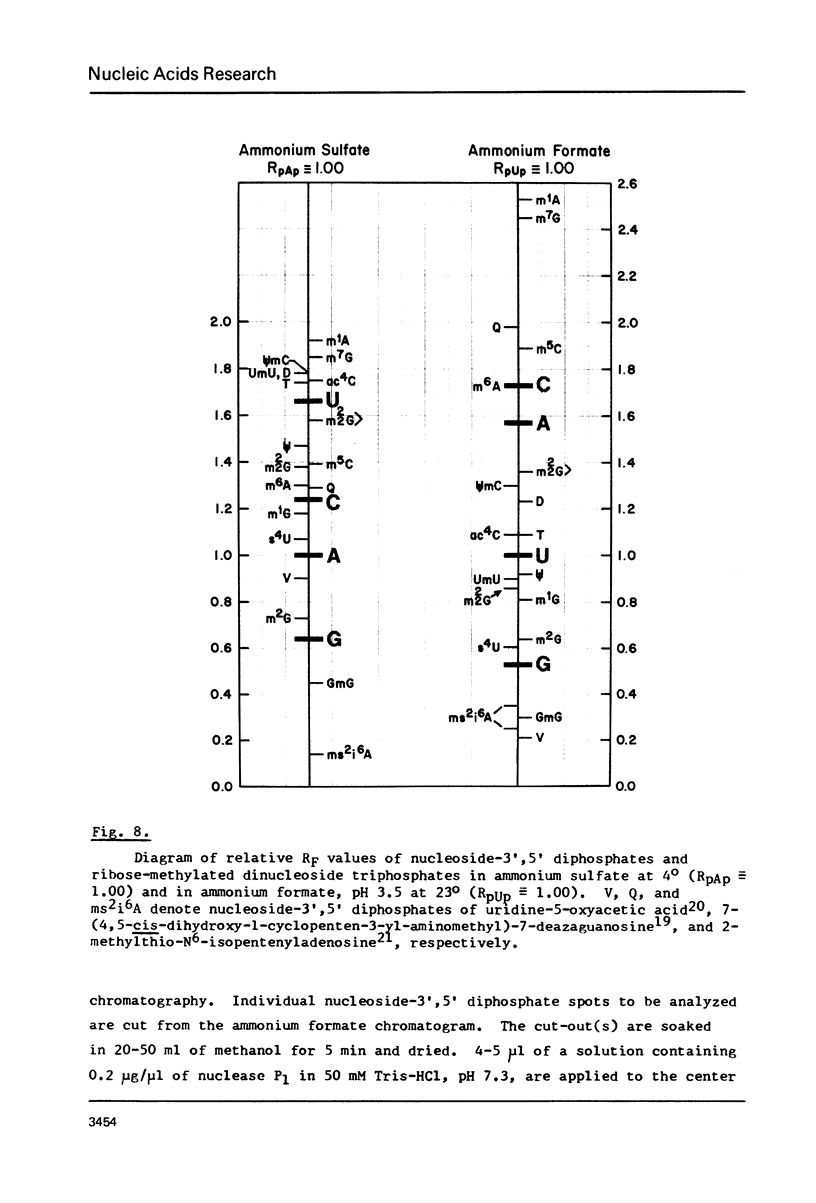




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang S. H., Kuo S., Hawkins E., Miller N. R. The corrected nucleotide sequence of yeast leucine transfer ribonucleic acid. Biochem Biophys Res Commun. 1973 Apr 16;51(4):951–955. doi: 10.1016/0006-291x(73)90019-3. [DOI] [PubMed] [Google Scholar]
- Chia L. L., Randerath K., Randerath E. Base analysis of ribopolynucleotides by tritium incorporation following analytical polyacrylamide gel electrophoresis. Anal Biochem. 1973 Sep;55(1):102–113. doi: 10.1016/0003-2697(73)90295-9. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman H. M., Abelson J. N., Landy A., Zadrazil S., Smith J. D. The nucleotide sequences of tyrosine transfer RNAs of Escherichia coli. Eur J Biochem. 1970 Apr;13(3):461–483. doi: 10.1111/j.1432-1033.1970.tb00950.x. [DOI] [PubMed] [Google Scholar]
- Gupta R. C., Randerath E., Randerath K. An improved separation procedure for nucleoside monophosphates on polyethyleneimine-(PEI-)cellulose thin layers. Nucleic Acids Res. 1976 Nov;3(11):2915–2921. doi: 10.1093/nar/3.11.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. C., Randerath K. Use of specific endonuclease cleavage in RNA sequencing-an enzymic method for distinguishing between cytidine and uridine residues. Nucleic Acids Res. 1977 Oct;4(10):3441–3454. doi: 10.1093/nar/4.10.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. C., Randerath K. Use of specific endonuclease cleavage in RNA sequencing. Nucleic Acids Res. 1977 Jun;4(6):1957–1978. doi: 10.1093/nar/4.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada F., Gross H. J., Kimura F., Chang S. H., Nishimura S., RajBhandary U. L. 2-Methylthio N6-(delta 2-isopentenyl) adenosine: a component of E. coli tyrosine transfer RNA. Biochem Biophys Res Commun. 1968 Oct 24;33(2):299–306. doi: 10.1016/0006-291x(68)90784-5. [DOI] [PubMed] [Google Scholar]
- Kasai H., Oashi Z., Harada F., Nishimura S., Oppenheimer N. J., Crain P. F., Liehr J. G., von Minden D. L., McCloskey J. A. Structure of the modified nucleoside Q isolated from Escherichia coli transfer ribonucleic acid. 7-(4,5-cis-Dihydroxy-1-cyclopenten-3-ylaminomethyl)-7-deazaguanosine. Biochemistry. 1975 Sep 23;14(19):4198–4208. doi: 10.1021/bi00690a008. [DOI] [PubMed] [Google Scholar]
- Lockard R. E., Alzner-Deweerd B., Heckman J. E., MacGee J., Tabor M. W., RajBhandary U. L. Sequence analysis of 5'[32P] labeled mRNA and tRNA using polyacrylamide gel electrophoresis. Nucleic Acids Res. 1978 Jan;5(1):37–56. doi: 10.1093/nar/5.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murao K., Saneyoshi M., Harada F., Nishimura S. Uridin-5-oxy acetic acid: a new minor constituent from E. coli valine transfer RNA I. Biochem Biophys Res Commun. 1970 Feb 20;38(4):657–662. doi: 10.1016/0006-291x(70)90631-5. [DOI] [PubMed] [Google Scholar]
- Randerath K., Chia L. S., Gupta R. C., Randerath E. Structural analysis of nonradioactive RNA by postlabeling: the primary structure of baker's yeast tRNA Leu/CUA. Biochem Biophys Res Commun. 1975 Mar 3;63(1):157–163. doi: 10.1016/s0006-291x(75)80024-6. [DOI] [PubMed] [Google Scholar]
- Randerath K., Randerath E., Chia L. S., Gupta R. C., Sivarajan M. Sequence analysis of nonradioactive RNA fragments by periodate-phosphatase digestion and chemical tritium labeling: characterization of large oligonucleotides and oligonucleotides containing modified nucleosides. Nucleic Acids Res. 1974 Sep;1(9):1121–1141. doi: 10.1093/nar/1.9.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randerath K. Separation of the constituent nucleotides of nucleic acids on ion-exchange thin-layers. Experientia. 1964 Jul 15;20(7):406–407. doi: 10.1007/BF02147995. [DOI] [PubMed] [Google Scholar]
- Roe B. A., Anandaraj M. P., Chia L. S., Randerath E., Gupta R. C., Randerath K. Sequence studies on tRNAPhe from placenta: comparison with known sequences of tRNAPhe from other normal mammalian tissues. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1097–1105. doi: 10.1016/0006-291x(75)90470-2. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Simoncsits A., Brownlee G. G., Brown R. S., Rubin J. R., Guilley H. New rapid gel sequencing method for RNA. Nature. 1977 Oct 27;269(5631):833–836. doi: 10.1038/269833a0. [DOI] [PubMed] [Google Scholar]
- Sivarajan M., Gupta R. C., Chia L. L., Randerath E., Randerath K. Tritium sequence analysis of oligoribonucleotides: a combination of post-labeling and thin-layer chromatographic techniques for the analysis of partial snake venom phosphodiesterase digests. Nucleic Acids Res. 1974 Oct;1(10):1329–1341. doi: 10.1093/nar/1.10.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley J., Vassilenko S. A different approach to RNA sequencing. Nature. 1978 Jul 6;274(5666):87–89. doi: 10.1038/274087a0. [DOI] [PubMed] [Google Scholar]
- Yaniv M., Barrell B. G. Nucleotide sequence of E. coli B tRNA1-Val. Nature. 1969 Apr 19;222(5190):278–279. doi: 10.1038/222278a0. [DOI] [PubMed] [Google Scholar]