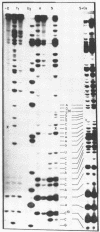
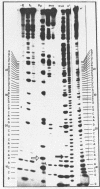
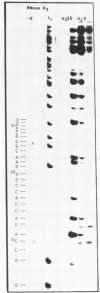
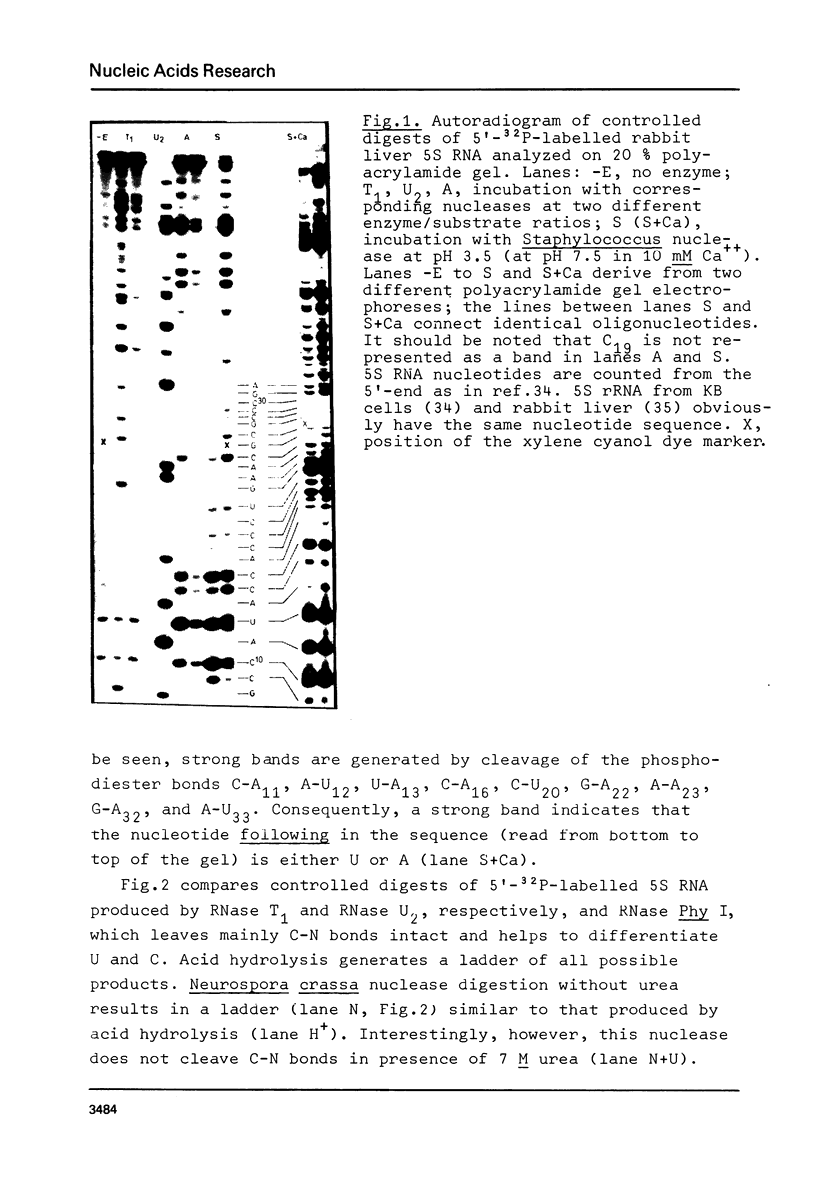
Abstract
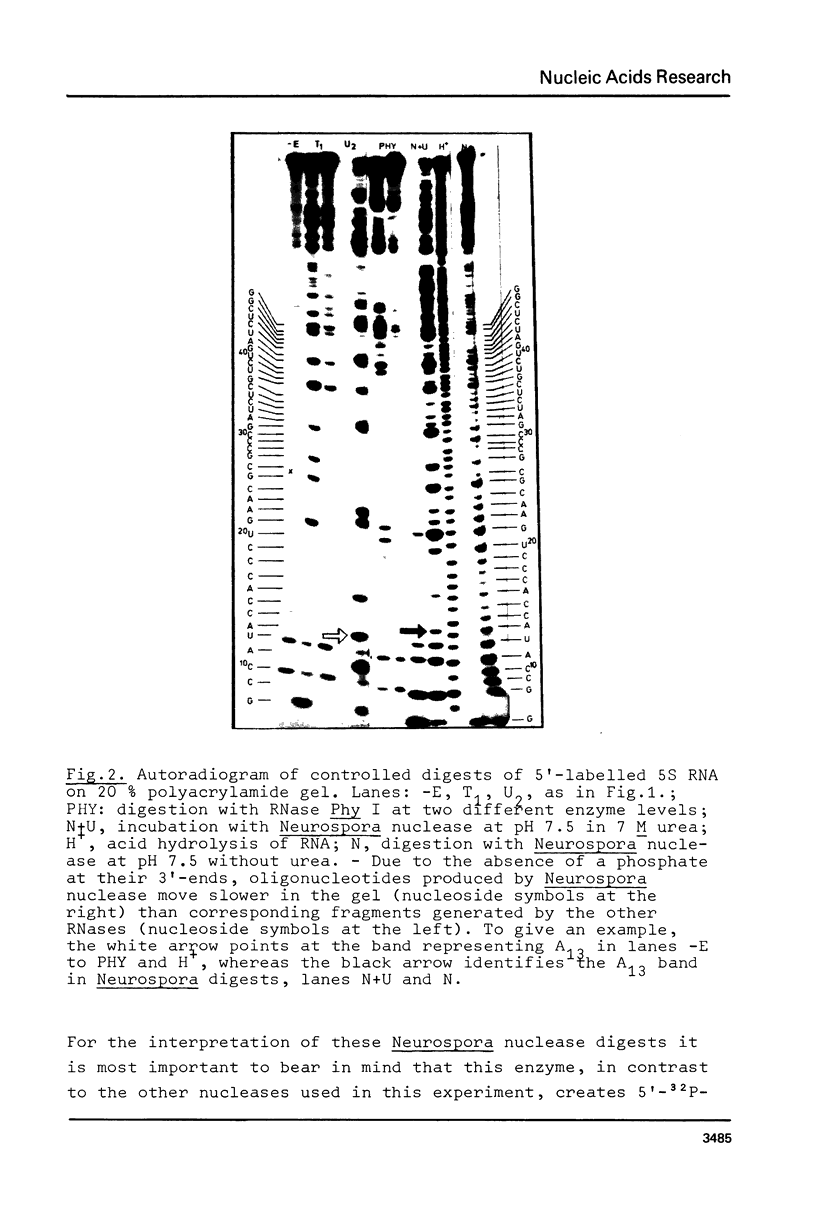
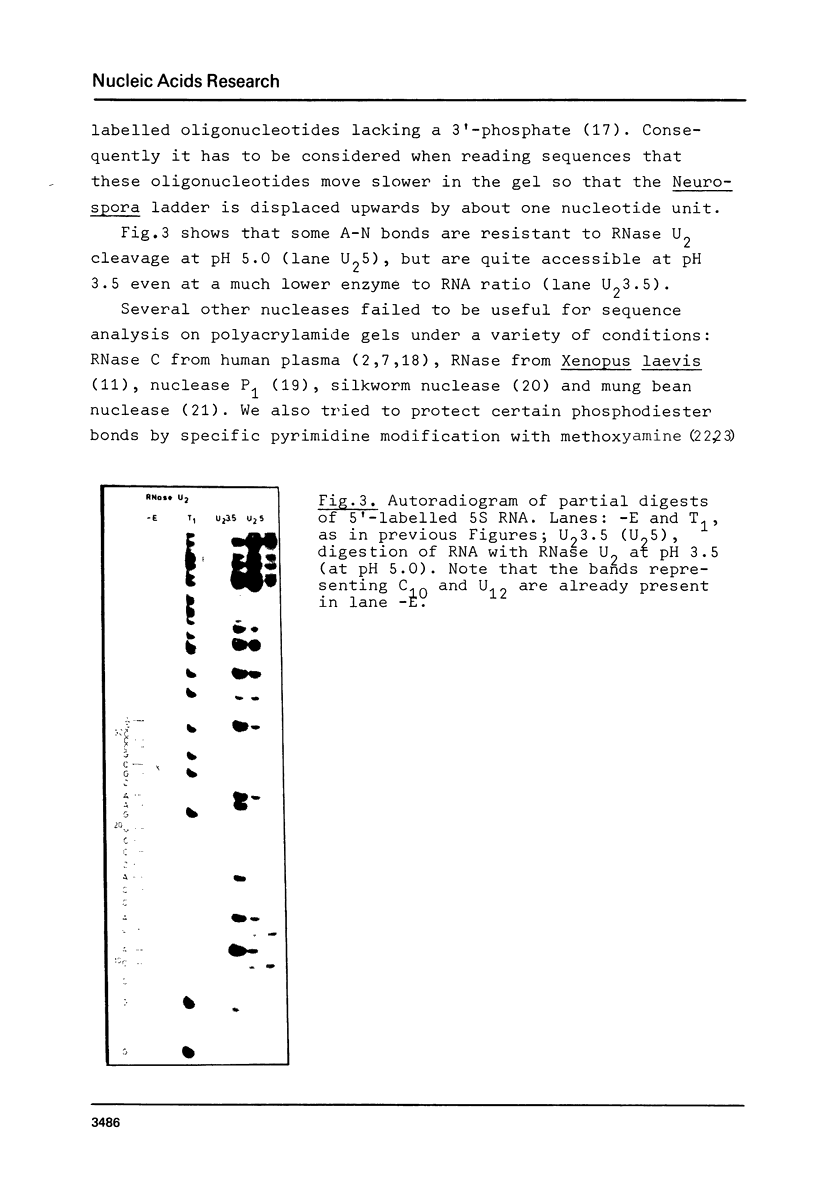
Using end-labelled RNA, significant changes in base specificity of three nucleases have been detected under defined conditions. Staphylococcus aureus nuclease at pH 3.5 without Ca++ cleaves all Pyr-N bonds more uniformly and efficiently than RNase A, without any preference for Pyr-A bonds. At pH 7.5 in 10 mM Ca++ this enzyme cleaves all N-C and N-G bonds slowly, whereas N-U and N-A bonds are hydrolyzed rapidly. Hence, the base at the 3'- or at the 5'-side of a phosphodiester bond can determine the base specificity of S. aureus nuclease. - In absence of urea, Neurospora crassa endonuclease cleaves all phosphodiester bonds, but leaves all C-N bonds intact in 7 M urea. - RNase U2 at pH 3.5 cleaves A-N bonds more efficiently than at pH 5.0.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. V., Loewensteiner D. D., Aronson A. I. Characterization of an endoribonuclease from Xenopus oocytes. Possible role in ribonucleic acid turnover. Biochemistry. 1974 Jun 4;13(12):2520–2527. doi: 10.1021/bi00709a007. [DOI] [PubMed] [Google Scholar]
- Carbon P., Ehresmann C., Ehresmann B., Ebel J. P. The sequence of Escherichia coli ribosomal 16 S RNA determined by new rapid gel methods. FEBS Lett. 1978 Oct 1;94(1):152–156. doi: 10.1016/0014-5793(78)80926-0. [DOI] [PubMed] [Google Scholar]
- Domdey H., Gross H. J. RNA sequence determination in the nanogram range by a combination of in vitro labeling procedures. Anal Biochem. 1979 Mar;93(2):321–328. doi: 10.1016/s0003-2697(79)80158-x. [DOI] [PubMed] [Google Scholar]
- Domdey H., Jank P., Sänger L., Gross H. J. Studies on the primary and secondary structure of potato spindle tuber viroid: products of digestion with ribonuclease A and ribonuclease T1, and modification with bisulfite. Nucleic Acids Res. 1978 Apr;5(4):1221–1236. doi: 10.1093/nar/5.4.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget B. G., Weissman S. M. The nucleotide sequence of ribosomal 5 S ribonucleic acid from KB cells. J Biol Chem. 1969 Jun 25;244(12):3148–3165. [PubMed] [Google Scholar]
- Gross H. J., Domdey H., Lossow C., Jank P., Raba M., Alberty H., Sänger H. L. Nucleotide sequence and secondary structure of potato spindle tuber viroid. Nature. 1978 May 18;273(5659):203–208. doi: 10.1038/273203a0. [DOI] [PubMed] [Google Scholar]
- Guilley H., Jonard G., Kukla B., Richards K. E. Sequence of 1000 nucleotides at the 3' end of tobacco mosaic virus RNA. Nucleic Acids Res. 1979 Apr;6(4):1287–1308. doi: 10.1093/nar/6.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. C., Randerath K. Use of specific endonuclease cleavage in RNA sequencing-an enzymic method for distinguishing between cytidine and uridine residues. Nucleic Acids Res. 1977 Oct;4(10):3441–3454. doi: 10.1093/nar/4.10.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. C., Randerath K. Use of specific endonuclease cleavage in RNA sequencing. Nucleic Acids Res. 1977 Jun;4(6):1957–1978. doi: 10.1093/nar/4.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht M. E., Busch H. Isolation of an electrophoretically homogeneous nonhistone nucleolar protein. Life Sci II. 1971 Nov 22;10(22):1297–1309. doi: 10.1016/0024-3205(71)90237-2. [DOI] [PubMed] [Google Scholar]
- Koper-Zwarthoff E. C., Lockard R. E., Alzner-deWeerd B., RajBhandary U. L., Bol J. F. Nucleotide sequence of 5' terminus of alfalfa mosaic virus RNA 4 leading into coat protein cistron. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5504–5508. doi: 10.1073/pnas.74.12.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINN S., LEHMAN I. R. AN ENDONUCLEASE FROM NEUROSPORA CRASSA SPECIFIC FOR POLYNUCLEOTIDES LACKING AN ORDERED STRUCTURE. II. STUDIES OF ENZYME SPECIFICITY. J Biol Chem. 1965 Mar;240:1294–1304. [PubMed] [Google Scholar]
- Labrie F., Sanger F. 32P-labellingof haemoglobin messenger and other reticulocyte ribonucleic acids with polynucleotide phosphokinase in iro. Biochem J. 1969 Sep;114(2):29P–29P. doi: 10.1042/bj1140029pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lockard R. E., Alzner-Deweerd B., Heckman J. E., MacGee J., Tabor M. W., RajBhandary U. L. Sequence analysis of 5'[32P] labeled mRNA and tRNA using polyacrylamide gel electrophoresis. Nucleic Acids Res. 1978 Jan;5(1):37–56. doi: 10.1093/nar/5.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. P., Sibler A. P., Schneller J. M., Keith G., Stahl A. J., Dirheimer G. Primary structure of yeast mitochondrial DNA-coded phenylalanine-tRNA. Nucleic Acids Res. 1978 Dec;5(12):4579–4592. doi: 10.1093/nar/5.12.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulski A. J., Laskowski M., Sr Mung bean nuclease I. 3. Purification procedure and (3') omega monophosphatase activity. J Biol Chem. 1970 Oct 10;245(19):5026–5031. [PubMed] [Google Scholar]
- Mukai J. I. An endonuclease from silkworm---purification and mode of action. Biochem Biophys Res Commun. 1965 Dec 21;21(6):562–567. doi: 10.1016/0006-291x(65)90522-x. [DOI] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilly D., Niemeyer A., Schmidt M., Bargetzi J. P. Enzymes for RNA sequence analysis. Preparation and specificity of exoplasmodial ribonucleases I and II from Physarum polycephalum. J Biol Chem. 1978 Jan 25;253(2):437–445. [PubMed] [Google Scholar]
- Ross A., Brimacombe R. Application of a rapid gel method to the sequencing of fragments of 16S ribosomal RNA from Escherichia coli. Nucleic Acids Res. 1978 Jan;5(1):241–256. doi: 10.1093/nar/5.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Schmukler M., Jewett P. B., Levy C. C. The effects of polyamines on a residue-specific human plasma ribonuclease. J Biol Chem. 1975 Mar 25;250(6):2206–2212. [PubMed] [Google Scholar]
- Silberklang M., Prochiantz A., Haenni A. L., Rajbhandary U. L. Studies on the sequence of the 3'-terminal region of turnip-yellow-mosaic-virus RNA. Eur J Biochem. 1977 Feb;72(3):465–478. doi: 10.1111/j.1432-1033.1977.tb11270.x. [DOI] [PubMed] [Google Scholar]
- Simoncsits A., Brownlee G. G., Brown R. S., Rubin J. R., Guilley H. New rapid gel sequencing method for RNA. Nature. 1977 Oct 27;269(5631):833–836. doi: 10.1038/269833a0. [DOI] [PubMed] [Google Scholar]
- Stanley J., Vassilenko S. A different approach to RNA sequencing. Nature. 1978 Jul 6;274(5666):87–89. doi: 10.1038/274087a0. [DOI] [PubMed] [Google Scholar]
- Tenenhouse H., Fraser M. J. The ribonuclease activities of the single-strand-specific nucleases of Neurospora crassa. Can J Biochem. 1973 May;51(5):569–580. doi: 10.1139/o73-071. [DOI] [PubMed] [Google Scholar]
- Verdlov E. D., Monastyrskaya G. S., Guskova L. I., Levitan T. L., Sheichenko V. I., Budowsky E. I. Modification of cytidine residues with a bisulfite-O-methylhydroxylamine mixture. Biochim Biophys Acta. 1974 Mar 8;340(2):153–165. [PubMed] [Google Scholar]
- Walker R. T., RajBhandary U. L. The nucleotide sequence of formylmethionine tRNA from Mycoplasma mycoides sp. capri. Nucleic Acids Res. 1978 Jan;5(1):57–70. doi: 10.1093/nar/5.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter G., Brownlee G. G. 3'End labelling of RNA with 32P suitable for rapid gel sequencing. Nucleic Acids Res. 1978 Sep;5(9):3129–3139. doi: 10.1093/nar/5.9.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]