Abstract
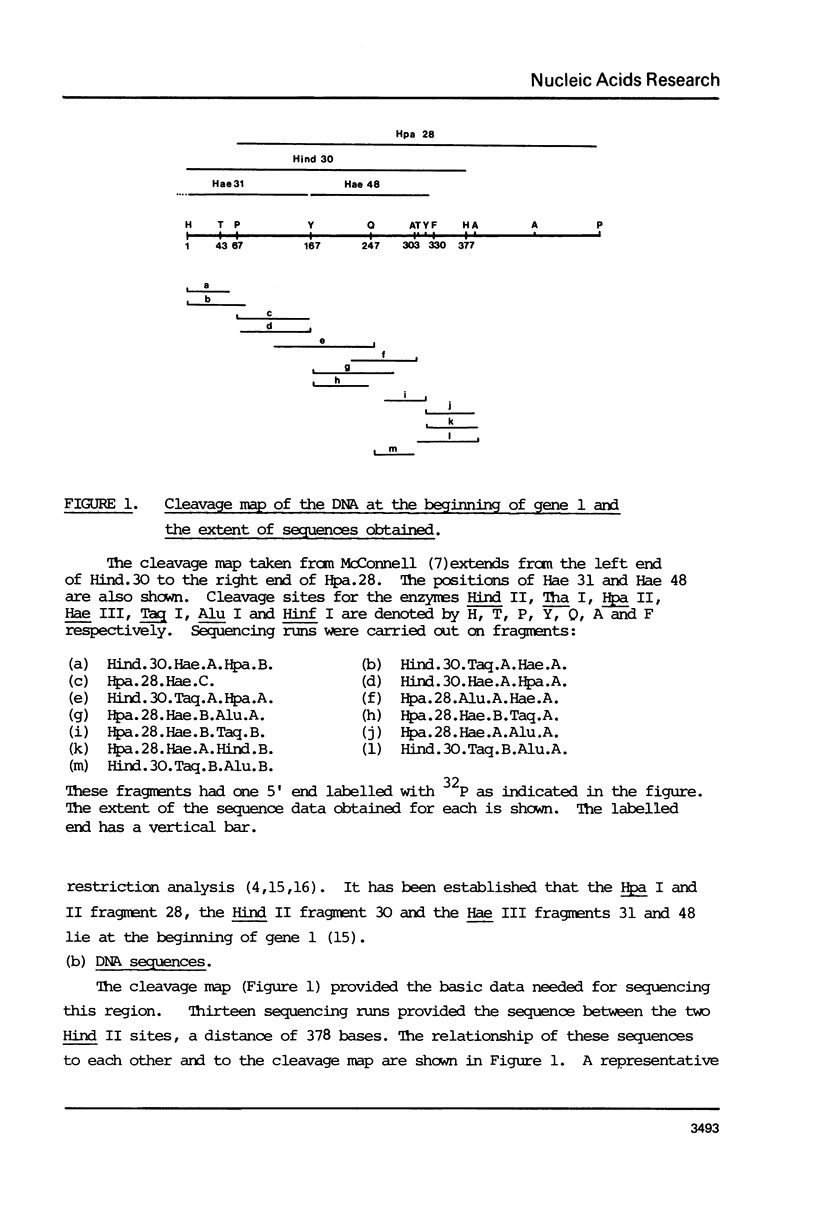
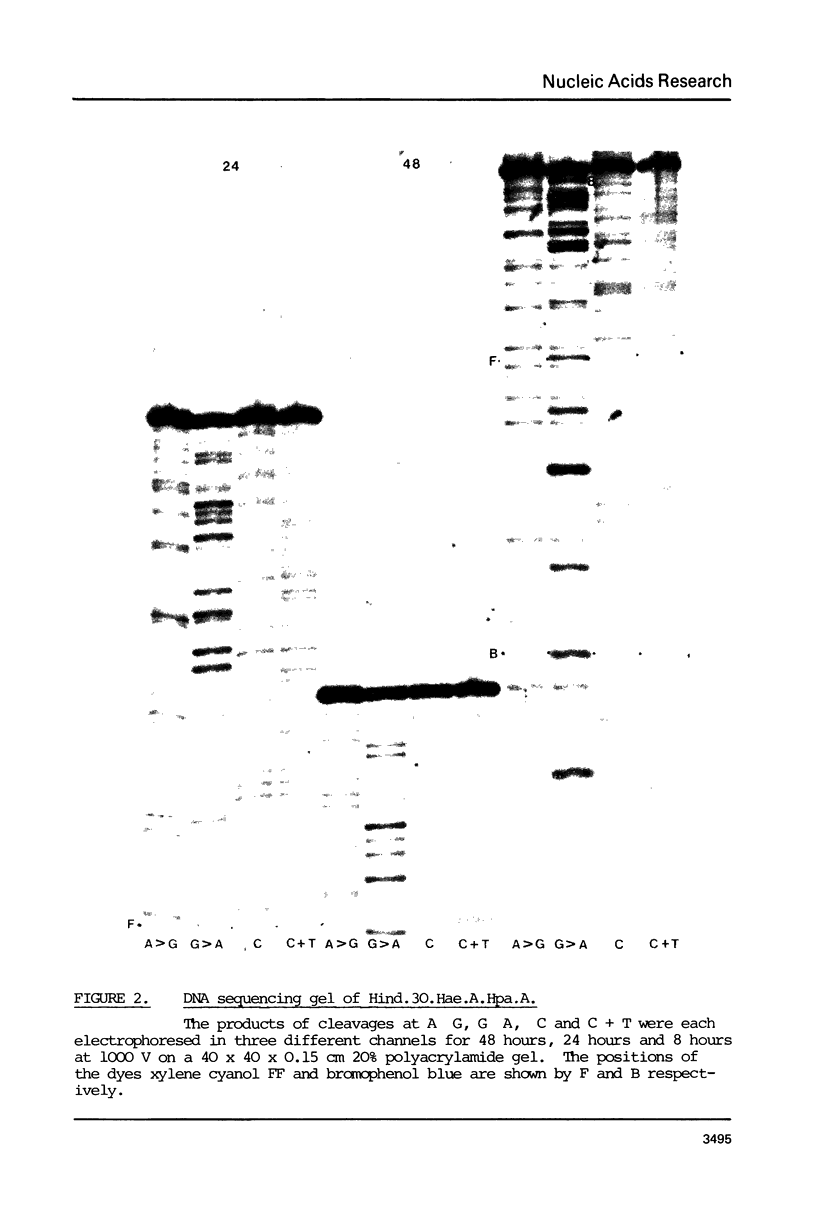
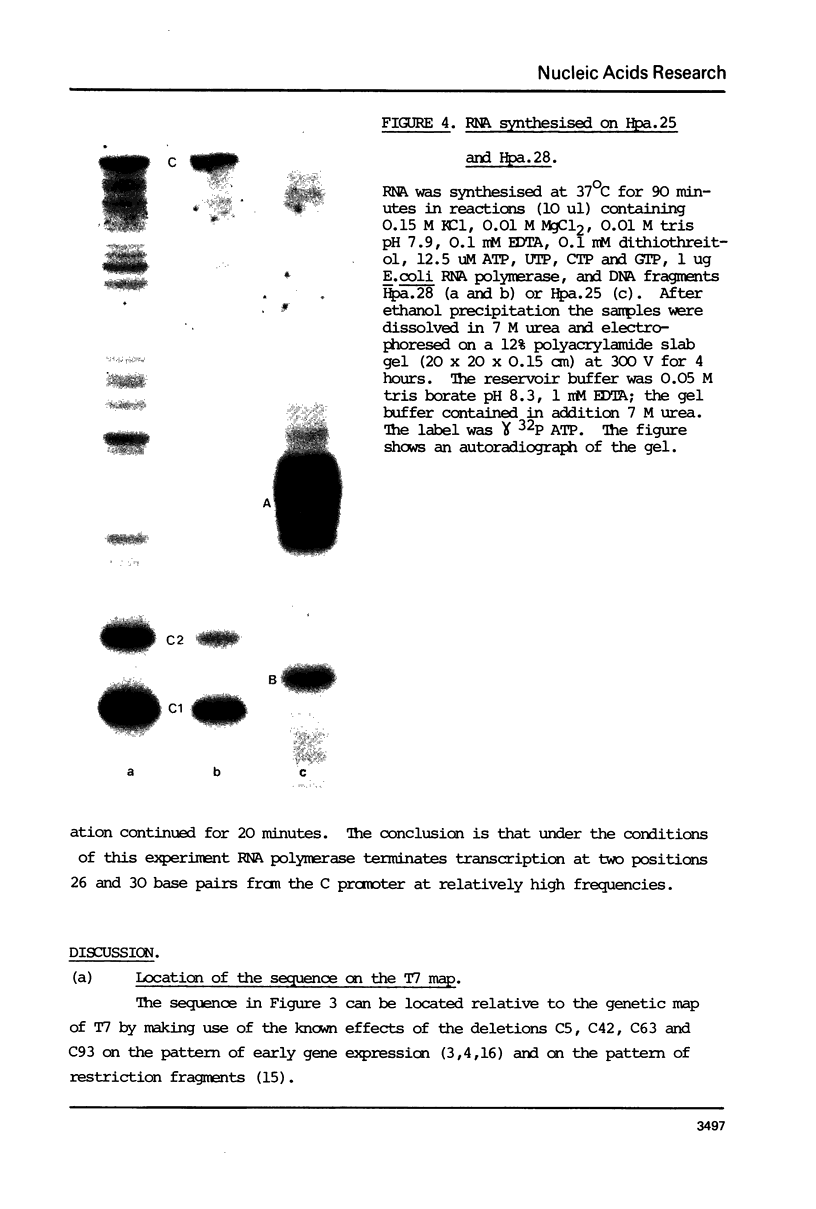
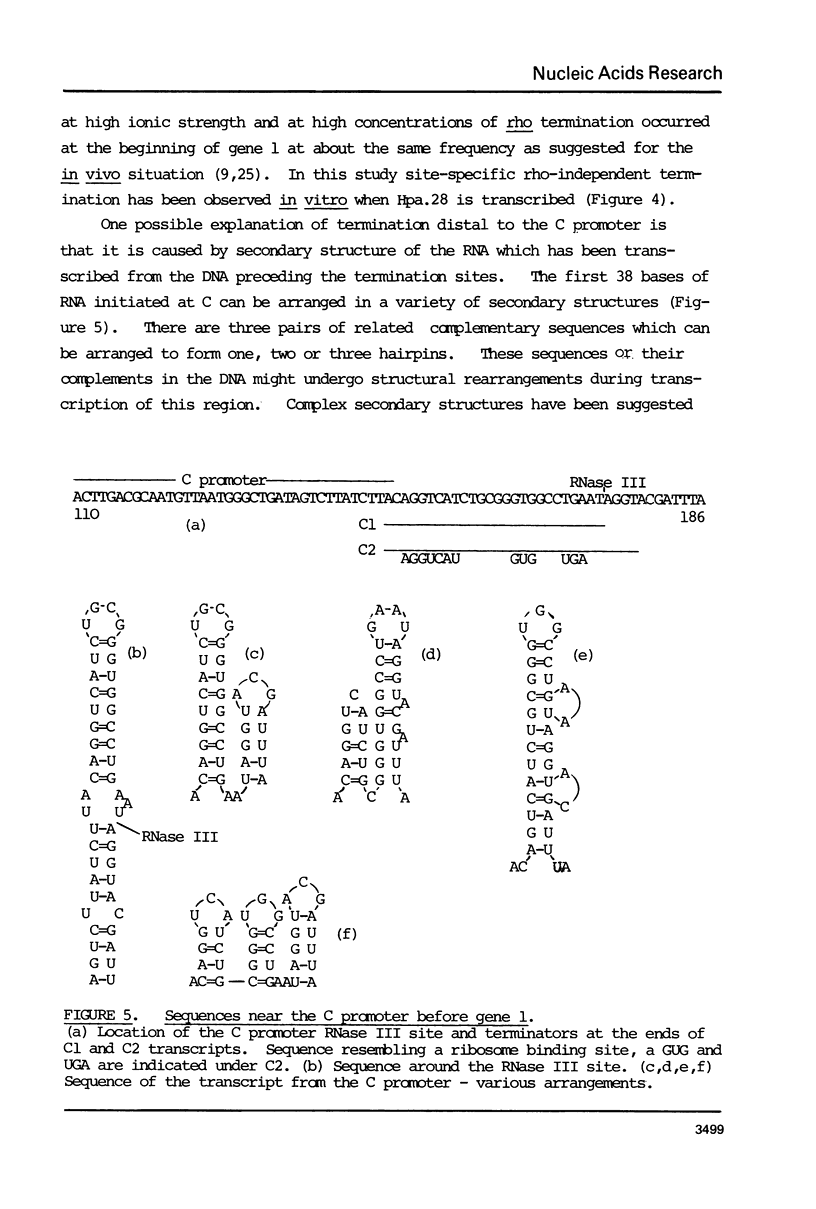
The DNA sequence of the fragment Hind.30, 378 bases long, from the beginning of gene 1 of T7 is presented. It contains the C promoter, two in vitro transcriptional terminator sites and a sequence of 171 bases which probably codes for the N terminus of the T7 RNA polymerase. The sequence also codes for the RNase III cleavage site before gene 1. The overlaps with the transcriptional terminators, The RNA transcript of the sequence about the terminators can be arranged in a set of alternative double-stranded hairpin structures. It is suggested that conversion between these structures may have a role in termination; this may be influenced by interactions with ribosomes and RNase III. The region of the C promoter between genes 0.7 and 1 thus contains several sites which may be involved in the control of transcription and translation.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Gottesman M. Control of transcription termination. Annu Rev Biochem. 1978;47:967–996. doi: 10.1146/annurev.bi.47.070178.004535. [DOI] [PubMed] [Google Scholar]
- Axelrod N. Transcription of bacteriophage phi-X174 in vitro: selective initiation with oligonucleotides. J Mol Biol. 1976 Dec 25;108(4):753–770. doi: 10.1016/s0022-2836(76)80115-5. [DOI] [PubMed] [Google Scholar]
- Barnes W. M. DNA sequence from the histidine operon control region: seven histidine codons in a row. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4281–4285. doi: 10.1073/pnas.75.9.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand K., Korn L., Lee F., Platt T., Squires C. L., Squires C., Yanofsky C. New features of the regulation of the tryptophan operon. Science. 1975 Jul 4;189(4196):22–26. doi: 10.1126/science.1094538. [DOI] [PubMed] [Google Scholar]
- Bordier C. Inhibition of rifampicin-resistant RNA synthesis by rifampicin-RNA polymerase complexes. FEBS Lett. 1974 Sep 1;45(1):259–262. doi: 10.1016/0014-5793(74)80857-4. [DOI] [PubMed] [Google Scholar]
- Chamberlin M., McGrath J., Waskell L. New RNA polymerase from Escherichia coli infected with bacteriophage T7. Nature. 1970 Oct 17;228(5268):227–231. doi: 10.1038/228227a0. [DOI] [PubMed] [Google Scholar]
- Darlix J. L., Horaist M. Existence and possible roles of transcriptional barriers in T7 DNA early region as shown by electron microscopy. Nature. 1975 Jul 24;256(5515):288–292. doi: 10.1038/256288a0. [DOI] [PubMed] [Google Scholar]
- Darlix J. L. Rho, a factor causing the modulation of early T7 genes transcription. Biochimie. 1974;56(5):693–701. doi: 10.1016/s0300-9084(74)80040-4. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. T7 early RNAs are generated by site-specific cleavages. Proc Natl Acad Sci U S A. 1973 May;70(5):1559–1563. doi: 10.1073/pnas.70.5.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon R. L., Humphries P., McConnell D. J. Restriction enzyme cleavage mapping of T7 virus early region. Mol Gen Genet. 1978 Jul 4;162(3):329–339. doi: 10.1007/BF00268859. [DOI] [PubMed] [Google Scholar]
- Hausmann R. Bacteriophage T7 genetics. Curr Top Microbiol Immunol. 1976;75:77–110. doi: 10.1007/978-3-642-66530-1_3. [DOI] [PubMed] [Google Scholar]
- Hayward DNA blockade by rifampicin-inactivated Escherichia coli RNA polymerase, and its amelioration by a specific mutation. Eur J Biochem. 1976 Dec;71(1):19–24. doi: 10.1111/j.1432-1033.1976.tb11084.x. [DOI] [PubMed] [Google Scholar]
- Hercules K., Jovanovich S., Sauerbrier W. Early gene expression in bacteriophage T7. I. In vivo synthesis, inactivation, and translational utilization of early mRNA's. J Virol. 1976 Feb;17(2):642–658. doi: 10.1128/jvi.17.2.642-658.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh T., Wang J. C. Physicochomecial studies on interactions between DNA and RNA polymerase. Isolation and mapping of a T7 DNA fragment containing the early promoters for Escherichia coli RNA polymerase. Biochemistry. 1976 Dec 28;15(26):5776–5783. doi: 10.1021/bi00671a014. [DOI] [PubMed] [Google Scholar]
- Humphries P., Gordon R. L., McConnell D. J., Connolly P. Endonuclease R. Hind fragments of T7 DNA. Virology. 1974 Mar;58(1):25–31. doi: 10.1016/0042-6822(74)90138-x. [DOI] [PubMed] [Google Scholar]
- Kramer R. A., Rosenberg M., Steitz J. A. Nucleotide sequences of the 5' and 3' termini of bacteriophage T7 early messenger RNAs synthesized in vivo: evidence for sequence specificity in RNA processing. J Mol Biol. 1974 Nov 15;89(4):767–776. doi: 10.1016/0022-2836(74)90051-5. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell D. J., Bonner J. Preparation of highly purified ribonucleic acid polymerase; separation from polynucleotide phosphorylase and polyphosphate kinase. Biochemistry. 1972 Nov 7;11(23):4329–4336. doi: 10.1021/bi00773a020. [DOI] [PubMed] [Google Scholar]
- McConnell D. J., Searcy D. G., Sutcliffe J. G. A restriction enzyme Tha I from the thermophilic mycoplasma Thermoplasma acidophilum. Nucleic Acids Res. 1978 Jun;5(6):1729–1739. doi: 10.1093/nar/5.6.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell D. J. The DNA sequence at the T7 C promoter. Nucleic Acids Res. 1979 Feb;6(2):525–544. doi: 10.1093/nar/6.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkley E. G., Jr Transcription of the early region of bacteriophage T7: specificity and selectivity in the in vitro initiation of RNA synthesis. J Mol Biol. 1974 Mar;83(3):305–331. doi: 10.1016/0022-2836(74)90282-4. [DOI] [PubMed] [Google Scholar]
- Minkley E. G., Pribnow D. Transcription of the early region of bacteriophage T7: selective initiation with dinucleotides. J Mol Biol. 1973 Jun 25;77(2):255–277. doi: 10.1016/0022-2836(73)90335-5. [DOI] [PubMed] [Google Scholar]
- Nierlich D. P. Regulation of bacterial growth, RNA, and protein synthesis. Annu Rev Microbiol. 1978;32:393–432. doi: 10.1146/annurev.mi.32.100178.002141. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D., Shimatake H., Brady C., Wulff D. L. The relationship between function and DNA sequence in an intercistronic regulatory region in phage lambda. Nature. 1978 Mar 30;272(5652):414–423. doi: 10.1038/272414a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Kramer R. A. Nucleotide sequence surrounding a ribonuclease III processing site in bacteriophage T7 RNA. Proc Natl Acad Sci U S A. 1977 Mar;74(3):984–988. doi: 10.1073/pnas.74.3.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U. Nucleotide sequence of the three major early promoters of bacteriophage T7. Nucleic Acids Res. 1979;6(5):1895–1907. doi: 10.1093/nar/6.5.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. N., Studier F. W. Physical mapping of the early region of bacteriophage T7 DNA. J Mol Biol. 1973 Sep 15;79(2):249–265. doi: 10.1016/0022-2836(73)90004-1. [DOI] [PubMed] [Google Scholar]
- Stahl S. J., Chamberlin M. J. An expanded transcriptional map of T7 bacteriophage. Reading of minor T7 promoter sites in vitro by Escherichia coli RNA polymerase. J Mol Biol. 1977 Jun 5;112(4):577–601. doi: 10.1016/s0022-2836(77)80165-4. [DOI] [PubMed] [Google Scholar]
- Stauffer G. V., Zurawski G., Yanofsky C. Single base-pair alterations in the Escherichia coli trp operon leader region that relieve transcription termination at the trp attenuator. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4833–4837. doi: 10.1073/pnas.75.10.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Gene 0.3 of bacteriophage T7 acts to overcome the DNA restriction system of the host. J Mol Biol. 1975 May 15;94(2):283–295. doi: 10.1016/0022-2836(75)90083-2. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Genetic analysis of non-essential bacteriophage T7 genes. J Mol Biol. 1973 Sep 15;79(2):227–236. doi: 10.1016/0022-2836(73)90002-8. [DOI] [PubMed] [Google Scholar]
- Zurawski G., Brown K., Killingly D., Yanofsky C. Nucleotide sequence of the leader region of the phenylalanine operon of Escherichia coli. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4271–4275. doi: 10.1073/pnas.75.9.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Elseviers D., Stauffer G. V., Yanofsky C. Translational control of transcription termination at the attenuator of the Escherichia coli tryptophan operon. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5988–5992. doi: 10.1073/pnas.75.12.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]