Abstract
OBJECTIVE
Branched-chain amino acids, such as leucine and glucose, stimulate protein synthesis and increase the phosphorylation and activity of the mammalian target of rapamycin (mTOR) and its downstream target p70S6 kinase (p70S6K). We examined in skeletal muscle whether the effects of leucine and glucose on these parameters and on insulin resistance are mediated by the fuel-sensing enzyme AMP-activated protein kinase (AMPK).
RESEARCH DESIGN AND METHODS
Rat extensor digitorum longus (EDL) muscle was incubated with different concentrations of leucine and glucose with or without AMPK activators. Muscle obtained from glucose-infused rats was also used as a model.
RESULTS
In the EDL, incubation with 100 or 200 μmol/l leucine versus no added leucine suppressed the activity of the α2 isoform of AMPK by 50 and 70%, respectively, and caused concentration-dependent increases in protein synthesis and mTOR and p70S6K phosphorylation. Very similar changes were observed in EDL incubated with 5.5 or 25 mmol/l versus no added glucose and in muscle of rats infused with glucose in vivo. Incubation of the EDL with the higher concentrations of both leucine and glucose also caused insulin resistance, reflected by a decrease in insulin-stimulated Akt phosphorylation. Coincubation with the AMPK activators AICAR and α-lipoic acid substantially prevented all of those changes and increased the phosphorylation of specific sites of mTOR inhibitors raptor and tuberous sclerosis complex 2 (TSC2). In contrast, decreases in AMPK activity induced by leucine and glucose were not associated with a decrease in raptor or TSC2 phosphorylation.
CONCLUSIONS
The results indicate that both leucine and glucose modulate protein synthesis and mTOR/p70S6 and insulin signaling in skeletal muscle by a common mechanism. They also suggest that the effects of both molecules are associated with a decrease in AMPK activity and that AMPK activation prevents them.
AMP-activated protein kinase (AMPK) is a fuel-sensing enzyme that has classically been defined in terms of its role in restoring ATP levels in energy-depleted cells. In skeletal muscle, AMPK is typically activated by such factors as glucose deprivation and contraction (exercise) (1,2). The activated AMPK in turn enhances processes that generate ATP, such as fatty acid oxidation and glucose transport, and downregulates others that consume ATP and can be diminished temporarily without jeopardizing the cell (e.g., protein and lipid synthesis). Much less studied is the notion that a decrease in AMPK below baseline values may also be a physiologically or pathophysiologically relevant event. In keeping with such a possibility, decreased AMPK activity has been observed in tissues of many obese insulin-resistant rodents (3) and in liver (4,5) and adipose tissue (6) of rats starved for 48 h when they are refed. One consequence of decreased AMPK activity could be increases in mammalian target of rapamycin (mTOR)/p70S6 kinase (p70S6K) signaling and protein synthesis because both are decreased by AMPK activation (7).
In the present study, we assessed whether fuel-induced increases in protein synthesis, mTOR/p70S6K signaling, and insulin resistance in skeletal muscle are mediated by decreases in AMPK activity. Toward this end, rat extensor digitorum longus (EDL) muscles were incubated for different time periods with various concentrations of leucine or glucose and the above parameters were assessed. The results indicate that elevated concentrations of leucine and glucose decrease AMPK activity, increase protein synthesis and mTOR/p70S6 phosphorylation, and cause insulin resistance and that activation of AMPK by pharmacological agents prevents these events from occurring. Finally, the data suggest that the decrease in AMPK activity caused by both leucine and glucose is not mediated by changes in the AMP-to-ATP ratio but is associated with an increase in the lactate/pyruvate ratio.
RESEARCH DESIGN AND METHODS
5-Aminoimidazole-4-carboxamide ribonucleoside (AICAR) was purchased from Toronto Research Chemicals (Toronto, ON, Canada). Compound C, a selective AMPK inhibitor, was purchased from Calbiochem (San Diego, CA). [γ-32P] ATP was purchased from NEN (Boston, MA) and protein A/G plus conjugate from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies for phosphorylated AMPK (P-AMPK) (Thr 172), total AMPK, phosphorylated mTOR (P-mTOR) (Ser 2448), phosphorylated p70S6K (P-p70S6K) (Thr 389), and phosphorylated 4EBP (P-4EBP) (Thr 70) were obtained from Cell Signaling (Danvers, MA) and for phosphorylated acetyl CoA carboxylase (P-ACC) (Ser 79) from Upstate Biotechnologies (Charlottesville, VA). Phosphorylated tuberous sclerosis complex 2 (TSC2) (Ser 1387) was purchased from LifeSpan Biosciences (Seattle, WA). Total and phosphorylated raptor (Ser 792), total TSC2, and rabbit polyclonal anti-SIRT1 (Silent Information Regulator T1) (H-300) were from Santa Cruz Biotechnology. SAMS peptide and polyclonal antibodies that immunoprecipitate the α1 and α2 catalytic subunit of AMPK were obtained from QCB Biotechnology (Hopkinton, MA). All other chemicals were purchased from either Sigma-Aldrich or Fisher.
Protocols for animal use were reviewed and approved by the Institutional Animal Care and Use Committee of Boston University Medical Center and were in accordance with National Institutes of Health guidelines. Male Sprague-Dawley rats weighing 55–65 g were purchased from Charles River Breeding Laboratories (Wilmington, MA). They were maintained on a 12:12-h light-dark cycle in a temperature-controlled (19–21°C) room and were fed standard Purina rat chow and water ad libitum. Food was withdrawn 16–20 h before the initiation of experimental protocols. Muscles were removed from rats anesthetized with pentobarbital (6 mg/100 g body wt).
Muscle incubation.
After removal from the rat EDL muscles, the samples were first equilibrated for 20 min at 37°C in oxygenated Krebs-Henseleit solution (95% O2/5% CO2) containing 5.5 mmol/l glucose (8). They were then incubated in media containing 0, 5.5, or 25 mmol/l glucose and different concentations of leucine (0–200 μmol/l) for varying time periods as indicated in the Fig. legends. (Physiological concentration of leucine is usually 70–120 μmol/l) [9,10].) At the end of the incubation, muscles were blotted, quick-frozen in liquid nitrogen, and stored at −80°C until they were used for analyses.
AMPK activity assay.
AMPK activity was measured in the EDL as described previously (8,11). In brief, frozen muscle was homogenized (20 mmol/l Tris, pH 7.4, 5 mmol/l EDTA, 10 mmol/l Na4P2O7, 100 mmol/l NaF, 2 mmol/l Na3VO4, 1% NP-40, 1 mmol/l phenylmethanesulfonyl fluoride (PMSF), 0.006 mg/ml aprotinin, and 0.006 mg/ml leupeptin), and the muscle lysate containing 200-μg protein was immunoprecipitated with specific antibodies to the α-2 or α-1 catalytic subunit of AMPK (11) and protein A/G agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA). Beads were washed five times, and the immobilized enzyme was assayed based on the phosphorylation of SAMS peptide (0.2 mmol/l) by 0.2 mmol/l ATP (containing 2 μCi [γ-32P] ATP) in the presence and absence of 0.2 mmol/l AMP. Label incorporation into the SAMS peptide was measured on a Racbeta 1214 scintillation counter.
Western blot analysis.
Protein homogenates (50 μg) were run on a SDS polyacrylamide gel (4–15% gradient; BioRad) and transferred onto a polyvinylidene fluoride membrane (Bio-Rad). Membranes were then stained with Ponceau S (1% in 5% acetic acid) to ensure even transfer and blocked in Tris-buffered saline (pH 7.5) containing 0.05% Tween-20 and 5% milk for 1 h at room temperature. After this, they were incubated overnight in primary antibodies (P-AMPK, P-ACC, total AMPK, P-mTOR, P-p70S6K, P-raptor, and P-TSC2) at a 1:1,000 dilution. They were then incubated with a secondary antibody conjugated to horseradish peroxidase (Amersham) at a 1:5,000 dilution and subjected to an enhanced chemiluminescence solution (Pierce). Densitometry was performed using Scion Image software (8).
Assessment of protein synthesis.
EDL were initially equilibrated in Krebs-Henseleit solution containing 5.5 mmol/l glucose for 20 min. They were then incubated for 1 h in fresh medium containing 2 mCi/ml of 14C-phenylalanine, unlabeled phenylalanine at a final concentration of 100 μmol/l/ml, and the indicated concentrations of glucose and leucine. At the end of the incubation, muscles were blotted and homogenized in 10% tricarboxlylic acid (TCA). Samples were centrifuged at 10,000g for 10 min at 4°C, and TCA-insoluble material was washed three times with 10% TCA. The resultant pellet was solubilized in 0.1 N NaOH at 37°C for 2 h and used for determination of protein abundance and phenylalanine incorporated into muscle protein (7). Protein mass was determined by the bicinchoninic acid procedure (see below) and protein-associated radioactivity by liquid scintillation counting. Protein synthesis was calculated by dividing the protein-bound radioactivity by the specific activity of free leucine in the incubation medium. The results are expressed as nanomoles of leucine incorporated per milligram protein per hour.
Glucose infusion.
Glucose infusion was carried out as described previously (12). Briefly, 7 days after cannulation surgery, rats were randomly divided into treatment groups. After a basal blood sample (600 μl) was taken, a 50% (wt/vol) glucose solution was infused for either 0 or 5 h using a peristaltic roller pump (101U/R; Watson-Marlow, Falmouth, U.K.). Blood samples were taken every 30 min, and the glucose infusion rate was altered to maintain a blood glucose concentration of 11 mmol/l (∼16–17 mmol/l plasma glucose).
Other analyses.
Protein concentrations were determined with the bicinchoninic acid reagent (Pierce, Rockford, IL) using bovine serum albumin (BSA) as the standard. ATP, AMP, ADP, and phosphocreatine were measured spectrophotometrically as previously described (8). Malonyl CoA was determined radioenzymatically using the method of McGarry et al. (13), though slightly modified. Lactate and pyruvate was determined spectrophotometrically using lactate dehydrogenase and NAD (8,14).
Statistics.
Results are expressed as means ± SEM. Statistical differences between two groups were determined by Student t test where multiple groups were compared by ANOVA followed by Student-Newman-Keuls post hoc analysis. Differences between groups were considered statistically significant at P < 0.05.
RESULTS
Incubation with leucine increases protein synthesis and the phosphorylation of mTOR, p70S6K, and 4EBP1.
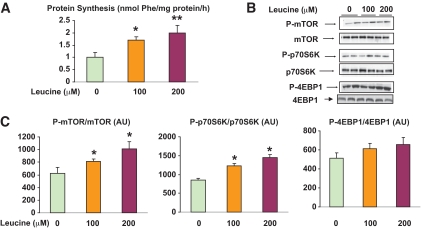
In keeping with previous reports in muscle (15) and pancreatic β-cells (16), incubation of the EDL with leucine (100 and 200 μmol/l) for 1 h significantly increased protein synthesis (Fig. 1A), an effect associated temporally with increases in the phosphorylation of mTOR and p70S6K but not another mTOR target, 4EBP1 (Fig. 1B and C). Twenty percent increases in both mTOR and p70S6K phosphorylation were found in the presence of 100 μmol/l isoleucine; however, neither achieved statistical significance (Fig. S1, available in an online appendix [http://diabetes.diabetesjournals.org/cgi/content/full/db09-1870/DC1]).
FIG. 1.
Effect of incubation with different concentrations of leucine on protein synthesis and P-mTOR, P-p70S6K, and P-4EBP1 phosphorylation. EDL muscles were incubated in Krebs-Henseleit solution containing 0, 100, and 200 μmol/l leucine for 1 h. Protein synthesis was determined at the end of the incubation on the basis of phenylalanine incorporation into protein (A) and the phosphorylation of mTOR (C, left panel), p70S6K (C, center panel), and 4EBP1 (C, right panel) by immunoblot analysis. Representative Western blots (B) and densitometric analysis (C). Results are means ± SE (n = 6). *P < 0.05 relative to no leucine or time 0. (A high-quality color representation of this figure is available in the online issue.)
Leucine concurrently diminishes AMPK phosphorylation and activity.
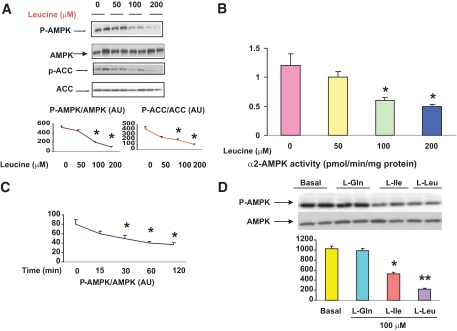
EDL incubated with leucine for 1 h also demonstrated a significant decrease in the phosphorylation of AMPK at Thr172. As shown in Fig. 2A, a progressive decrease in the abundance of both P-AMPK and its downstream target acetyl CoA carboxylase (ACC) occurred as the concentration of leucine in the medium was increased from 0 to 50, 100, and 200 μmol/l. An almost identical pattern was observed when the activity of the α2 isoform of AMPK was measured (Fig. 2B). In contrast, the activity of α1 AMPK was unchanged (data not shown). Time course studies revealed that 100 μmol/l leucine decreased AMPK phosphorylation by 20% at 15 min, 40% by 30 min, and 80% after 2 h (Fig. 2C). Incubation with 100 μmol/l isoleucine, another branched chain amino acid, also decreased AMPK activity, although not to the same extent as leucine. In contrast, incubation with 100 μmol/l glutamine had no effect (Fig. 2D).
FIG. 2.
Dose response and time course of the effects of leucine on AMPK phosphorylation and activity. EDL muscles were incubated in the absence (0) or presence (50, 100, or 200 μmol/l) of leucine for 1 h and muscle lysates analyzed for the phosphorylation of AMPK and its downstream target P-ACC using SDS-PAGE (A) and for α2-AMPK activity (B). C: The time course (15–120 min) of changes in the abundance of P-AMPK in muscles incubated with 100 μmol/l leucine. D: Comparison of effects of leucine, isoleucine, and glutamine (100 μmol/l) on total AMPK and P-AMPK. Results are means ± SE (n = 6). *P < 0.05 relative to 0 μmol/l leucine or time 0. (A high-quality color representation of this figure is available in the online issue.)
Incubation with glucose mimics the effects of leucine on protein synthesis, mTOR/p70S6K phosphorylation, and AMPK phosphorylation and activity.
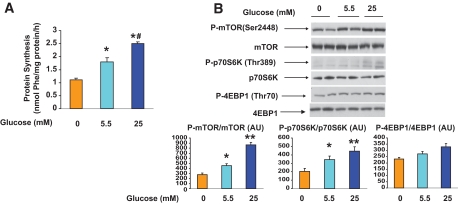
Incubation with glucose had effects very similar to those of leucine. As shown in Fig. 3A, protein synthesis increased by more than twofold when the medium concentration of glucose was increased from 5.5 to 25 mmol/l, as did the phosphorylation of mTOR and p70S6K. Conversely, eliminating glucose from the medium decreased all of these parameters (Fig. 3B). As with leucine, no change in the phosphorylation of 4EBP1 was observed.
FIG. 3.
Effect of incubation with different concentrations of glucose on protein synthesis (A), mTOR (B, lower left panel), p70S6K (B, lower center panel), and 4EBP1 (B, lower right panel) phosphorylation. EDL muscles were incubated for 1 h in media containing 0, 5.5, or 25 mmol/l glucose. B: Representative Western blots for P-mTOR, P-p70S6K, and P-4EBP1 (top panel). Quantification of Western blots (bottom panel). Results are means ± SE (n = 4). *P < 0.05 vs. no glucose and **P < 0.01 vs. 0 and 5.5 mmol/l glucose. #P < 0.01 vs. 0 and 5.5 mmol/l glucose. (A high-quality color representation of this figure is available in the online issue.)
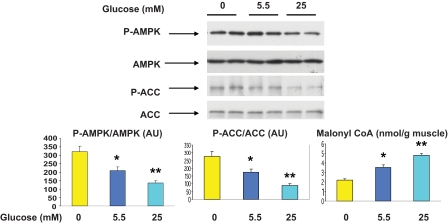
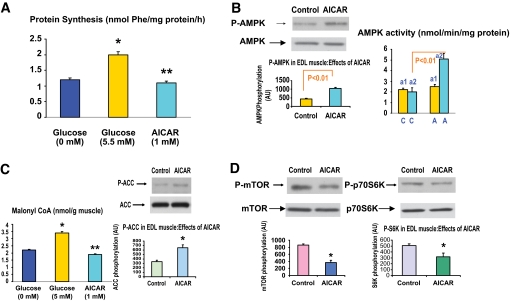
Incubation of the EDL with higher concentrations of glucose (25 vs. 5.5 vs. 0 mmol/l) for 1 h also decreased the phosphorylation of AMPK and ACC (Fig. 4) and the activity of the α2 isoform of AMPK (data not shown). In contrast, eliminating glucose from the medium increased all of these parameters. No change in the activity of α1 AMPK was observed under these conditions (data not shown). The concentration of malonyl CoA changed inversely with the activity and phosphorylation of AMPK as previously described (11).
FIG. 4.
Effect of incubation with different concentrations of glucose on AMPK and ACC phosphorylation, AMPK abundance, and malonyl CoA content. EDL muscles were incubated for 1 h in media containing 0, 5.5, or 25 mmol/l glucose. Representative Western blots (upper panel). Densitometric analysis of blots and malonyl CoA content (lower panel). Results are means ± SE (n = 4). *P < 0.05 vs. 0 mmol/l glucose and **P < 0.01 vs. 0 and 5.5 mmol/l glucose. (A high-quality color representation of this figure is available in the online issue.)
AICAR and another AMPK activator, α-lipoic acid, inhibit glucose-induced changes in protein synthesis and AMPK and mTOR/p70S6K phosphorylation.
The above-mentioned results indicate that the ability of both leucine and glucose to increase mTOR signaling and protein synthesis is associated with a decrease in AMPK phosphorylation and α2-AMPK activity. To assess whether AMPK downregulation might be the cause of these changes, studies were performed in which AMPK downregulation was prevented by coincubation with the AMPK activators, AICAR, or α-lipoic acid (ALA). In muscles incubated with 5.5 mmol/l glucose for 1 h, AICAR (1 mmol/l) decreased protein synthesis by 50% to a rate similar to that observed in muscles incubated in a glucose-free medium (Fig. 5A). As shown in Fig. 5B, AICAR activated α2-AMPK but not α1-AMPK and it increased the phosphorylation of AMPK and ACC and decreased the concentration of malonyl CoA (Fig. 5C). In addition, it diminished the phosphorylation of both mTOR and p70S6K (Fig. 5D).
FIG. 5.
Effect of AICAR on protein synthesis; AMPK α1 and α2 activity and phosphorylation; mTOR, p70S6K, and ACC phosphorylation; and malonyl CoA content. EDL muscles were preincubated with Krebs-Henseleit solution containing 5.5 mmol/l glucose for 20 min and then for an additional 1 h in the presence of 1 mmol/l AICAR. Protein synthesis was measured at 0 or 5.5 mmol/l glucose or 5.5 mmol/l glucose and AICAR (1 mmol/l) (A). Muscle supernatants were subjected to immunopecipitation with specific antibodies to the α1 and α2 of AMPK (B). AMPK (B), ACC (C), and mTOR and p70S6K (D) phosphorylation were determined. Malonyl CoA concentrations (C) were determined as described in research design and methods. P-AMPK, P-ACC, P-mTOR, and P-p70S6K at 0 mmol/l glucose are shown in Figs. 3 and 4. Results are the means ± SE of three experiments. *P < 0.05 vs. 5.5 mmol/l glucose. *P < 0.01 vs. 5.5 mmol/l glucose. (A high-quality color representation of this figure is available in the online issue.)
Almost identical findings were observed when the EDL was incubated with ALA, a naturally occurring short-chain fatty acid that has been shown to activate AMPK in muscle by increasing Ca2+/calmodulin-dependent protein kinase kinase (CAMKK)β (17). Thus, at 5 mmol/l glucose, incubation with 100 μmol/l ALA increased the phosphorylation of AMPK by threefold (Fig. S2A) and decreased both mTOR phosphorylation (Fig. S2B) and protein synthesis by 50% (Fig. S2C). As shown below in Fig. 7, AICAR prevented the inhibition of insulin-stimulated protein kinase B (PKB)/Akt phosphorylation caused by high glucose and leucine. To determine whether this effect is not specific to AICAR, we treated EDL muscle with ALA in the presence of high glucose. As shown in the Fig. S2D, the decrease in Akt phosphorylation by high glucose was completely prevented by ALA.
FIG. 7.
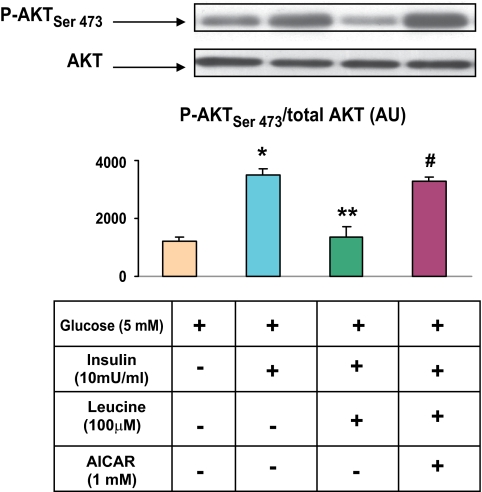
AICAR prevents the inhibition of insulin-stimulated PKB/Akt phosphorylation caused by leucine. EDL muscles were preincubated with 100 μmol/l leucine for 30 min and then AICAR for 1 h. They were then incubated with insulin (10 mU/ml) for 10 min. Muscle lysates were analyzed for PKB/Akt using SDS-PAGE and quantified as described in research design and methods. Results are means ± SE (n = 6). *P < 0.05 vs. basal. **P < 0.01 vs. insulin. #P < 0.01 vs. insulin plus leucine. (A high-quality color representation of this figure is available in the online issue.)
Effects of AICAR on mTOR phosphorylation and protein synthesis in EDL incubated with 25 mmol/l glucose or leucine.
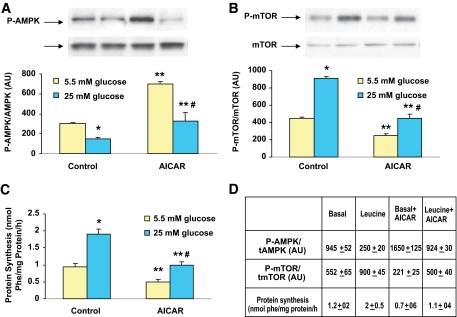
As shown in Fig. 6A, AICAR prevented, although not completely, the decreases in AMPK phosphorylation and the increases in mTOR phosphorylation (Fig. 6B) and protein synthesis (Fig. 6C) caused by incubating muscle with 25 vs. 5 mmol/l glucose. It also prevented the changes in these paramaters caused by incubation with leucine. (Fig. 6D).
FIG. 6.
Comparison of effect of 1 mmol/l AICAR on AMPK and mTOR phosphorylation and protein synthesis in muscles incubated with 5 or 25 mmol/l glucose or leucine. EDL muscles were preincubated with Krebs-Henseleit solution containing 5.5 and 25 mmol/l glucose or 100 μmol/l leucine for 30 min and then for an additional 1 h in the presence or absence of AICAR (1 mmol/l). Results are means ± SE of four experiments. *P < 0.05 vs. 5 mmol/l glucose. **P < 0.01 vs. 5 mmol/l glucose alone. #P < 0.05 vs. 5 mmol/l glucose plus AICAR. (A high-quality color representation of this figure is available in the online issue.)
AMPK activation also diminishes leucine- and glucose-induced insulin resistance.
Incubation with leucine has been shown to cause insulin resistance in skeletal muscle (15). In keeping with this observation, we found that the ability of insulin to increase the phosphorylation of Akt on Ser 473 was diminished by 50% in EDL incubated with leucine at a concentration of 100 μmol/l. As shown in Fig. 7, this effect of leucine was completely prevented by coincubation with 1 mmol/l AICAR. An identical observation was observed when EDL was incubated with 25 mmol/l glucose and AICAR prevented the decrease in AMPK phosphoryation caused by high glucose (data not shown).
Effect of compound C on AMPK and mTOR phosphorylation at 5 mmol/l glucose.
The studies with AICAR and ALA suggest that AMPK activation prevents or partially prevents both the decrease in AMPK activity and the increase in mTOR/p70S6K phosphorylation and protein synthesis caused by leucine and glucose. To assess further whether decreased AMPK plays a causal role in mediating these changes, we next evaluated whether the effects of glucose and leucine on these parameters could be mimicked by incubating the EDL with 50 μmol/l compound C, a selective AMPK inhibitor. As shown in Table S1 in the online appendix, compound C did not affect P-AMPK or mTOR phosphorylation and protein synthesis under baseline conditions indicating it could not be used for this purpose.
Examination of factors that could mediate the downregulation of AMPK by leucine and high glucose
Energy state and lactate and pyruvate concentration.
In a search for factors responsible for the decrease in AMPK phosphorylation caused by leucine and glucose, we first assessed cellular energy state. Incubation with 100 μmol/l leucine for 30 min (not shown) or 1 h did not have an effect on whole tissue levels of creatine phosphate (CrP), ATP, ADP, or AMP (Table 1); however, it increased muscle lactate and, to a lesser extent, pyruvate. The net result was a nearly twofold increase in the lactate/pyruvate ratio (Table 2). In keeping with our previous report (8), there were not any differences observed in the whole-tissue concentrations of CrP, ATP, ADP, or AMP in muscles incubated with 25 vs. 5 mmol/l glucose for 1 h, and here, too, tissue lactate and pyruvate were increased, as was the lactate-to-pyruvate ratio (Table 2).
TABLE 1.
Effects of leucine and high glucose on adenine nucleotide
| ATP | AMP | ADP | CrP | |
|---|---|---|---|---|
| Control (5 mm glucose) | 3.9 ± 0.01 | 0.04 ± 0.001 | 0.5 ± 0.03 | 13 ± 2 |
| Leucine (100 μmol/l) | 3.8 ± 0.01 | 0.035 ± 0.001 | 0.4 ± 0.02 | 14.5 ± 4 |
| Glucose (25 mmol/l) | 4.2 ± 0.2 | 0.045 ± 0.002 | 0.6 ± 0.03 | 14 ± 1 |
Data are means ± SE. Nucleotide values were expressed as millimoles per gram muscle.
TABLE 2.
Effects of leucine and high glucose on lactate and pyruvate
| Lactate | Pyruvate | Lactate/pyruvate ratio | |
|---|---|---|---|
| Control (5 mmol/l glucose) | 12 ± 2 | 1.1 ± 0.1 | 11 ± 2 |
| Leucine (100 μmol/l) | 29 ± 4* | 1.4 ± 0.1* | 21 ± 3* |
| Glucose (25 mmol/l) | 30 ± 2* | 1.7 ± 0.2* | 18 ± 1.5* |
Data are means ± SE (n = 5–6). Lactate and pyruvate are expressed as micromoles per milligram muscle.
*Significantly different from control at 5 mmol/l glucose (P < 0.05).
The observed changes in the lactate-to-pyruvate ratio suggests that leucine and high glucose both cause increases in NADH relative to NAD+ in muscle. For this reason, we examined the effects of incubation with glucose or leucine on the abundance of the NAD+-dependent, redox-sensitive histone protein deacetylase SIRT1. In a previous study in HepG2 cells (18), we found that incubation with 25 vs. 5 mmol/l glucose caused similar changes in cell lactate and significantly diminished SIRT1 abundance (by 20%). In the present study, SIRT1 protein was also decreased by 20% in the muscle incubated with 25 vs. 5.5 glucose; however, the decrease was not statistically significant (P < 0.12) (Fig. S4).
Downregulation of mTOR/p70S6K signaling by rapamycin.
We next assessed whether the decrease in AMPK phosphorylation caused by leucine was dependent on its ability to activate mTOR. Toward this end, EDL was incubated with 200 μmol/l leucine in the presence or absence of rapamycin, an inhibitor of mTOR signaling. As shown in Fig. S3, rapamycin inhibited the increased phosphorylation of mTOR and p70S6K induced by leucine; however, it did not prevent the decrease of AMPK phosphorylation caused by leucine (Fig. S2).
Phosphorylation of raptor and TSC2 in EDL muscle treated with AICAR, high glucose, and leucine.
Recently, AICAR-induced activation in myocytes and other cells has been reported to impair target of rapamycin complex 1 (TORC1) kinase activity by increasing the phosphorylation of raptor on Ser 792 (19). As shown in Fig. S5A, Ser 792 phosphorylation of raptor in EDL incubated with 1 mmol/l AICAR was threefold higher than in the control group. In contrast, high glucose and leucine at concentrations that diminished AMPK phosphorylation and activity did not decrease raptor phosphorylation or diminish the increase in raptor phosphorylation caused by AICAR (Fig. S5B).
A second mechanism by which AMPK might diminish TORC1 signaling and protein synthesis is phosphorylation of TSC2 on Ser 1387 (20). In the incubated EDL, AICAR increased TSC2 phosphorylation by fourfold under basal conditions and two- to threefold in the presence of high glucose and leucine. However, neither high glucose nor leucine by themselves diminished TSC2 phosphorylation from control values (Fig. S6).
Effects of glucose infusion in vivo.
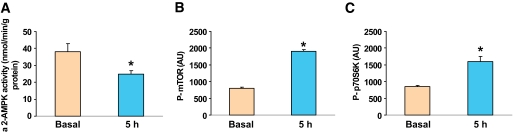
To determine whether the effects of glucose observed in the incubated EDL also occur in vivo, rats were infused with 50% glucose for 5 h with the rate of infusion adjusted to maintain the plasma glucose concentration at 16–17 mmol/l. The plasma insulin concentration was 250 μU/l during the infusion versus 50 μU/l prior to its start. As shown in Fig. 8, AMPK α2 activity in red gastrocnemius muscle decreased after 5 h of glucose infusion compared with baseline values, and the phosphorylation of mTOR and p70S6K increased. It has previously been demonstrated that the glucose infusion also caused insulin resistance by 5 h (12).
FIG. 8.
AMPK α2 activity and phosphorylation of mTOR and p70S6K following glucose infusion. Red gastrocnemius muscle was frozen in liquid nitrogen after 0 or 5 h of glucose infusion, and α2-AMPK activity and phosphorylation of mTOR and p70S6K were determined as described in research design and methods. Results are means ± SE (n = 4–6 rats per group). *P < 0.05 vs. basal.
DISCUSSION
The physiological and biochemical relevance of AMPK activation has been well described (21–24). In contrast, less is known about the consequences of AMPK downregulation. In the present study, we show that excesses of glucose and the branched-chain amino acid leucine stimulated protein synthesis and cause insulin resistance in skeletal muscle and that both effects were paralleled by decreases in AMPK activity. Furthermore, activation of AMPK with both AICAR and ALA prevented these events from occurring.
That leucine at physiological concentrations (70–120 μmol/l) stimulates protein synthesis (25,26) and causes insulin resistance (27) in skeletal muscle and that it does so by increasing mTOR/p70S6K signaling (28) has been reported previously. The novel findings of the present study are that these changes are associated with a decrease in AMPK activity and that they are prevented by incubation with two AMPK activators that work by different mechanisms. Similar observations have recently been made in the pancreatic β-cell (16) suggesting that a decrease in AMPK activity might also contribute to the well-documented stimulation of insulin synthesis and secretion by branched-chain amino acids. The results also indicate that these effects are not unique to leucine, given that incubation of the EDL with progressively higher concentrations of glucose produced an almost identical pattern of events. Collectively, these findings suggest the existence of a common fuel sensing and signaling mechanism by which leucine and glucose downregulate AMPK and alter protein synthesis, insulin sensitivity, and presumably other processes as their concentration is increased (Fig. S7).
The question of whether the decreased AMPK activity induced by leucine and glucose mediated their effects on mTOR/p70S6K signaling and, secondarily, protein synthesis is unresolved. On the one hand, increased AMPK activity has been shown to inhibit protein synthesis both by phosphorylating and activating TSC2 and raptor (19,20)—findings that we confirmed in the incubated EDL. Likewise, the decrease in AMPK activity correlated very strongly with the increases in both protein synthesis and mTOR/p70S6K signaling. On the other hand, if decreased AMPK modulated these effects, one would predict that phosphorylation of the AMPK-sensitive sites on TSC2 and raptor would be diminished. This was not found. Incubation with high glucose and leucine failed to decrease both the phosphorylation of raptor on Ser 792 and TSC2 on Ser 1387. Because baseline phosphorylation of these molecules was very low the possibility that a significant decrease was missed cannot be excluded. Likewise, it is possible that the decrease in AMPK caused by leucine and glucose altered other molecules that could upregulate mTOR/p70S6K (rev. in 20). Clearly, the role of decreased AMPK in mediating the increase in protein synthesis caused by these molecules will require further study.
The findings also raise the following question: by what mechanism does an excess of two different fuels lead to a decrease in AMPK activity? The decreases in AMPK activity induced by both leucine and glucose were not associated with an increase in energy state; indeed, the whole tissue concentrations of creatine-P and the AMP-to-ATP ratio were the same at all glucose and leucine concentrations tested. That a local decrease in the AMP-to-ATP ratio occurred that was not reflected in the whole tissue cannot be ruled out, however. Perhaps relevant to this discussion, we found two- to threefold increases in the lactate-to-pyruvate ratio in muscle incubated with both high glucose and leucine (Table 2), indicating increases in the cytosolic state and, presumably, the nuclear redox state (i.e., a decreased NAD+-to-NADH ratio). This in turn was associated with a 20% decrease in the abundance of the NAD+-dependent histone/protein deacetylase SIRT1, an enzyme reported to deacetylate the AMPK kinase liver kinase B 1, leading to an increase in its activity and that of AMPK (29–31). On the other hand, the decrease in SIRT1 abundance observed here was not statistically significant (P < 0.12; n = 6). This contrasts with an earlier study, in which we incubated HepG2 cells in a high-glucose medium (25 vs. 5 mmol/l) and observed similar decreases in AMPK phosphorylation and SIRT1 abundance (20%)—both of which were statistically significant (18). Clearly, further investigations are needed to assess whether glucose and leucine affect SIRT1 in the EDL. Finally, incubation of the EDL with the mTOR inhibitor, rapamycin, had no effect on the ability of leucine to diminish AMPK phosphorylation (Fig. S3). Thus, the decrease in AMPK activity was not a consequence of mTOR activation.
With respect to the relevance of the findings to muscle in vivo, the infusion of glucose at a rate sufficient to increase its plasma concentration to 16–17 mmol/l and plasma insulin levels from 50 to 250 mU/ml increased both mTOR/p70S6K signaling and decreased AMPK activity after 5 h (Fig. 8). Others have shown that feeding a high-protein diet (ingestion of branched-chain amino acids) causes similar changes in AMPK and mTOR/p70S6K in liver (32). Presumably, these events occur in muscle and liver physiologically after meals dependent on their composition. Whether the glucose- and leucine-induced decreases in AMPK activity contribute to the postprandial increases in protein (mTOR/p70S6K) synthesis is open to debate—as it is in the incubated EDL. On the other hand, it is highly likely that concurrent changes in lipid synthesis in muscle during a glucose infusion (12) are due to the decrease in AMPK because they are associated with decreased phosphorylation and increased activity of the AMPK target ACC. Also, in keeping with this conclusion, in the present study we found that the concentration of the ACC product malonyl CoA was elevated in the EDL when it was incubated with leucine or high glucose (Figs. 4 and 5).
As already noted, incubation of muscles with elevated concentrations of leucine (27) or glucose (8) have been shown to cause insulin resistance. The results presented here suggest that the insulin resistance correlates with a decrease in AMPK activity; however, for the same reason discussed in the context of mTOR/p70S6K signaling and protein synthesis, it is unclear whether the decrease in AMPK was a causal event. Whatever the mechanism, we would propose that such fuel-induced insulin resistance in vivo develops postprandially when the muscle or liver cell senses its need to synthesize glycogen or protein has been met and that it is to the benefit of the organism to shunt glucose and amino acids to other tissues (e.g., adipose tissue) for storage. By virtue of the setting in which it occurs, and its likely reversibility, one might refer to this phenomenon as “physiological insulin resistance.”
Whether a more sustained decrease in AMPK activity caused by glucose and leucine can lead to pathological insulin resistance (i.e., insulin-resistance associated with less reversible cellular abnormalities) in vivo remains to be determined. In keeping with such a notion, the insulin resistance caused by exposure of cultured endothelial cells to a high-glucose medium for 24 h is associated with mitochondrial dysfunction, apoptosis, oxidative stress, and inflammation (33–35), all of which are prevented by AMPK activation. The early observation that amino acid levels are elevated in the plasma of obese individuals (36,37) first raised the possibility that they may be involved in the development of obesity-linked or diet-induced insulin resistance. Subsequently, infusion of the branched-chain amino acid leucine was found to impair glucose uptake in humans despite elevated plasma insulin levels (38), and in rodents fed a high-fat diet, branched-chain amino acids have been implicated in the development of obesity-associated insulin resistance (39). Whether decreases in AMPK activity or an impairment of its activation occurred in these settings was not examined. Finally, decreased AMPK activity has been observed in many rodents with chronic insulin resistance including ob/ob mice (40), fa/fa and ZDF rats (41,42), and the IL-6 KO mouse (43). However, in all of these rodents it was attributable to a genetic lack of a hormone or its receptor.
In summary, we have demonstrated that incubation of rat skeletal muscle with moderately elevated concentrations of leucine or glucose suppresses AMPK activation and concomitantly increases mTOR/p70S6K signaling and protein synthesis and leads to insulin resistance. All of these changes were associated with a decrease in AMPK activity and were prevented by incubating the muscles with the AMPK activators AICAR or α-lipoic acid. Although collectively these findings suggest that a decrease in AMPK activity mediates the effects of high glucose and leucine, direct evidence for this is still lacking.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by U.S. Public Health Service grants RO1DK19514 and RO1DK67509 (to N.R. and A.K.S.) and by a mentor-based fellowship from the American Diabetes Association (to N.R.).
No potential conflicts of interest relevant to this article were reported.
A.K.S. wrote the manuscript, contributed to the discussion, and reviewed and edited the manuscript. X.J.X. researched data. E.L. researched data. R.D. researched data. A.E.B. researched data and reviewed and edited the manuscript. E.W.K. contributed to the discussion and reviewed and edited the manuscript. N.B.R. wrote the manuscript, contributed to the discussion, and reviewed and edited the manuscript.
Parts of this study were presented at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–29 June 2010.
The authors thank Emma Heart for her technical assistance and Meenakshi Sivaraman for her assistance in typing the manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1. Hardie DG, Hawley SA, Scott JW. AMP-activated protein kinase: development of the energy sensor concept. J Physiol 2006;574:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hutber CA, Hardie DG, Winder WW. Electrical stimulation inactivates muscle acetyl-CoA carboxylase and increases AMP-activated protein kinase. Am J Physiol 1997;272:E262–E666 [DOI] [PubMed] [Google Scholar]
- 3. Richter EA, Ruderman NB. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Biochem J 2009;418:261–27519196246 [Google Scholar]
- 4. Munday MR, Milic MR, Takhar S, Holness MJ, Sugden MC. The short-term regulation of hepatic acetyl-CoA carboxylase during starvation and re-feeding in the rat. Biochem J 1991;280:733–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Assifi MM, Suchankova G, Constant S, Prentki M, Saha AK, Ruderman NB. AMP Activated protein kinase and the coordination of hepatic fatty acid metabolism of starved/carbohydrate-refed rats. Am J Physiol 2005;289:E794–E800 [DOI] [PubMed] [Google Scholar]
- 6. Sponarova J, Mustard KJ, Horakova O, Flachs P, Rossmeisl M, Brauner P, Bardova K, Thomason-Hughes M, Braunerova R, Janovska P, Hardie DG, Kopecky J. Involvement of AMP-activated protein kinase in fat depot-specific metabolic changes during starvation. FEBS Letts 2005;579:6105–6110 [DOI] [PubMed] [Google Scholar]
- 7. Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem 2002;277:23977–23980 [DOI] [PubMed] [Google Scholar]
- 8. Itani SA, Saha AK, Kurowski TG, Coffin HR, Tornheim K, Ruderman NB. Glucose autoregulates its uptake in skeletal muscle: involvement of AMP-activated protein kinase. Diabetes 2003;52:1635–1640 [DOI] [PubMed] [Google Scholar]
- 9. Fafournoux P, Remesy C, Demigne C. Fluxes and membrane transport of amino acids in rat liver under different protein diets. Am J Physiol 1990;259:E614–E625 [DOI] [PubMed] [Google Scholar]
- 10. Gomez-Angelats M, Ruiz-Montasell B, Felipe A, Marin JJ, Casado FJ, Anglada MP. Effect of protein malnutrition on neutral amino acid transport by rat hepatocyte during development. Am J Physiol 1995;268:E368–E374 [DOI] [PubMed] [Google Scholar]
- 11. Park H, Kaushik VK, Constant S, Prentki M, Przybytkowski E, Ruderman NB, Saha AK. Coordinate regulation of malonyl-CoA decarboxylase, sn-glycerol-3-phosphate acyltransferase and acetyl-CoA carboxylase by AMP-activated protein kinase in rat tissues in response to exercise. J Biol Chem 2002;277:32571–32577 [DOI] [PubMed] [Google Scholar]
- 12. Kraegen EW, Saha AK, Preston E, Wilks D, Hoy AJ, Cooney GJ, Ruderman NB. Increased malonyl CoA and diacylglycerol content and reduced AMPK activity accompany insulin resistance induced by glucose infusion in muscle and liver of rats. Am J Physiol 2006;290:E471–E479 [DOI] [PubMed] [Google Scholar]
- 13. McGarry JD, Stark MJ, Foster DW. Hepatic malonyl CoA levels of fed, fasted and diabetic rats as measured using a simple radioisotopic assay. J Biol Chem 1997;253:8291–8294 [PubMed] [Google Scholar]
- 14. Maizels EZ, Ruderman NB, Goodman MN, Lau D. Effect of acetoacetate on glucose metabolism in the soleus and extensor digitorum longus muscles of the rat. Biochem J 1977;162:557–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lang CH. Elevated plasma free fatty acids decrease basal protein synthesis, but not the anabolic effect of leucine, in skeletal muscle. Am J Physiol 2006;291:E666–E674 [DOI] [PubMed] [Google Scholar]
- 16. Gleason CE, Lu D, Witters LA, Newgard CB, Birnbaum MJ. The role of AMPK and mTOR in nutrient sensing in pancreatic beta-cells. J Biol Chem 2007;282:10341–10351 [DOI] [PubMed] [Google Scholar]
- 17. Shen QW, Zhu MJ, Tong J, Ren J, Du M. Ca2+/calmodulin-dependent protein kinase kinase is involved in AMP-activated protein kinase activation by alpha-lipoic acid in C2C12 myotubes. Am J Physiol Cell Physiol 2007;293:C1395–C1403 [DOI] [PubMed] [Google Scholar]
- 18. Suchankova G, Nelson LE, Gerhart-Hines Z, Kelly M, Gauthier MS, Saha AK, Ido Y, Puigserver P, Ruderman NB. Concurrent regulation of AMP-activated protein kinase and SIRT1 in mammalian cells. Biochem Biophys Res Commun 2009;378:836–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 2008;30:214–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang J, Manning BD. The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem J 2008;412:179–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 2005;1:15–25 [DOI] [PubMed] [Google Scholar]
- 22. Steinberg GR, Kemp BE. AMPK in health and disease. Physiol Rev 2009;89:1025–78 [DOI] [PubMed] [Google Scholar]
- 23. Hardie DG. AMPK and the biochemistry of exercise: implications for human health and disease. Nat Rev Mol Cell Biol 2007;8:774–785 [DOI] [PubMed] [Google Scholar]
- 24. Witczak CA, Sharoff CG, Goodyear LJ. AMP-activated protein kinase in skeletal muscle: from structure and localization to its role as a master regulator of cellular metabolism. Cell Mol Life Sci 2008;65:3737–3755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suryawan A, Jeyapalan AS, Orellana RA, Wilson FA, Nguyen HV, Davis TA. Leucine stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing mTORC1 activation. Am J Physiol Endocrinol Metab 2008;295:E868–E875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Crozier SJ, Kimball SR, Emmert SW, Anthony JC, Jefferson LS. Oral leucine administration stimulates protein synthesis in rat skeletal muscle. J Nutr 2005;135:376–382 [DOI] [PubMed] [Google Scholar]
- 27. Patti ME, Brambilla E, Luzi L, Landaker EJ, Kahn CR. Bidirectional modulation of insulin action by amino acids. J Clin Invest 1998;101:1519–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Drummond MJ, Rasmussen BB. Leucine-enriched nutrients and the regulation of mammalian target of rapamycin signalling and human skeletal muscle protein synthesis. Curr Opin Clin Nutr Metab Care 2008;11:222–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ruderman NB, Xu J, Nelson LE, Cacicedo JM, Saha AK, Lan F, Ido Y: AMPK and SIRT1: a longstanding partnership? Am J Physiol 2010;298:E751–E760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1: possible role in AMP-activated protein kinase activation. J Biol Chem 2008;283:27628–27635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, Lan F, Walsh K, Wierzbicki M, Verbeuren TJ, Cohen RA, Zang M. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem 2008;283:20015–20026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chotechuang N, Azzout-Marniche D, Bos C, Chaumontet C, Gausserès N, Steiler T, Gaudichon C, Tomé D. mTOR, AMPK, and GCN2 coordinate the adaptation of hepatic energy metabolic pathways in response to protein intake in the rat. Am J Physiol Endocrinol Metab 2009;297:E1313–E1323 [DOI] [PubMed] [Google Scholar]
- 33. Ido Y, Carling D, Ruderman N. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: inhibition by the AMP-activated protein kinase activation. Diabetes 2002;51:159–167 [DOI] [PubMed] [Google Scholar]
- 34. Kukidome D, Nishikawa T, Sonoda K, Imoto K, Fujisawa K, Yano M, Motoshima H, Taguchi T, Matsumura T, Araki E. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes 2006;55:120–127 [PubMed] [Google Scholar]
- 35. Hattori Y, Suzuki K, Hattori S, Kasai K. Metformin inhibits cytokine-induced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension 2006;47:1183–1188 [DOI] [PubMed] [Google Scholar]
- 36. Felig P, Wahren J, Hendler R, Brundin T, Splanchnic A. Glucose and amino acid metabolism in obesity. J Clin Invest 1974;53:582–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Felig P, Owen OE, Wahren J, Cahill GF., Jr Amino acid metabolism during prolonged starvation. J Clin Invest 1969;48:584–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Abumrad NN, Robinson RP, Gooch BR, Lacy WW. The effect of leucine infusion on substrate flux across the human forearm. J Surg Res 1982;32:453–463 [DOI] [PubMed] [Google Scholar]
- 39. Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Ha AM, Shah SH, Arlotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wenner BR, Yancy WS, Jr, Eisenson H, Musante G, Surwit RS, Millington DS, Butler MD, Svetkey LP. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009;9:311–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Halseth AE, Ensor NJ, White TA, Ross SA, Gulve EA. Acute and chronic treatment of ob/ob and db/db mice with AICAR decreases blood glucose concentrations. Biochem Biophys Res Commun 2002;294:798–805 [DOI] [PubMed] [Google Scholar]
- 41. Yu X, McCorkle S, Wang M, Lee Y, Li J, Saha AK, Unger RH, Ruderman NB. Leptinomimetic effects of the AMP kinase activator AICAR in leptin-resistant rats: prevention of diabetes and ectopic lipid deposition. Diabetologia 2004;47:2012–2021 [DOI] [PubMed] [Google Scholar]
- 42. Pold R, Jensen LS, Jessen N, Buhl ES, Schmitz O, Flyvbjerg A, Fujii N, Goodyear LJ, Gotfredsen CF, Brand CL, Lund S. Long-term AICAR administration and exercise prevents diabetes in ZDF rats. Diabetes 2005;54:928–934 [DOI] [PubMed] [Google Scholar]
- 43. Kelly M, Keller C, Avilucea PR, Keller P, Luo Z, Xiang X, Giralt M, Hidalgo J, Saha AK, Pedersen BK, Ruderman NB. AMPK activity is diminished in tissues of IL-6 knockout mice: the effect of exercise. Biochem Biophys Res Commun 2004;320:449–454 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.