Abstract
OBJECTIVE
It has long been recognized that autoimmunity is often associated with immunodeficiency. The mechanism underlying this paradox is not well understood. Bcl-3 (B-cell lymphoma 3) is an atypical member of the IκB (inhibitor of the nuclear factor-κB) family that is required for lymphoid organogenesis and germinal center responses. Mice deficient in Bcl-3 are immunodeficient because of the microarchitectural defects of their lymphoid organs. The goal of this study is to define the potential roles of Bcl-3 in type 1 diabetes.
RESEARCH DESIGN AND METHODS
Bcl-3–deficient NOD mice were generated by backcrossing Bcl-3–deficient C57BL/6 mice to NOD mice. Spontaneous and induced type 1 diabetes were studied in these mice by both pathologic and immunologic means. The effect of Bcl-3 on inflammatory gene transcription was evaluated in a promoter reporter assay.
RESULTS
We found that Bcl-3–deficient NOD and C57BL/6 mice were, paradoxically, more susceptible to autoimmune diabetes than wild-type mice. The increase in diabetes susceptibility was caused by Bcl-3 deficiency in hematopoietic cells but not nonhematopoietic cells. Bcl-3 deficiency did not significantly affect anti-islet Th1 or Th2 autoimmune responses, but markedly increased inflammatory chemokine and T helper 17 (Th17)-type cytokine expression. Upon transfection, Bcl-3 significantly inhibited the promoter activities of inflammatory chemokine and cytokine genes.
CONCLUSIONS
These results indicate that in addition to mediating lymphoid organogenesis, Bcl-3 prevents autoimmune diabetes by inhibiting inflammatory chemokine and cytokine gene transcription. Thus, a single Bcl3 gene mutation leads to both autoimmunity and immunodeficiency.
Type 1 diabetes, or insulin-dependent diabetes, is an inflammatory disease of the pancreatic islets that afflicts millions of people worldwide. Although the genetic and environmental factors that trigger the disease vary, the common pathologic outcome of type 1 diabetes is the destruction of insulin-producing β-cells by inflammatory cells (activated lymphoid and myeloid cells) through a process called insulitis. Development of insulitis requires coordinated expression of a large number of genes that mediate the activation, migration and effector functions of inflammatory cells (1,2). These include genes that encode cytokines, chemokines, and cytotoxic enzymes. Although it is well recognized that expression of these genes is tightly controlled at the transcriptional level, the nature of the transcription factors involved and the mechanisms of their action in type 1 diabetes are not well understood. Recent studies from several laboratories, including ours, indicate that the nuclear factor-κB (NF-κB) family of transcription factors plays crucial roles in type 1 diabetes. Thus, in both mice and humans, type 1 diabetes is associated with heightened NF-κB activation (3–6), whereas NF-κB deficiency in mice renders them resistant to the disease (7,8). Importantly, inhibiting NF-κB activities is highly effective in suppressing models of type 1 diabetes (9–11). Therefore, NF-κB has emerged as a long sought-after transcriptional regulator of type 1 diabetes.
In mammals, there are five NF-κB genes, NFKB1, NFKB2, RELA, cREL, and RELB, which in turn encode seven proteins: p105, p50, p100, p52, p65 (RelA), c-Rel, and RelB (12,13). The protein p50 is generated from limited proteasomal processing of its p105 precursor, as is p52 from its p100 precursor. The p50 and p52 proteins lack the transactivating domain found in the COOH-terminal regions of other NF-κB proteins. Therefore, their homodimers function primarily as repressors of gene transcription. The activities of NF-κB are tightly regulated by several IκB (inhibitor of κB) proteins that share high sequence and structural homologies (14). These include IκBα, IκBβ, IκBγ, IκBε, IκBζ, IκBNS, and Bcl-3. Additionally, the COOH-terminal regions of p100 and p105 also serve as IκBs. These IκB proteins contain repeated sequences of ∼30 amino acids long termed ankyrin repeats, which are essential for binding to NF-κB. Unlike other IκB proteins that are located primarily in the cytoplasm, IκBζ, IκBNS, and Bcl-3 are found mainly in the nucleus and therefore are involved chiefly in regulating nuclear NF-κB activities. These nuclear IκB proteins exhibit significant differences in their NF-κB binding. Bcl-3 binds to only p50 and p52 homodimers (15,16); IκBζ binds to both p50 homodimers and p65/p50 dimers, whereas IκBNS appears to show little subunit preference (17,18). Importantly, the nuclear IκB proteins are not degraded after IKK (IκB kinase) activation, and their primary function appears to be the modulation of gene transcription in a gene- and NF-κB subunit–specific manner. Bcl-3 has been reported to be able to either activate or repress gene transcription after binding to p50 or p52 homodimers (19–22). Mice deficient in Bcl-3 are more susceptible to infectious diseases because of developmental defects of their lymphoid organs and reduced germinal center responses (23,24). These defects appear to be caused by Bcl-3 deficiency in nonhematopoietic stromal cells, and they reflect a role of Bcl-3 in activating organogenic chemokine genes (25,26). Similar to p52, Bcl-3 may also regulate thymic stromal cell development, indirectly controlling thymic tolerance (26). On the other hand, Bcl-3 in combination with p50 can inhibit cytokine gene expression after Toll-like receptor (TLR) activation, preventing septic shock (27). Here, we describe a novel role of Bcl-3 in models of type 1 diabetes.
RESEARCH DESIGN AND METHODS
C57BL/6 (B6) mice that carry a Bcl3 gene mutation were generated as described previously (24,28). NOD.BDC2.5 TCR transgenic mice and NOD.scid mice were purchased from Jackson Laboratory (Bar Harbor, ME). To generate BDC2.5 transgenic mice with Bcl3 gene null mutation, Bcl3−/− B6 mice were crossed with NOD.BDC2.5 transgenic mice, and progenies were genotyped for MHC and Bcl3 by PCR. BDC2.5 TCR expression was determined by flow cytometry using monoclonal antibodies against CD4 and Vβ4. The B6xNOD.BDC2.5 TCR transgenic mice used in this study were all H-2b/g7. Only wild-type littermates were used as controls. To generate NOD mice with the Bcl3 gene null mutation, Bcl3−/− B6 mice were backcrossed to NOD mice for nine generations. All mice were housed in the University of Pennsylvania animal care facilities under pathogen-free conditions, and all procedures were preapproved by the Institutional Animal Care and Use Committee.
Induction and evaluation of diabetes.
To induce diabetes, B6 mice were injected intraperitoneally for 5 consecutive days with 40 mg/kg/day of streptozotocin, whereas NOD and BDC2.5 transgenic mice were injected intraperitoneally with 200 mg/kg of cyclophosphamide once weekly for 2 weeks. Both streptozotocin and cyclophosphamide were purchased from Sigma (St. Louis, MO). Mice were tested in a blinded manner every other day for urinary glucose levels using the Keto-Diastix kit (Bayer, Elkhart, IN) and every other week for blood glucose levels. They were considered diabetic if the urinary glucose levels were >500 mg/dl or the blood glucose levels were >250 mg/dl on at least two consecutive tests. To obtain histologic profiles of the pancreas, pancreatic sections were fixed in 10% formalin, embedded in paraffin, sectioned, stained with hematoxylin/eosin, and examined by microscopy.
Flow cytometry and antibodies.
Flow cytometric analyses were performed on freshly prepared splenocytes or CD4+ T-cells after Th17 cell differentiation in vitro. For intracellular staining, cells were fixed in fixation and permeabilization solution (BD Biosciences), and intracellular cytokine staining was performed per the manufacturer's protocol. Stained cells were analyzed on a fluorescence-activated cell sorter-calibur (BD Biosciences). Data were analyzed with FlowJo software.
Cell culture and cytokine assay.
For cytokine assays, splenocytes were cultured at 1.5 × 106 cells/well in 0.2 ml of Dulbecco's modified Eagle's medium (DMEM) with 10% FBS in the presence or absence of 20 μg/ml GAD65 peptide or 50 μg/ml insulin, anti-CD3 mAb, and/or anti-CD28 mAb. Culture supernatants were collected 48 h later, and cytokine concentration was determined by quantitative ELISA per the manufacturer's recommendations. All purified antibodies and recombinant cytokines were purchased from BD Biosciences (San Diego, CA).
Real-time RT-PCR.
Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Reverse transcription was performed using oligo dT primers. Real-time PCR was performed in an Applied Biosystems 7500 system using Power SYBR Green PCR Master Mix or TaqMan Fast Universal PCR Master Mix (Applied Biosystems). Relative levels of gene expression was determined using GAPDH as the control. Taqman probe for TNF-α, Rantes, and CCL5 were purchased from Applied Biosystems. The primers for Eotaxin, MIP1α, MIP1β, IP10, interleukin (IL)-17A, Aire, Foxp3, Fabp, and GAD65 were synthesized as previously described (29).
Preparation of tissue extract.
Spleen and pancreas were aseptically removed from the mice and homogenized in RPMI-1640 containing 1% CHAPS (3-[(3-cholamidopropyl)dimethyammonio]-1-propanesulfonate) (Calbiochem, La Jolla, CA) at a ratio of 1:10 (wt/vol) using a Dounce grinder. The homogenates were centrifuged at 2,000g for 20 min, and the supernatant was collected and stored at −80°C before the cytokine assay.
Immunoblotting.
Serially diluted tissue extract samples were spotted onto a nitrocellulose membrane. The membrane was then blocked using 5% nonfat milk in Tris-buffered saline Tween-20 (TBS-T), and incubated with 1:2,000 diluted rabbit anti–MCP-1 (eBioscience) or 1:1,000 diluted rabbit anti-Rantes (eBioscience) and mouse anti–β-actin for 30 min at room temperature. After washing with TBS-T, the membrane was incubated with 1:20,000 diluted secondary antibody conjugated with horseradish peroxidase at room temperature for 30 min. Color was developed using ECL Western Blotting Detection Reagents (Amersham Pharmacia Biotech).
Promoter luciferase assay.
The pGL3-based murine p19 promoter construct containing the genomic fragment −1,180 to +110 of the p19 gene has been previously described (30). The 2,730-bp genomic DNA (from −2,660 to +70) of the murine MCP1 promoter and 629-bp genomic DNA (from −569 to +60) of the murine IP10 promoter were cloned into the pGL3-basic vector (Promega). Mouse full-length Bcl-3 cDNA was cloned into the pEF4 mammalian expression vector. RAW264.7 cells were transiently transfected with p19, MCP1, or IP10 promoter-luciferase construct together with the Bcl-3 expression vector or empty vector using Lipofectamine LTX transfection reagent (Invitrogen). After 24 h, cells were treated with or without 200 ng/ml lipopolysaccharide for 8 h, and the luciferase activities of the total cell lysates were measured using the dual-luciferase reporter assay system (Promega). Cotransfection of the Renilla-luciferase expression vector pRL-TK (Promega) was used as an internal control for all reporter assays.
Th17 cell differentiation.
Splenic CD4+ T-cells were purified by MACS (Miltenyi Biotec) and cultured at 1.5 × 106/well in 24-well plates containing plate-bound anti-CD3 (5 μg/ml) and soluble anti-CD28 (1 μg/ml) in DMEM supplemented with 10% FBS, 10 units/ml mouse IL-2, 5 μg/ml anti–IL-4 (BD Pharmingen), 5 μg/ml anti–γ-interferon (IFN-γ) (BD Pharmingen) with or without 20 ng/ml IL-6 (BD Pharmingen), and 5 ng/ml TGF-β1 (R&D Systems). Three days later, cells were washed and treated for 4–5 h with 50 ng/ml phorbol myristic acid (PMA) (Sigma) and 750 ng/ml ionomycin (Sigma) in the presence of GolgiStop (1:1,500 dilution, BD Pharmingen) at 37°C before being examined by flow cytometry.
Bone marrow and T-cell transfer.
Bcl3−/− mice have severe defects in the microarchitecture of their secondary lymphoid organs, including reduced germinal centers and follicular dendritic cell networks (23,24), which in turn affect the immune competence of these animals. The structural defect is not present in chimeric mice reconstituted with Bcl3−/− bone marrow (25). In this study, bone marrow chimeric mice were generated by irradiating wild-type or Bcl3−/− C57BL/6 mice twice with 500 rad spaced 3 h apart, followed by intravenous injection of 107 bone marrow cells from wild-type or Bcl3−/− C57BL/6 mice. Repopulation of the immune system was monitored by flow cytometric analysis of the blood. As we reported, in the chimeric mice so generated, ∼90% of the T-cells and >95% of the B-cells and myeloid cells were derived from donor bone marrow 8–9 weeks after the cell transfer (31). For T-cell transfer, 1.5 × 107 T-cells isolated from spleens were injected into NOD.scid mice through the tail vein.
Statistical analysis.
The significance of the differences in disease severity and immune parameters was determined by the Kaplan-Meier test, paired Student t test, or ANOVA.
RESULTS
Increased diabetes susceptibility of Bcl-3–deficient NOD and C57BL/6 mice.
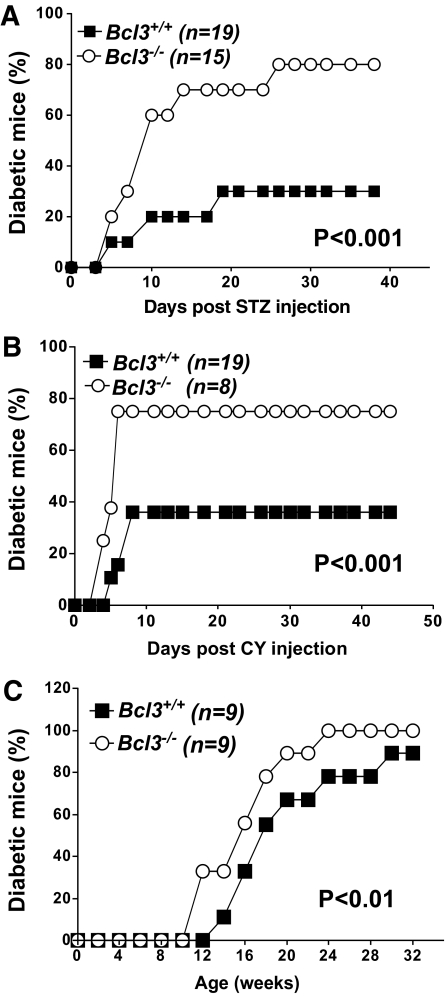
To study the potential roles of Bcl-3 in type 1 diabetes, we generated three strains of Bcl-3–deficient mice: Bcl3−/− C57BL/6, NOD, and B6xNOD.BDC2.5 TCR transgenic mice as described in research design and methods. NOD mice were monitored for the development of spontaneous diabetes by weekly testing of blood glucose levels. To induce diabetes in C57BL/6 and B6xNOD.BDC2.5 mice, we administered low-dose streptozotocin and cyclophosphamide, respectively (Fig. 1). Age- and sex-matched wild-type littermates were used as controls. As shown in Fig. 1A and B, <40% of C57BL/6 and B6xNOD.BDC2.5 mice developed diabetes. By contrast, the incidence of the disease was markedly increased in Bcl3−/− groups (∼80%). The NOD.BDC2.5 is a transgenic mouse line expressing the diabetogenic T-cell receptor BDC2.5 in the NOD background. Young NOD.BDC2.5 mice are significantly more sensitive to cyclophosphamide-induced diabetes than their nontransgenic counterparts. It is a unique model of type 1 diabetes that is different from B6 or conventional NOD mice in TCR usage and incidence. Similarly, spontaneous diabetes was also significantly accelerated in Bcl3−/− NOD mice compared with their wild-type controls (Fig. 1C). However, because the incidence of spontaneous type 1 diabetes was very high (80–85%) in wild-type NOD mice, the disease-promoting effect of the Bcl3 gene mutation in this model was not as striking as in other models.
FIG. 1.
Bcl3−/− NOD and C57BL/6 mice are more susceptible to type 1 diabetes. A: Bcl3+/+ (n = 19) and Bcl3−/− (n = 15) C57BL/6 male mice were injected with low-dose streptozotocin as described in research design and methods. B: Bcl3+/+ (n = 19) and Bcl3−/− (n = 8) B6xNOD.BDC2.5 transgenic mice were injected with cyclophosphamide as described in research design and methods. C: Bcl3+/+ (n = 9) and Bcl3−/− (n = 9) NOD mice were monitored for the development of spontaneous diabetes for 32 weeks. Data presented are accumulated diabetes incidence pooled from two independent experiments. The differences between the two groups are statistically significant for all panels, as determined by the Kaplan-Meier test.
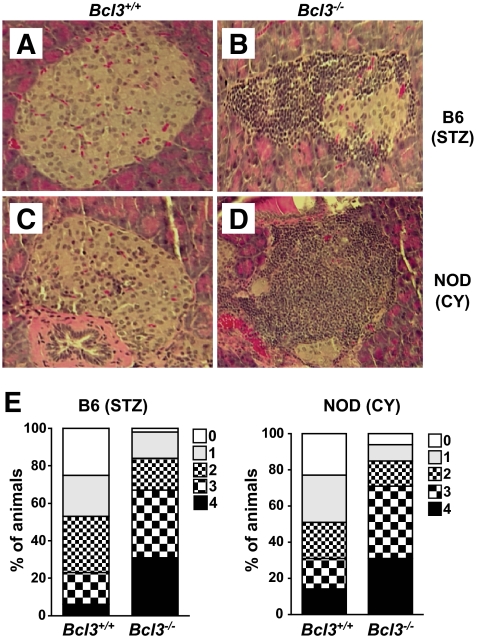
Consistent with these clinical findings, histochemical analysis of pancreatic sections of wild-type and Bcl3−/− mice revealed significant differences. Insulitis, characterized by peri- and intraislet infiltration by inflammatory cells, was observed frequently in Bcl3−/−, but not wild-type mice (Fig. 2). Taken together, these results indicate that Bcl-3 plays a crucial role in preventing type 1 diabetes.
FIG. 2.
Histologic profiles of pancreas. Mice were treated as described in Fig. 1 and killed 45 days after the first streptozotocin (STZ) or cyclophosphamide (CY) injection. Pancreata were collected, fixed in 10% formalin, and embedded in paraffin. Paraffin sections (5-μm thick) were stained with hematoxylin and eosin. Pancreatic sections of B6 (A) and B6xNOD (C) Bcl3+/+ mice showed little or no insulitis, whereas pancreatic sections of B6 (B) and B6xNOD (D) Bcl3−/− mice showed severe insulitis. Magnification: ×200. E: Insulitis scores. Mice were treated as in Fig. 1, and pancreatic inflammation was graded as follows: 0, no inflammation; 1, peri-insulitis with mononuclear cell infiltration affecting 25% of the circumference; 2, peri-insulitis with mononuclear cell infiltration affecting 25% of the circumference; 3, mild-to-moderate insulitis with intraislet mononuclear cell filtration but good preservation of islet architecture; 4, severe insulitis with numerous intraislet inflammatory cells and loss of normal islet architecture. (A high-quality digital representation of this figure is available in the online issue.)
Increased Th17, but not Th1 or Th2, cytokine gene expression in Bcl3−/− mice.
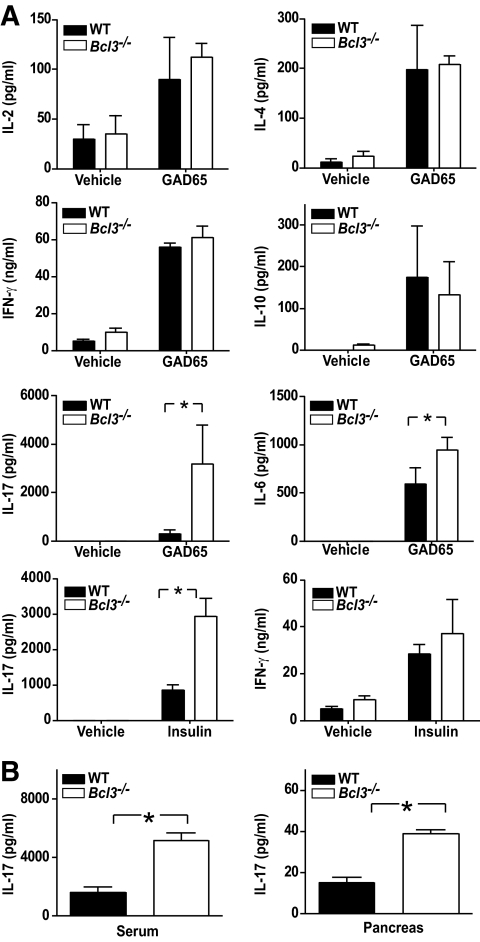
T-cells play important roles in type 1 diabetes. To determine the potential effect of Bcl-3 deficiency on T-cells during type 1 diabetes, we examined their cytokine expression in vitro upon stimulation with self-GAD65 peptide and insulin. We found that Bcl3−/− NOD splenocytes produced significantly more Th17 (IL-17A and IL-6), but not Th1-(IL-2 and IFN-γ) or Th2-type cytokines (IL-4 and IL-10) (Fig. 3A). Consistent with this finding, IL-17A but not Th1- or Th2-type cytokines was significantly increased in the blood and pancreas of 6- to 8-week-old Bcl3−/− NOD mice (Fig. 3B and data not shown).
FIG. 3.
Increased Th17, but not Th1 or Th2, type cytokines in Bcl3−/− mice. A: Splenocytes from 6- to 8-week-old NOD mice (n = 3) were cultured with or without GAD65 peptide (20 μg/ml) or insulin (50 μg/ml) for 40 h. The cytokine concentrations in the culture supernatants were determined by ELISA. B: Sera and pancreatic extracts of 6- to 8-week-old NOD mice (n = 3) were tested for IL-17A by ELISA. *P < 0.01. Data presented are representative of two independent experiments.
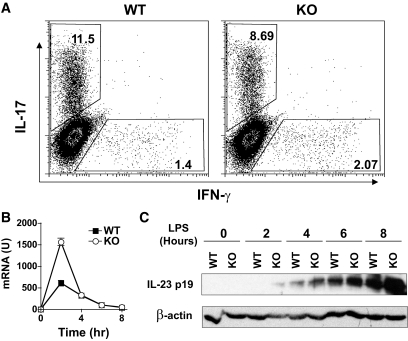
These results indicate that Bcl-3 may regulate Th17 cell differentiation. To test this possibility, wild-type and Bcl-3–deficient splenic CD4+ T-cells were purified and cultured under Th17-inducing conditions (with anti–IL-4, anti–IFN-γ, IL-6, and TGF-β1) (Fig. 4A). Five days later, cells were washed and restimulated with PMA and ionomycin, stained with respective antibodies, and examined by flow cytometry. We found that Bcl-3 deficiency did not significantly affect the differentiation of Th17 cells. This indicates that the increased Th17 response in Bcl-3–deficient mice is likely related to the Bcl-3 effect on Th17 cell activation or survival, but not differentiation. Consistent with this view, IL-23, a cytokine important for Th17 cell expansion and survival, was upregulated at both mRNA and protein levels in Bcl3−/− cultures (Fig. 4B). Thus, Bcl-3 may regulate Th17 cell response through IL-23.
FIG. 4.
Th17 cell differentiation and IL23 gene expression in the absence of Bcl-3. A: Bcl3+/+ and Bcl3−/− splenic CD4+ T-cells were purified from 6- to 8-week-old B6 mice (n = 3) and cultured with IL-2 (10 units/ml), anti-CD28 (1 μg/ml), and anti-CD3 (5 μg/ml) under Th17-inducing conditions (5 μg/ml anti–IL-4, 5 μg/ml anti–IFN-γ, 20 ng/ml IL-6, and 5 ng/ml TGF-β1). Three days later, cells were restimulated with 50 ng/ml PMA and 1 μmol/l ionomycin in the presence of GolgiStop for 5 h, stained with anti–IL-17A and anti–IFN-γ, and examined by flow cytometry. The difference in the frequency of Th17 cells between the two groups is not statistically significant (P > 0.05). B and C: Bone marrow–derived dendritic cells (30) from 6- to 8-week-old B6 mice (n = 3) were cultured with 100 ng/ml lipopolysaccharide for the indicated times. IL-23p19 mRNA (B) and protein (C) were measured by real-time PCR and Western blot, respectively. Data presented are representative of two independent experiments.
Increased chemokine gene expression in Bcl3−/− mice.
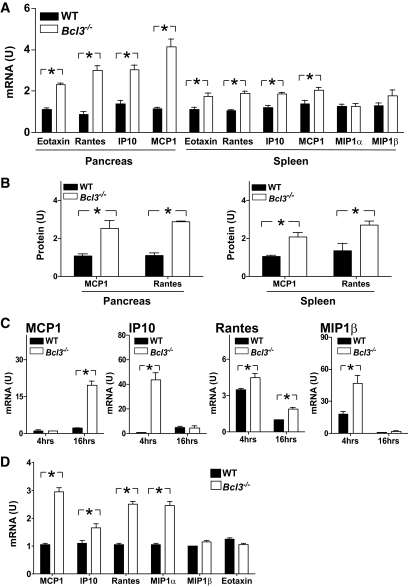
Chemokines play crucial roles in the development of type 1 diabetes. To determine whether Bcl3 gene mutation alters chemokine gene expression, we examined the levels of chemokines in the spleen and pancreas of Bcl3+/+ and Bcl3−/− NOD mice. We found that the mRNAs of MCP1, RANTES, IP10, and Eotaxin, but not those of MIP1a, MIP1b, MIP2, or CCR7, were significantly increased in the pancreata of Bcl3−/− mice, whereas those of RANTES and MIP1β were also increased in the spleens of Bcl3−/− mice (Fig. 5A and data not shown). As expected, proteins encoded by these genes were also increased in Bcl3−/− mice (Fig. 5B). Similarly, increased expression of chemokines was also found in Bcl3−/− CD4+ T-cells treated with anti-CD3 antibody (Fig. 5C) and Bcl3−/− insulin-specific T-cells (Fig. 5D). Although IP10, RANTES, and MIP1b were early response genes, MCP1 was a late response gene under these experimental conditions. These results indicate that Bcl-3 plays a crucial role in controlling chemokine gene expression in type 1 diabetes.
FIG. 5.
Increased inflammatory chemokine expression in Bcl3−/− mice. A: Total RNA was extracted from the pancreata and spleens of 6- to 8-week-old Bcl3+/+ and Bcl3−/− NOD mice (n = 3 mice). Chemokine expression was evaluated by real-time PCR. B: Chemokine levels in the splenic and pancreatic extracts of 6- to 8-week-old Bcl3+/+ and Bcl3−/− NOD mice (n = 3 mice) were tested by immunoblotting and quantified by desitometry using β-actin as a control. C and D: CD4+ T-cells (C) or splenocyte (D) were isolated from 6- to 8-week-old Bcl3+/+ and Bcl3−/− mice and stimulated with plate-bound anti-CD3 (2 μg/ml) for 4 and 16 h (C) or insulin (50 μg/ml) for 16 h (D). Chemokine expression was evaluated by real-time RT-PCR. Data presented are representative of two independent experiments. U, arbitrary unit. *P < 0.01.
Repression of cytokine and chemokine gene promoters by Bcl-3.
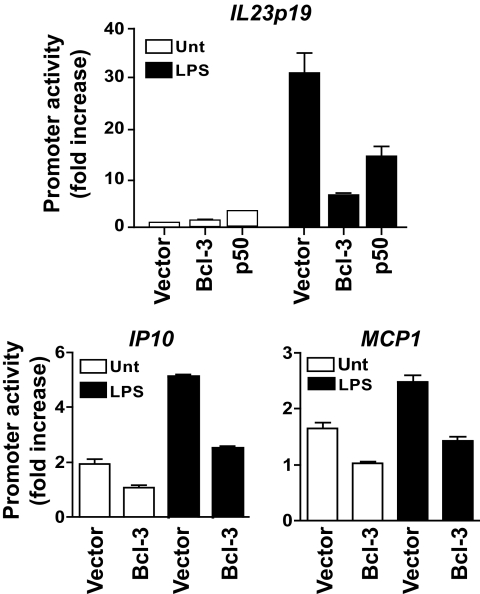
Bcl-3 has been reported to be able to either increase or decrease gene promoter activities. To determine the effect of Bcl-3 on the promoters of genes involved in type 1 diabetes, we next performed promoter luciferase reporter assays. RAW264.7 cells were transfected with IL23p19, IP10, or MCP1 luciferase reporter construct, together with a Bcl-3 expression construct. We found that Bcl-3 transfection significantly decreased the promoter activities of all these genes (Fig. 6). These results indicate that Bcl-3 is able to repress inflammatory cytokine and chemokine gene promoters.
FIG. 6.
Bcl-3 represses the promoter activities of IL23p19, IP10, and MCP1 genes. RAW264.7 cells were transiently transfected with IL23p19, IP10, and MCP1 promoter-luciferase constructs and the expression vector for full-length Bcl-3 or p50, or the empty vector, as indicated. After 24 h, cells were treated with or without lipopolysaccharide for 8 h and the luciferase activities were measured. The promoter activity is presented as fold increase over cells transfected with Bcl-3 plasmid but not treated with lipopolysaccharide. All data were normalized for transfection efficiency by dividing the activity of the firefly luciferase by that of the Renilla luciferase. Data are representative of three independent experiments.
Bcl-3 expressed by hematopoietic cells versus that expressed by nonhematopoietic cells in type 1 diabetes.
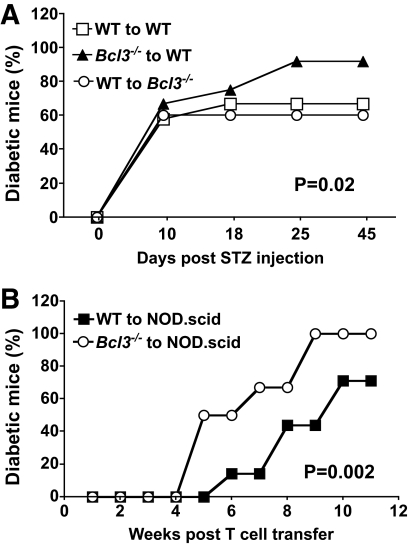
Bcl-3 is constitutively expressed by both hematopoietic and nonhematopoietic cells. Bcl-3 expressed by nonhematopoietic cells is important for lymphoid organogenesis, but its role in autoimmunity is not clear. Experiments presented in Fig. 1 do not directly address this issue because Bcl-3 deficiency involved all cell lineages. To elucidate lineage-specific roles of Bcl-3 in type 1 diabetes, an experimental system in which Bcl-3 is selectively depleted in one cell type but not others is needed. This can be created by generating bone marrow chimeric mice in which hematopoietic and nonhematopoietic cells are generated from different progenitor cells. Thus, chimeric mice were generated by injecting Bcl3+/+ or Bcl3−/− bone marrow cells into irradiated Bcl3+/+ or Bcl3−/− recipients (Fig. 7A). Mice were then injected with low-dose streptozotocin to induce diabetes. Bcl-3 deficiency in hematopoietic cells significantly exacerbated the diabetes, whereas Bcl-3 deficiency in nonhematopoietic cells had no effect on the disease (Fig. 7A). The accumulated incidence of diabetes was increased from 67% in mice that received wild-type bone marrow cells to 92% in mice that received Bcl3−/− bone marrow cells (P = 0.02). Similarly, upon adoptive transfer into NOD.scid mice, purified Bcl3−/− T- cells were significantly more effective than wild-type T- cells in inducing spontaneous type 1 diabetes (Fig. 7B). Taken together, these results indicate that Bcl-3 expressed by hematopoietic cells, but not that expressed by nonhematopoietic cells, prevents autoimmune diabetes.
FIG. 7.
Bcl-3 deficiency in hematopoietic cells exacerbates type 1 diabetes. A: Bone marrow chimeric B6 mice were generated by injecting wild-type or Bcl3−/− bone marrow cells into wild-type or Bcl3−/− mice (n = 12) as described in research design and methods. Eight weeks later, mice were injected with low-dose streptozotocin to induce diabetes. Mice were monitored for the development of diabetes for 45 days. Results are representative of two independent experiments. B: A quantity of 1.5 × 107 splenic T-cells isolated from wild-type or Bcl3−/− NOD mice were injected into NOD.scid mice (n = 6–7) through the tail vein. Mice were monitored for the development of spontaneous diabetes for 11 weeks. Results are representative of two independent experiments.
Normal central tolerance in Bcl3−/− mice.
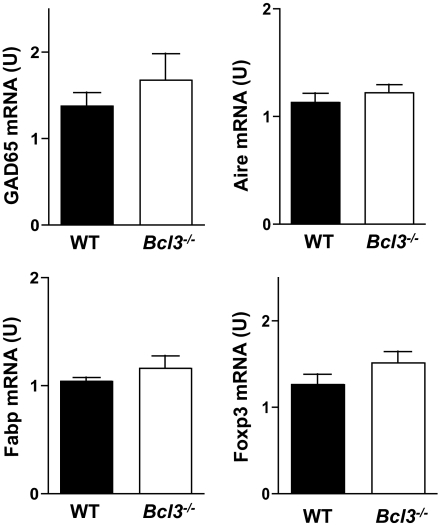
Mice doubly deficient in Bcl-3 and NF-κBp52 have a severe defect in thymic tolerance because of an arrest in thymic stroma development and reduced self-antigen expressions in the thymus, which can be corrected by wild-type thymic transplants (26). Mice deficient in only Bcl-3 or NF-κBp52 do not suffer from thymic developmental disorders. Consistent with this finding, quantitative PCR analysis of thymus revealed that Bcl-3 deficiency did not affect GAD65, fatty acid binding protein, or Aire expression (Fig. 8), indicating that the medullary thymic epithelial cells of Bcl3−/− mice developed normally. Furthermore, Foxp3 expression was not changed in Bcl3−/− thymus (Fig. 8) or anti-CD3–treated Bcl3−/− CD4+ T-cells (data not shown), indicating that Bcl-3 deficiency may not affect Treg cell development.
FIG. 8.
Normal thymic autoantigen, Aire, and Foxp3 expression in Bcl3−/− NOD mice. Total RNA was extracted from the thymus of 4- to 6-week-old wild-type and Bcl3−/− NOD mice (n = 3). Quantitative real-time PCR was used to determine the levels of GAD65, Fabp, Aire, and Foxp3 mRNA.
DISCUSSION
The relationship between immunodeficiency and autoimmunity is poorly understood. Our finding that Bcl-3–deficient mice are more susceptible to autoimmune diabetes despite their immunodeficient status provides a rare opportunity to address this issue. On one hand, Bcl-3 is required for lymphoid organogenesis, and in its absence, mice suffer from immunodeficiency as a result of disrupted lymphoid organ microarchitecture. On the other hand, as revealed from studies reported here, Bcl-3 is also required for inhibiting inflammatory chemokine and Th17-type cytokine expression. Bone marrow chimeric experiments indicate that the immunodeficient status of Bcl3−/− mice resulted from Bcl-3 deficiency in stromal cells (25), whereas increased autoimmunity was caused by Bcl-3 deficiency in hematopoietic cells (Fig. 7). Thus, by acting through two different cell lineages, Bcl-3 controls both lymphoid organogenesis and autoimmunity.
Bcl-3 has been reported to mediate IL-10–induced anti-inflammatory effect by suppressing the expression of IL-23p19 (32). Lipopolysaccharide-induced Bcl-3 expression was strongly impaired in IL10−/− cells, which had enhanced RelA binding to the IL23p19 promoter. Bcl-3 overexpression decreased lipopolysaccharide-induced IL-23p19 gene expression (32). This is consistent with our demonstration that Bcl-3 controlled the promoter activity of IL23p19. Bcl-3 has also been previously reported to regulate Th2 cell responses in anti-CD3–treated cultures (33). Consistent with that report, we observed reduced IL-4 production in anti-CD3–treated Bcl3−/− T-cell cultures (data not shown). However, GAD65-induced IL-4 production was not affected by Bcl-3 deficiency (Fig. 3A), indicating that the effect of Bcl-3 on Th2 cytokine expression could be stimulus-specific.
Understanding the molecular mechanisms of type 1 diabetes requires investigation of at least two types of genes: the susceptibility genes and the pathogenic genes. The susceptibility genes are those that dictate who develops type 1 diabetes, whereas the pathogenic genes mediate the insulitic process in those individuals who develop the disease, although certain genes may fall into both categories. Because type 1 diabetes is a multigenic disease heavily influenced by environmental factors, correcting the genetic defect in patients may have a limited impact on the disease. By contrast, halting the function of pathogenic genes may be more effective for controlling the disease. Indeed, most immune therapeutic strategies developed for type 1 diabetes target pathogenic genes or their products. The NF-κB family of transcription factors represents one of the most attractive targets for anti-inflammatory therapy. Because NF-κB directly controls the expression of multiple inflammatory genes, its blockade is more effective for controlling inflammation than blocking one or a few downstream inflammatory genes or proteins. The first-generation Rel/NF-κB drugs that block the entire Rel/NF-κB family have already been tested in both humans and animals. These include proteasome inhibitors (e.g., the FDA-approved PS-341), NF-κB decoy oligodeoxynucleotides, and the nemo-binding domain peptides, which are highly effective in preventing and treating models of autoimmune diseases (9–11,34–40). Additionally, glucocorticoids, which are currently used to control acute inflammation, mediate their immunosuppressive effects, at least in part, through inhibiting NF-κB (glucocorticoids upregulate IκB expression and bind directly to NF-κB). Results reported here indicate that Bcl-3, a natural regulator of NF-κB, is critical for preventing type 1 diabetes, and therefore may be harnessed to control the disease. However, the precise molecular mechanisms through which Bcl-3 inhibits type 1 diabetes need to be further investigated. Although Bcl-3 may directly repress the promoters of IL23p19, IP10, and MCP1 genes as we showed in this study, whether it does so to other target genes tested here is not clear. Additionally, although the increased production of IL-17 and chemokines correlated with the increased incidence of diabetes in Bcl3−/− mice, whether and to what degree these molecules contribute to type 1 diabetes in this model remain to be established. The latter issue may be difficult to address because multiple cytokines and chemokines are likely regulated by Bcl-3 in this model.
NOD mice spontaneously develop autoimmune diabetes that shares many immunologic and pathologic features with human type 1 diabetes (2,41). The disease is characterized by mononuclear cell infiltration and subsequent destruction of the pancreatic islets. Both lymphocytes and myeloid cells play important roles in initiating and propagating the autoimmune process. The incidence of the disease is higher in female NOD mice than in males, although it is heavily influenced by environmental conditions. The cyclophosphamide-induced diabetes in NOD mice used in this study is an accelerated model of type 1 diabetes. The disease can be precipitated by a single injection of cyclophosphamide in young nondiabetic NOD mice. Similar to spontaneous diabetes, it shares many clinical and histologic features with human type 1 diabetes and requires the participation of both T-cells and macrophages (42,43). Low-dose streptozotocin-induced diabetes is another animal model for type 1 diabetes. Diabetes can be induced in susceptible strains of mice or rats by multiple injections of low doses of streptozotocin. Low-dose streptozotocin-induced diabetes also shares many clinical and histologic features with human type 1 diabetes and may require the participation of both lymphocytes and macrophages. However, the degree to which lymphocytes contribute to diabetes in this model is not well understood. It has been reported that mice deficient in T-cells did not develop low-dose streptozotocin-induced diabetes and that splenocytes from low-dose streptozotocin-administered mice were able to transfer the disease to normal or immune-deficient recipients. On the other hand, low-dose streptozotocin has also been reported to induce diabetes in severe combined immunodeficient (SCID) mice that have myeloid but not lymphoid cells (44,45). However, scid gene mutation also increases the sensitivity of the cells to apoptosis. Therefore, further studies are required to elucidate the pathogenic mechanisms of diabetes in this model. STZ may release nitric oxide (NO) upon degradation, which can directly induce β-cell apoptosis. This may in turn trigger the activation of macrophages and islet-specific T-cells, leading to the destruction of more islet cells. Results reported here indicate that Bcl-3 plays a crucial role in both the NOD and the B6 models of type 1 diabetes. Therefore, a role of Bcl-3 in the development of human type 1 diabetes needs to be determined.
ACKNOWLEDGMENTS
This study was supported by grants from the National Institutes of Health (GM0 85112, AI 50059, DK0 70691, and AI0 69289).
No potential conflicts of interest relevant to this article were reported.
Q.R. researched data and wrote the manuscript. S.-J.Z., S.P., and R.J.C. researched data and contributed to the discussion. Y.H.C. supervised the project and edited the manuscript.
The authors thank Dr. Roland Tisch (University of North Carolina) for the GAD65 peptide.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1. Santamaria P. Effector lymphocytes in autoimmunity. Curr Opin Immunol 2001;13:663–669 [DOI] [PubMed] [Google Scholar]
- 2. Thomas HE, Kay TW. β Cell destruction in the development of autoimmune diabetes in the non-obese diabetic (NOD) mouse. Diabete Metab Res Rev 2000;16:251–261 [DOI] [PubMed] [Google Scholar]
- 3. Bierhaus A, Schiekofer S, Schwaninger M, Andrassy M, Humpert PM, Chen J, Hong M, Luther T, Henle T, Kloting I, Morcos M, Hofmann M, Tritschler H, Weigle B, Kasper M, Smith M, Perry G, Schmidt AM, Stern DM, Haring HU, Schleicher E, Nawroth PP. Diabetes-associated sustained activation of the transcription factor nuclear factor-κB. Diabetes 2001;50:2792–2808 [DOI] [PubMed] [Google Scholar]
- 4. Weaver DJ, Jr, Poligone B, Bui T, Abdel-Motal UM, Baldwin AS, Jr, Tisch R. Dendritic cells from nonobese diabetic mice exhibit a defect in NF-κ B regulation due to a hyperactive IκB kinase. J Immunol 2001;167:1461–1468 [DOI] [PubMed] [Google Scholar]
- 5. Poligone B, Weaver DJ, Jr, Sen P, Baldwin AS, Jr, Tisch R. Elevated NF-κB activation in nonobese diabetic mouse dendritic cells results in enhanced APC function. J Immunol 2002;168:188–196 [DOI] [PubMed] [Google Scholar]
- 6. Guo D, Li M, Zhang Y, Yang P, Eckenrode S, Hopkins D, Zheng W, Purohit S, Podolsky RH, Muir A, Wang J, Dong Z, Brusko T, Atkinson M, Pozzilli P, Zeidler A, Raffel LJ, Jacob CO, Park Y, Serrano-Rios M, Larrad MT, Zhang Z, Garchon HJ, Bach JF, Rotter JI, She JX, Wang CY. A functional variant of SUMO4, a new IκBα modifier, is associated with type 1 diabetes. Nat Genet 2004;36:837–841 [DOI] [PubMed] [Google Scholar]
- 7. Lamhamedi-Cherradi SE, Zheng S, Hilliard BA, Xu L, Sun J, Alsheadat S, Liou HC, Chen YH. Transcriptional regulation of type I diabetes by NF-κB. J Immunol 2003;171:4886–4892 [DOI] [PubMed] [Google Scholar]
- 8. Mabley JG, Hasko G, Liaudet L, Soriano F, Southan GJ, Salzman AL, Szabo C. NFκB1 (p50)-deficient mice are not susceptible to multiple low-dose streptozotocin-induced diabetes. J Endocrinol 2002;173:457–464 [DOI] [PubMed] [Google Scholar]
- 9. Ho E, Chen G, Bray TM. Supplementation of N-acetylcysteine inhibits NFκB activation and protects against alloxan-induced diabetes in CD-1 mice. FASEB J 1999;13:1845–1854 [PubMed] [Google Scholar]
- 10. Ma L, Qian S, Liang X, Wang L, Woodward JE, Giannoukakis N, Robbins PD, Bertera S, Trucco M, Fung JJ, Lu L. Prevention of diabetes in NOD mice by administration of dendritic cells deficient in nuclear transcription factor-κB activity. Diabetes 2003;52:1976–1985 [DOI] [PubMed] [Google Scholar]
- 11. Ho E, Chen G, Bray TM. Alpha-phenyl-tert-butylnitrone (PBN) inhibits NFκB activation offering protection against chemically induced diabetes. Free Radic Biol Med 2000;28:604–614 [DOI] [PubMed] [Google Scholar]
- 12. Carmody RJ, Chen YH. Nuclear factor-κB: activation and regulation during toll-like receptor signaling. Cell Mol Immunol 2007;4:31–41 [PubMed] [Google Scholar]
- 13. Lenardo MJ, Baltimore D. NF-κB: a pleiotropic mediator of inducible and tissue-specific gene control. Cell 1989;58:227–229 [DOI] [PubMed] [Google Scholar]
- 14. Gilmore TD. Introduction to NF-κB: players, pathways, perspectives. Oncogene 2006;25:6680–6684 [DOI] [PubMed] [Google Scholar]
- 15. Inoue J, Takahara T, Akizawa T, Hino O. Bcl-3, a member of the IκB proteins, has distinct specificity towards the Rel family of proteins. Oncogene 1993;8:2067–2073 [PubMed] [Google Scholar]
- 16. Nolan GP, Fujita T, Bhatia K, Huppi C, Liou HC, Scott ML, Baltimore D. The bcl-3 proto-oncogene encodes a nuclear IκB-like molecule that preferentially interacts with NF-κB p50 and p52 in a phosphorylation-dependent manner. Mol Cell Biol 1993;13:3557–3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bates PW, Miyamoto S. Expanded nuclear roles for IκBs. Sci STKE 2004:pe48. [DOI] [PubMed] [Google Scholar]
- 18. Yamamoto M, Yamazaki S, Uematsu S, Sato S, Hemmi H, Hoshino K, Kaisho T, Kuwata H, Takeuchi O, Takeshige K, Saitoh T, Yamaoka S, Yamamoto N, Yamamoto S, Muta T, Takeda K, Akira S. Regulation of Toll/IL-1-receptor-mediated gene expression by the inducible nuclear protein IκBζ. Nature 2004;430:218–222 [DOI] [PubMed] [Google Scholar]
- 19. Hatada EN, Nieters A, Wulczyn FG, Naumann M, Meyer R, Nucifora G, McKeithan TW, Scheidereit C. The ankyrin repeat domains of the NF-κB precursor p105 and the protooncogene bcl-3 act as specific inhibitors of NF-κB DNA binding. Proc Natl Acad Sci U S A 1992;89:2489–2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kerr LD, Duckett CS, Wamsley P, Zhang Q, Chiao P, Nabel G, McKeithan TW, Baeuerle PA, Verma IM. The proto-oncogene bcl-3 encodes an IκB protein. Genes Dev 1992;6:2352–2363 [DOI] [PubMed] [Google Scholar]
- 21. Wulczyn FG, Naumann M, Scheidereit C. Candidate proto-oncogene bcl-3 encodes a subunit-specific inhibitor of transcription factor NF-κB. Nature 1992;358:597–599 [DOI] [PubMed] [Google Scholar]
- 22. Bours V, Franzoso G, Azarenko V, Park S, Kanno T, Brown K, Siebenlist U. The oncoprotein Bcl-3 directly transactivates through κB motifs via association with DNA-binding p50B homodimers. Cell 1993;72:729–739 [DOI] [PubMed] [Google Scholar]
- 23. Franzoso G, Carlson L, Scharton-Kersten T, Shores EW, Epstein S, Grinberg A, Tran T, Shacter E, Leonardi A, Anver M, Love P, Sher A, Siebenlist U. Critical roles for the Bcl-3 oncoprotein in T cell-mediated immunity, splenic microarchitecture, and germinal center reactions. Immunity 1997;6:479–490 [DOI] [PubMed] [Google Scholar]
- 24. Schwarz EM, Krimpenfort P, Berns A, Verma IM. Immunological defects in mice with a targeted disruption in Bcl-3. Genes Dev 1997;11:187–197 [DOI] [PubMed] [Google Scholar]
- 25. Poljak L, Carlson L, Cunningham K, Kosco-Vilbois MH, Siebenlist U. Distinct activities of p52/NF-κB required for proper secondary lymphoid organ microarchitecture: functions enhanced by Bcl-3. J Immunol 1999;163:6581–6588 [PubMed] [Google Scholar]
- 26. Zhang X, Wang H, Claudio E, Brown K, Siebenlist U. A role for the IκB family member Bcl-3 in the control of central immunologic tolerance. Immunity 2007;27:438–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wessells J, Baer M, Young HA, Claudio E, Brown K, Siebenlist U, Johnson PF. BCL-3 and NF-κB p50 attenuate lipopolysaccharide-induced inflammatory responses in macrophages. J Biol Chem 2004;279:49995–50003 [DOI] [PubMed] [Google Scholar]
- 28. Carmody RJ, Ruan Q, Palmer S, Hilliard B, Chen YH. Negative regulation of toll-like receptor signaling by NF-κB p50 ubiquitination blockade. Science 2007;317:675–678 [DOI] [PubMed] [Google Scholar]
- 29. Kunikata T, Yamane H, Segi E, Matsuoka T, Sugimoto Y, Tanaka S, Tanaka H, Nagai H, Ichikawa A, Narumiya S. Suppression of allergic inflammation by the prostaglandin E receptor subtype EP3 [see comment]. Nat Immunol 2005;6:524–531 [DOI] [PubMed] [Google Scholar]
- 30. Carmody RJ, Ruan Q, Liou H-C, Chen YH. Essential roles of c-Rel in Toll-like receptor-induced interleukin-23 p19 gene expression in dendritic cells. J Immunol 2007;178:186–191 [DOI] [PubMed] [Google Scholar]
- 31. Carmody RJ, Hilliard B, Maguschak K, Chodosh LA, Chen YH. Genomic scale profiling of autoimmune inflammation in the central nervous system: the nervous response to inflammation. J Neuroimmunol 2002;133:95–107 [DOI] [PubMed] [Google Scholar]
- 32. Muhlbauer M, Chilton PM, Mitchell TC, Jobin C. Impaired Bcl3 up-regulation leads to enhanced lipopolysaccharide-induced interleukin (IL)-23P19 gene expression in IL-10(−/−) mice. J Biol Chem 2008;283:14182–14189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Corn RA, Hunter C, Liou HC, Siebenlist U, Boothby MR. Opposing roles for RelB and Bcl-3 in regulation of T-box expressed in T cells, GATA-3, and Th effector differentiation. J Immunol 2005;175:2102–2110 [DOI] [PubMed] [Google Scholar]
- 34. Rehman K, Bertera S, Bottino R, Balamurugan A, Mai J, Mi Z, Trucco M, Robbins P. Protection of islets by in situ peptide-mediated transduction of the IκB kinase inhibitor Nemo-binding domain peptide. J Biol Chem 2003;278:9862–9868 [DOI] [PubMed] [Google Scholar]
- 35. May MJ, D'Acquisto F, Madge LA, Glockner J, Pober JS, Ghosh S. Selective inhibition of NF-κB activation by a peptide that blocks the interaction of NEMO with the IκB kinase complex. Science 2000;289:1550–1554 [DOI] [PubMed] [Google Scholar]
- 36. Ethridge RT, Hashimoto K, Chung DH, Ehlers RA, Rajaraman S, Evers BM. Selective inhibition of NF-κB attenuates the severity of cerulein-induced acute pancreatitis. J Am Coll Surg 2002;195:497–505 [DOI] [PubMed] [Google Scholar]
- 37. Thomas RP, Farrow BJ, Kim S, May MJ, Hellmich MR, Evers BM. Selective targeting of the nuclear factor-κB pathway enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated pancreatic cancer cell death. Surgery 2002;132:127–134 [DOI] [PubMed] [Google Scholar]
- 38. Dasgupta S, Jana M, Zhou Y, Fung YK, Ghosh S, Pahan K. Antineuroinflammatory effect of NF-κB essential modifier-binding domain peptides in the adoptive transfer model of experimental allergic encephalomyelitis. J Immunol 2004;173:1344–1354 [DOI] [PubMed] [Google Scholar]
- 39. Vanderlugt CL, Rahbe SM, Elliott PJ, Dal Canto MC, Miller SD. Treatment of established relapsing experimental autoimmune encephalomyelitis with the proteasome inhibitor PS-519. J Autoimmun 2000;14:205–211 [DOI] [PubMed] [Google Scholar]
- 40. Suzuki J, Isobe M. NFκB decoy for clinical applications [in Japanese]. Nippon Rinsho 2003;61:1829–1835 [PubMed] [Google Scholar]
- 41. Delovitch TL, Singh B. The nonobese diabetic mouse as a model of autoimmune diabetes: immune dysregulation gets the NOD. Immunity 1997;7:727–738 [DOI] [PubMed] [Google Scholar]
- 42. Kolb-Bachofen V, Epstein S, Kiesel U, Kolb H. Low-dose streptozocin-induced diabetes in mice. Electron microscopy reveals single-cell insulitis before diabetes onset. Diabetes 1988;37:21–27 [DOI] [PubMed] [Google Scholar]
- 43. Harada M, Makino S. Promotion of spontaneous diabetes in non-obese diabetes-prone mice by cyclophosphamide. Diabetologia 1984;27:604–606 [DOI] [PubMed] [Google Scholar]
- 44. Reddy S, Wu D, Elliott RB. Low dose streptozotocin causes diabetes in severe combined immunodeficient (SCID) mice without immune cell infiltration of the pancreatic islets. Autoimmunity 1995;20:83–92 [DOI] [PubMed] [Google Scholar]
- 45. Gerling IC, Friedman H, Greiner DL, Shultz LD, Leiter EH. Multiple low-dose streptozocin-induced diabetes in NOD-scid/scid mice in the absence of functional lymphocytes. Diabetes 1994;43:433–440 [DOI] [PubMed] [Google Scholar]