Abstract
OBJECTIVE
Given the pleiotropic effect of eicosapentaenoic acid (EPA), it is interesting to know whether EPA is capable of improving obesity. Here we examined the anti-obesity effect of EPA in mice with two distinct models of obesity.
RESEARCH DESIGN AND METHODS
Male C57BL/6J mice were fed a high-fat/high-sucrose diet (25.0% [w/w] fat, 32.5% [w/w] sucrose) (HF/HS group) or a high-fat diet (38.1% [w/w] fat, 8.5% [w/w] sucrose) (HF group) for 4–20 weeks. A total of 5% EPA was administered by partially substituting EPA for fat in the HF/HS + EPA and HF + EPA groups.
RESULTS
Both the HF/HS and HF groups similarly developed obesity. EPA treatment strongly suppresses body weight gain and obesity-related hyperglycemia and hyperinsulinemia in HF/HS-fed mice (HF/HS + EPA group), where hepatic triglyceride content and lipogenic enzymes are increased. There is no appreciable effect of EPA on body weight in HF-fed mice (HF + EPA group) without enhanced expression of hepatic lipogenic enzymes. Moreover, EPA is capable of reducing hepatic triglyceride secretion and changing VLDL fatty acid composition in the HF/HS group. By indirect calorimetry analysis, we also found that EPA is capable of increasing energy consumption in the HF/HS + EPA group.
CONCLUSIONS
This study is the first demonstration that the anti-obesity effect of EPA in HF/HS-induced obesity is associated with the suppression of hepatic lipogenesis and steatosis. Because the metabolic syndrome is often associated with hepatic lipogenesis and steatosis, the data suggest that EPA is suited for treatment of the metabolic syndrome.
The metabolic syndrome has been defined as a cluster of visceral fat obesity, impaired glucose metabolism, atherogenic dyslipidemia (high plasma triglyceride and low HDL cholesterol), and hypertension (1). There is considerable evidence that visceral fat obesity is a key etiological factor in the metabolic syndrome (2). Enhanced hepatic lipogenesis and hepatic steatosis also appear to play an important role in the pathogenesis of the metabolic syndrome (3). Indeed, nonalcoholic fatty liver disease may constitute the common features of the metabolic syndrome.
Numerous epidemiological studies and clinical trials have revealed that fish oil and n-3 polyunsaturated fatty acids (PUFAs) reduce the risk of coronary heart disease (4). Eicosapentaenoic acid (EPA), one of the major n-3 PUFAs contained in fish oil, has a variety of pharmacological effects such as lipid-lowering (5), anti-platelet (6), anti-inflammatory (7), and anti-atherogenic effects (8,9). Recently, the Japan EPA Lipid Intervention Study (JELIS), a large-scale prospective randomized clinical trial, demonstrated that EPA delays the onset of cardiovascular events via cholesterol-independent mechanisms (10,11), but the molecular mechanisms remain to be elucidated. In a recent sub-analysis of the JELIS, EPA had a great risk reduction of coronary artery events of 53% in patients with high triglycerides and low HDL cholesterol (11), suggesting that EPA may be effective to reduce the incidence of atherosclerosis in the metabolic syndrome. These findings are supported by our recent observations that EPA administration results in decreases in remnant-like particle-triglyceride, small dense LDL, and C-reactive protein and an increase in adiponectin in patients with the metabolic syndrome (12,13).
Given the pleiotropic effect of EPA, it is interesting to know whether highly purified EPA is capable of improving obesity. There is currently a controversy as to the anti-obesity effect of EPA; it has been effective (13,14), has been ineffective (15), or has even increased visceral fat accumulation (16). On the other hand, it is noteworthy that EPA suppresses hepatic lipogenesis and steatosis by reducing mRNA and active protein of sterol regulatory element binding protein-1c (SREBP-1c) (17–19). We, therefore, examined the impact of hepatic lipogenesis on the anti-obesity effect of highly purified EPA.
Here, we demonstrate that EPA strongly suppresses body weight gain and obesity-related hyperglycemia and hyperinsulinemia in high-fat (HF)/high-sucrose (HS)-induced obese mice with enhanced hepatic lipogenesis but not in HF-induced obese mice without enhanced hepatic lipogenesis. This study is the first demonstration that the anti-obesity effect of EPA is related to the suppression of hepatic lipogenesis. Given that the metabolic syndrome is often associated with hepatic lipogenesis and steatosis, the data of this study suggest that EPA is suited for the treatment of the metabolic syndrome.
RESEARCH DESIGN AND METHODS
Highly purified EPA ethyl ester (purity >98%) was obtained from Nippon Suisan Kaisha (Tokyo, Japan). Ethyl palmitate (purity >95%) was purchased from Wako (Tokyo). Nine-week-old male C57BL/6J mice were obtained from CLEA Japan (Tokyo) and acclimated for 1 week before the experiment. Mice were housed under controlled temperature and lighting (0730–1930 light, 1930–0730 dark cycle) with free access to water and a fish meal-free diet (fish meal-free F1: 4.4% fat; Funabashi Farm, Funabashi, Japan). All experiments were carried out in accordance with the guidelines for the use and care of laboratory animals of Mochida Pharmaceutical (numbers 1523, 1524, 2354, and 2389).
Diets.
The outline of experiments and composition of diets are in supplementary Tables 1 and 2, respectively (available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db09-1554/DC1).
Plasma analysis.
Blood samples were collected via the retro-orbital sinus of nonfasted mice under light anesthesia every 2 weeks. Plasma glucose, total cholesterol, triglycerides, free fatty acid, and insulin were determined by commercially available kits. Plasma concentrations of leptin and adiponectin were measured with the respective enzyme-linked immunosorbent assay kits (leptin: Morinaga, Yokohama, Japan; adiponectin: R&D Systems, Minneapolis, MN).
Hepatic triglyceride content.
Liver lipids were extracted by the method of Folch et al. (20). Hepatic triglyceride content was measured with a reagent from Wako.
Gene expression analysis.
Expression levels of some genes were determined by quantitative real-time PCR using primers and probes shown in supplementary Table 3. Expression of other genes was determined using TaqMan gene expression assays (Applied Biosystems, Foster City, CA). The assay IDs of TaqMan (R) gene expression assays are shown in supplementary Table 4. 18s rRNA was measured using TaqMan (R) rRNA control reagents (Applied Biosystems) as a control.
Enzymatic activities.
Acyl-CoA oxidase, fatty acid synthase (FAS), and hydroxyacyl-CoA dehydrogenase activities were measured spectrophotometrically (21–23).
Cytoplasmic and nuclear protein extracts and Western blot analysis.
Cytoplasmic and nuclear proteins were extracted as described (24,25) with slight modifications. The samples were equally pooled from all the mice of each group (n = 7–10), and then 30 μg protein per lane was separated by SDS-PAGE. Western blot analysis was performed using anti–SREBP-1 antibody (H160; Santa Cruz Biotechnology, Santa Cruz, CA). We used anti–β-actin (Cell Signaling Technology, Beverly, MA) and anti-TATA binding protein (TBP) (Abcam, Cambridge, U.K.) antibodies as cytoplasmic and nuclear controls, respectively. The blots were visualized with the ECL Western blotting analysis system (GE Healthcare, Buckinghamshire, U.K.).
Indirect calorimetry analysis.
The volumes of consumed O2 (VO2) and produced CO2 (VCO2) were measured by indirect calorimetry using an Oxymax system (Columbus Instruments, Columbus, OH). The O2 and CO2 contents were recorded every 10 min from 1130 to 0900. Mice had unrestricted access to food and water. The respiratory exchange ratio (RER) was calculated by VCO2/VO2.
Glycerol release from white adipose tissue.
Glycerol release from the epididymal fat ex vivo was measured according to the method of Schweiger et al. (26) with slight modifications.
Hepatic triglyceride secretion rate.
A dose of 500 mg/kg tyloxapol (also known as Triton WR-1339; Sigma-Aldrich, St. Louis, MO) in saline was injected intravenously. Mice were fasted overnight before the injection. Immediately before and 1 h after the injection, blood samples were collected and plasma triglyceride concentrations were determined as described above. The hepatic triglyceride secretion rate was calculated as described by Steiner et al. (27).
Fatty acid composition of VLDL.
Blood samples were collected via the inferior vena cava of mice 4 h after the tyloxapol injection. Plasma was isolated, and VLDL fraction was obtained by ultracentrifugation, according to the method of Werner et al. (28). Fatty acid composition was analyzed by gas chromatography.
Statistical analysis.
Data are presented as means ± SE. ANOVA using a split-plot model was used for the line plots of indirect calorimetry analysis (Fig. 6C and E). Other data were assessed by a t test. P < 0.05 was considered a significant difference.
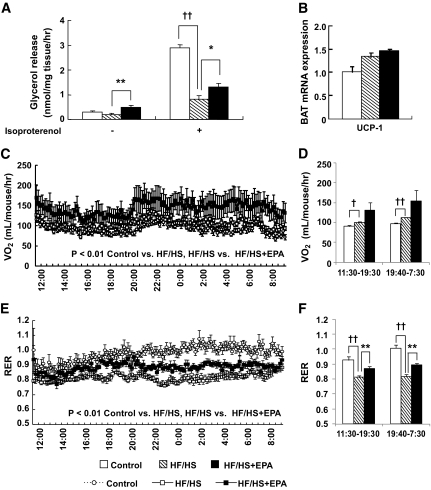
FIG. 6.
Effect of EPA on WAT lipolysis and energy consumption in the HS/HF group. A: Glycerol release from epididymal WAT with or without 10 μmol/l isoproterenol. B: UCP-1 mRNA expression in BAT. Indirect calorimetry analysis of VO2 (C and D) and RER (E and F). n = 9. ††P < 0.01 vs. control group. *P < 0.05; **P < 0.01 vs. HF/HS group.
RESULTS
Effect of EPA on HF/HS- and HF-induced obesity.
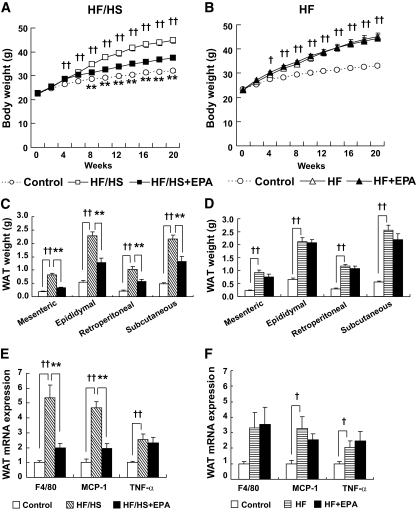
Both HF/HS and HF groups gained weight with increases in the mesenteric, epididymal, retroperitoneal, and subcutaneous white adipose tissue (WAT) weights relative to the control group after a 20-week feeding (experiments 1 and 2) (Fig. 1A–D). There were no appreciable differences between HF/HS and HF groups. The HF/HS-induced increases in body weight and WAT weights were markedly suppressed by treatment with EPA (HF/HS + EPA group). By contrast, EPA treatment did not affect an HF-induced increase in body weight and WAT weights (HF + EPA group). In the HF/HS group, obesity-induced WAT inflammation was evident, as revealed by elevated expression of mRNAs for F4/80, monocyte chemoattractant protein-1 (MCP-1), and tumor necrosis factor-α. EPA treatment markedly reduced F4/80 and MCP-1 mRNA expression in WAT (Fig. 1E). In WAT from the HF group, expression of F4/80, MCP-1, and tumor necrosis factor-α mRNAs was elevated, which was not affected by EPA (Fig. 1F). In this study, caloric intake in the HF/HS group was roughly equivalent to that in the HF/HS + EPA group (Table 1).
FIG. 1.
Effect of EPA on HF/HS- and HF-induced obesity. A and B: Body weight change. C and D: WAT weights. E and F: Proinflammatory gene expression in the epididymal WAT. n = 7–10. †P < 0.05; ††P < 0.01 vs. control group. **P < 0.01 vs. HF (/HS) group.
TABLE 1.
Food intake and plasma parameters in experiment 1 and 2 at 18 weeks of feeding
| Experiment 1 |
Experiment 2 |
|||||
|---|---|---|---|---|---|---|
| Control | HF/HS | HF/HS + EPA | Control | HF | HF + EPA | |
| Food intake (kcal/day/mouse) | 8.56 | 11.15 | 10.58 | 9.84 | 12.72 | 13.96 |
| Leptin (ng/ml) | 4.60 ± 0.82 | 44.60 ± 5.43†† | 15.46 ± 2.74** | 6.46 ± 0.67 | 47.02 ± 5.39†† | 36.24 ± 5.55 |
| Adiponectin (μg/ml) | 10.05 ± 0.59 | 10.78 ± 0.51 | 15.00 ± 1.45* | 10.85 ± 0.92 | 14.51 ± 0.76†† | 15.33 ± 1.03 |
| Total cholesterol (mg/dl) | 96.8 ± 5.8 | 236.4 ± 9.6†† | 104.6 ± 5.5** | 101.6 ± 3.7 | 193.6 ± 7.3†† | 109.2 ± 7.2** |
| Triglyceride (mg/dl) | 157.2 ± 9.2 | 105.6 ± 8.1†† | 80.6 ± 3.7* | 143.6 ± 11.6 | 113.2 ± 12.4 | 88.0 ± 4.6 |
| Free fatty acid (mEq/l) | 0.695 ± 0.060 | 0.771 ± 0.053 | 0.593 ± 0.038* | 0.670 ± 0.022 | 0.681 ± 0.059 | 0.663 ± 0.025 |
Data are means ± SE (n = 7–10), except for food intake. The results of the food intake are presented as means of two cages.
††P < 0.01 vs. the control group;
*P < 0.05;
**P < 0.01 vs. the HF (/HS) group.
Effect of EPA on HF/HS- and HF-induced metabolic abnormalities.
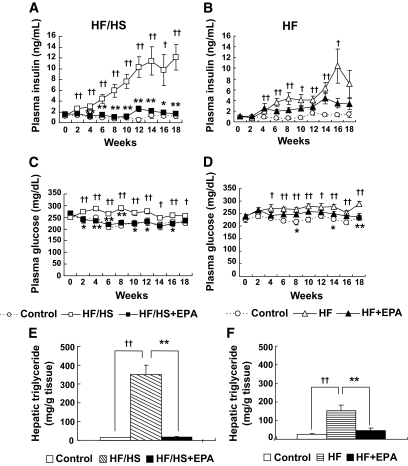
In experiment 1, the HF/HS group developed obvious hyperinsulinemia, hyperglycemia, and hepatic steatosis, which were significantly improved by EPA (HF/HS + EPA group) (Fig. 2A, C, and E). The liver weights after a 20-week feeding were 1.33 ± 0.03, 3.10 ± 0.30, and 1.42 ± 0.06 g in the control, HF/HS, and HF/HS + EPA groups, respectively (n = 10 for the control and HF/HS groups, n = 7 for the HF/HS + EPA group; P < 0.01 for the control group versus HF/HS group and the HF/HS group versus HF/HS + EPA group). Similarly, the HF group developed hyperglycemia and hyperinsulinemia; however, EPA had a marginal impact on HF-induced hyperglycemia and hyperinsulinemia (Fig. 2B and D). Although EPA treatment ameliorated hepatic steatosis in the HF group (Fig. 2F), liver weight was not decreased by EPA (data not shown). Plasma leptin concentrations were markedly elevated in the HF/HS group relative to the control group and were significantly reduced by EPA (P < 0.01) (Table 1). Although HF/HS did not affect plasma adiponectin concentration, EPA treatment resulted in an ∼1.5-fold elevation of plasma adiponectin (Table 1), which is consistent with our previous report (13).
FIG. 2.
Effect of EPA on HF/HS- and HF-induced metabolic abnormalities. A and B: Plasma insulin. C and D: Plasma glucose. E and F: Hepatic triglyceride content. †P < 0.05; ††P < 0.01 vs. control group. *P < 0.05; **P < 0.01 vs. HF (/HS) group.
Effect of EPA on gene expression in the HF/HS group.
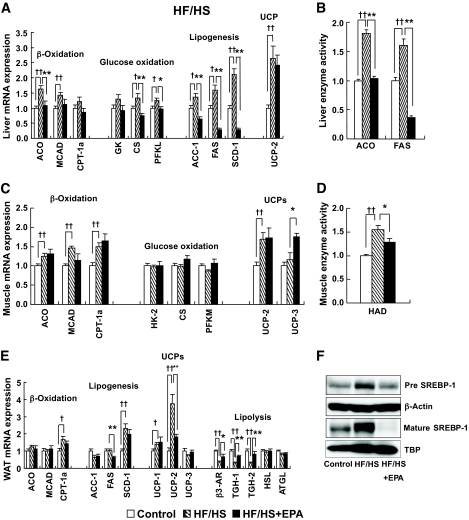
Enhanced expression of genes related to β-oxidation (29), uncoupling proteins (UCPs) (30), and glucose oxidation (31) are known to suppress obesity. However, they were not reduced or rather elevated in the liver and WAT from the HF/HS group relative to the control group (Fig. 3A and E). Moreover, in the skeletal muscle, except UCP-3, gene expression was not increased by EPA treatment (HF/HS + EPA group) relative to the HF/HS group (Fig. 3C). These observations suggest that enhancement of β-oxidation, UCPs, and glucose oxidation does not play a major role in the anti-obesity effect of EPA.
FIG. 3.
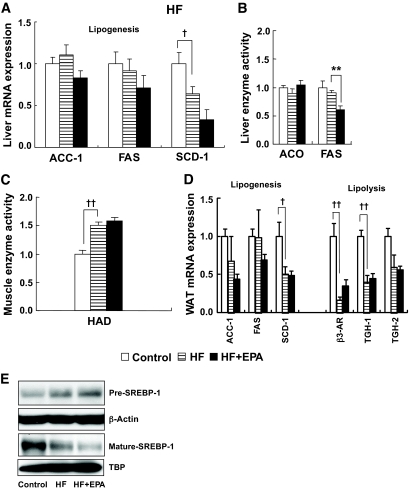
Effect of EPA on energy metabolism–related genes in the HF/HS groups. A and B: Liver. C and D: Skeletal muscle. E: Epididymal WAT. F: Hepatic SREBP-1 protein in the HF/HS group. n = 7–10. †P < 0.05; ††P < 0.01 vs. control group. *P < 0.05; **P < 0.01 vs. HF/HS group. ACO, acyl-CoA oxidase; ATGL, adipose triglyceride lipase; CPT-1a, carnitine palmitoyltransferase-1a; CS, citrate synthase; GK, glucokinase; HAD, hydroxyacyl-CoA dehydrogenase; HK-2, hexokinase-2; HSL, hormone-sensitive lipase; MCAD, acetyl-CoA dehydrogenase, medium chain; PFKL, phosphofructokinase, liver; PFKM, phosphofructokinase, muscle, B-type.
Enhanced lipolysis may promote the degradation of triglycerides accumulated in WAT, thereby leading to the suppression of obesity. Expression of mRNAs for hormone-sensitive lipase and adipose triglyceride lipase in WAT from the HF/HS group did not differ significantly from that in the control group. On the other hand, expression of mRNAs for the β3-adrenergic receptor (β3-AR), triglyceride hydrolase (TGH)-1, and TGH-2 in the HF/HS group was significantly lower than in the control group (P < 0.01), which was reversed in the HF/HS + EPA group (Fig. 3E).
It is also possible that enhanced lipogenesis promotes energy accumulation through triglyceride synthesis from diet-derived carbohydrates and fatty acids, thereby stimulating obesity and WAT accumulation. In WAT, there was no appreciable difference in acetyl-CoA carboxylase (ACC)-1 and FAS mRNA expression between the HF/HS and control groups. Expression of ACC-1 and FAS mRNAs was not increased in the HF/HS + EPA group relative to the HF/HS group. Stearoyl-CoA desaturase (SCD)-1 mRNA expression in the HF/HS group was elevated relative to the control group, which was unaffected by EPA treatment (Fig. 3E). In the liver, ACC-1, FAS, and SCD-1 mRNA expression in the HF/HS group was elevated relative to the control group, which was reduced by EPA treatment (HF/HS + EPA group) (Fig. 3A). In this study, hepatic SREBP-1 protein, a master regulator of lipogenic enzymes, was also increased in the HF/HS group, which was reduced by EPA treatment (Fig. 3F).
Effect of EPA on enzymatic activity in the HF/HS group.
In this study, elevated enzymatic activities of hepatic FAS and acyl-CoA oxidase in the HF/HS group were reduced by EPA treatment (Fig. 3B). Elevated activities of hydroxyacyl-CoA dehydrogenase in the skeletal muscle from the HF/HS group was also reduced by EPA treatment (Fig. 3D).
Effect of EPA on gene expression and enzymatic activity in the HF group.
We next examined mRNA levels and activities of lipogenic enzymes in the HF group, where EPA did not improve obesity and WAT accumulation (Fig. 1B and D). Notably, expression of mRNA for lipogenic enzymes and FAS activity in the liver were not elevated in the HF group relative to the control group (Fig. 4A and B). Moreover, the hepatic nuclear SREBP-1 protein was reduced by a HF diet (Fig. 4E), suggesting that HF diet induces obesity without enhancement of hepatic lipogenesis. Although EPA reduced FAS activity, mRNA of lipogenic enzymes was not affected by EPA (Fig. 4A and B). Treatment with EPA did not affect the activities of β-oxidation enzymes in the liver and muscle or the mRNA expression of lipogenesis or lipolysis-related proteins in WAT from the HF group (Fig. 4B–D). Expression of mRNAs for β3-AR and TGH-1 in WAT from the HF group was lower than in the control group. TGH-2 mRNA expression tended to be low relative to the control group, but the difference was not statistically significant (Fig. 4D).
FIG. 4.
Effect of EPA on energy metabolism–related genes in the HF groups. A and B: Liver. C: Skeletal muscle. D: Epididymal WAT. E: Hepatic SREBP-1 protein in the HF group. n = 10. †P < 0.05; ††P < 0.01 vs. control group. **P < 0.01 vs. HF group. ACO, acyl-CoA oxidase.
Effect of EPA on triglyceride secretion and VLDL fatty acid composition in the HF/HS group.
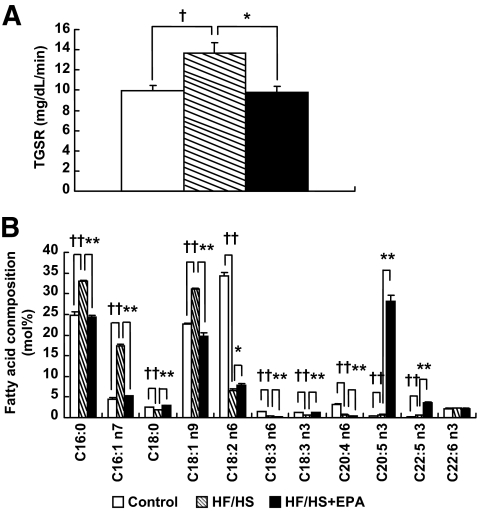
In the liver, triglycerides are synthesized via lipogenesis and secreted as VLDL, which in turn delivered to peripheral tissues such as WAT. Evidence has suggested that some species of fatty acids affect triglyceride accumulation in adipocytes (32). We therefore examined hepatic triglyceride secretion and VLDL fatty acid composition in the HF/HS group (experiment 3). We found the enhanced secretion of triglycerides in the HF/HS group relative to the control group (Fig. 5A). Analysis of VLDL fatty acid composition revealed that palmitic (C16:0), palmitoleic (C16:1 n-7), and oleic acids (C18:1 n-9) are increased and stearic acid (C18:0) is decreased in the HF/HS group relative to the control group (Fig. 5B). In this study, EPA (C20:5 n-3) was markedly increased in VLDL obtained from the HF/HS + EPA group relative to the HF/HS and control groups. Increased hepatic triglyceride secretion and the aberrant VLDL fatty acid composition in the HF/HS group were all reversed by EPA treatment (Fig. 5A and B). Linoleic acid (C18:2 n-6) was markedly decreased in both HF/HS and HF/HS + EPA groups relative to the control group.
FIG. 5.
Effect of EPA on triglyceride secretion rate (TGSR) and VLDL fatty acid composition in the HF/HS group. A: TGSR. B: VLDL fatty acid composition. n = 6. †P < 0.05; ††P < 0.01 vs. control group. *P < 0.05; **P < 0.01 vs. HF/HS group.
Effect of EPA on WAT lipolysis in the HF/HS group.
Because reduced expression of β3-AR, TGH-1, and TGH-2 mRNAs in WAT from the HF/HS group was restored by EPA treatment (Fig. 3E), we examined the effect of EPA on WAT lipolysis in the HF/HS group (experiment 4). There was no significant difference in glycerol release ex vivo between HF/HS and control groups (Fig. 6A). Treatment with isoproterenol, a β-AR agonist, stimulated glycerol release in the control group, which was markedly reduced in the HF/HS group. Interestingly, glycerol release with or without isoproterenol stimulation was higher in the HF/HS + EPA group than in the HF/HS group (Fig. 6A).
Effect of EPA on energy consumption in the HF/HS group.
There was a marked difference in energy accumulation between HF/HS and HF/HS + EPA groups, despite similar caloric intake (Fig. 1 and Table 1). However, except UCP-3 in the skeletal muscle, gene expression of β-oxidation enzymes and UCPs were unchanged by EPA treatment (Fig. 3A, C, and E). Furthermore, the activities of β-oxidation–related enzymes were reduced by EPA (Fig. 3B and D). We, therefore, examined the effect of EPA on O2 consumption and RER using indirect calorimetry (experiment 4). HF/HS feeding increased O2 consumption and significantly reduced RER 6 weeks after the experiment (Fig. 6C–F). In this study, EPA treatment significantly increased the HF/HS-induced O2 consumption and inhibited the HF/HS-induced decrease in RER throughout the experimental period (P < 0.01; 130 data points per mouse) (Fig. 6C and E). The mean values of RER for the light and dark cycles in the HF/HS + EPA group were significantly higher than those of the HF/HS group (P < 0.01) (Fig. 6F), whereas there was no significant difference in mean values of VO2 between HF/HS and HF/HS + EPA groups (Fig. 6D). EPA also significantly increased O2 consumption 2 weeks after the experiment (P < 0.01) (data not shown).
After 8 weeks of feeding, expression levels of mRNA of UCP-1 in brown adipose tissue (BAT) were unchanged by the HF/HS and HF/HS + EPA groups (Fig. 6B). There was no significant difference in UCP-3 mRNA expression in the skeletal muscle among control, HF/HS, and HF/HS + EPA groups (data not shown). EPA also suppressed body weight gain (control group, 28.47 ± 0.64 g; HF/HS group, 35.73 ± 0.99 g; HF/HS + EPA group, 30.78 ± 0.59 g) and epididymal WAT accumulation (control group, 0.36 ± 0.04 g; HF/HS group, 1.51 ± 0.14 g; HF/HS + EPA group, 0.80 ± 0.07 g) (experiment 4). There was no appreciable difference in caloric intake among control, HF/HS, and HF/HS + EPA groups (control group, 14.48 ± 2.03 kcal/day/mouse; HF/HS group, 13.76 ± 0.24 kcal/day/mouse; HF/HS + EPA group, 12.36 ± 0.67 kcal/day/mouse).
DISCUSSION
This study demonstrates that hepatic steatosis is more severe in the HF/HS group than in the HF group. Hyperinsulinemia also develops more rapidly in the HF/HS group than in the HF group, although both HF/HS and HF groups similarly develop obesity and WAT accumulation. In this study, we found that EPA ameliorates HF/HS-induced obesity, WAT inflammation, fatty liver, hyperinsulinemia, and hyperglycemia. By contrast, there is no effect of EPA on obesity in the HF group. These observations indicate the differential effect of EPA on metabolic parameters between HF/HS and HF groups. Expression and activities of hepatic lipogenic enzymes are increased in the HF/HS group, which are abolished by EPA treatment. By contrast, expression of hepatic lipogenic enzymes is not increased in the HF group, where EPA is ineffective against visceral fat accumulation and obesity. It seems that the lower expression of SREBP-1 in the HF/HS + EPA group results in the reduction of hepatic lipogenic enzymes. These observations, taken together, suggest that suppression of enhanced hepatic lipogenesis contributes to the anti-obesity effect of EPA. It is noteworthy that mice with liver-specific disruption of SCD-1 are resistant to high-carbohydrate diet–induced obesity but are sensitive to HF diet–induced obesity (33). In this study, we confirmed that EPA markedly reduces hepatic lipogenic enzymes including SCD-1. The phenotypic effect of liver-specific deficiency of SCD-1 is similar to that observed in this study, thereby supporting the concept that EPA exerts the anti-obesity effect at least in part through the suppression of hepatic lipogenesis.
Deficit of lipogenic enzymes or administration of lipogenic enzyme inhibitors has been reported to inhibit obesity. For instance, administration of FAS inhibitors lowers body weight by reducing food intake (34). Global deficiency of ACC-2 (35) or SCD-1 (36) or administration of antisense oligonucleotide against SCD-1 (37) also shows resistance to obesity through enhanced expression of β-oxidation enzymes or UCPs. On the other hand, neither anorectic effect nor upregulation of β-oxidation enzymes or UCPs has been reported with EPA. Then, how does reduced hepatic lipogenesis inhibit obesity or triglyceride accumulation in WAT? Here, we demonstrated that EPA markedly suppresses the HF/HS-induced hepatic triglyceride secretion and palmitic, palmitoleic, and oleic acids in VLDL. In this study, despite the enhanced hepatic lipogenesis and triglyceride secretion, plasma triglyceride concentrations are reduced in the HF/HS group relative to the control group. This may be because of HF/HS-induced activation of lipoprotein lipase in WAT, as suggested elsewhere (38). Previous studies with mice lacking VLDL receptor or apolipoprotein E demonstrated that VLDL metabolism is closely related to obesity (39,40). There are also several previous reports showing the relationship between triglycerides delivered from the liver and fat accumulation in the adipose tissue. For instance, obese subjects have exhibited enhanced VLDL-triglyceride secretion from the liver (41). Moreover, adenoviral overexpression of diacylglycerol-acyl transferase-1, which catalyzes the final step of hepatic triglyceride synthesis, has resulted in enhanced VLDL-triglyceride secretion and thus obesity (42). On the other hand, there is a report that triglyceride accumulation in WAT is enhanced by a certain species of fatty acids such as oleic and palmitic acids, among which, oleic acid tends to induce triglyceride accumulation in 3T3-L1 adipocytes (32). Moreover, plasma content of palmitoleic acid is positively correlated with obesity in humans (43,44). In this study, we showed that hepatic expression of SCD-1 is enhanced in the HF/HS group and is markedly suppressed by EPA. SCD-1 is known to catalyze the conversion from stearic and palmitic acids to oleic and palmitoleic acids, respectively. Increased oleic and palmitoleic acids in VLDL and hepatic gene expression of SCD-1 in the HF/HS group are both suppressed by EPA. It is, therefore, conceivable that increases in the quantity (i.e., secretion rate) and quality (i.e., fatty acid composition) of triglycerides delivered from the liver contribute to triglyceride accumulation and thus obesity in the HF/HS group. Collectively, we speculate that the anti-obesity effect of EPA is due at least in part to its impact on the quantity and quality of triglycerides through the suppression of hepatic lipogenesis.
Lipogenesis is an efficient means to transform the energy accumulated in the body but not to consume energy. Because EPA results in marked reduction of body weight and fat accumulation in the HF/HS group, it is obligatory that EPA consumes energy. Here, we demonstrated that EPA enhances energy consumption and reverses the decreased RER in the HF/HS group. In this regard, Rustan et al. (45) reported that treatment with docosahexaenoic acid + EPA increases RER, but they did not show increased energy consumption. These observations suggest that energy accumulated not as triglycerides is consumed inside and/or outside the liver. Enhanced energy consumption accompanied by enhanced expression of β-oxidation enzymes and UCPs was reported in SCD-1 knockout mice (36) and mice treated with antisense oligonucleotides of SCD-1 (37). In this study, although EPA strongly suppresses hepatic SCD-1 mRNA expression, it does not enhance β-oxidation enzymes and UCPs. There are appreciable changes in gene expression, which suggests the EPA-induced increase in energy consumption. Expression of β-oxidation and glucose oxidation genes in the liver, skeletal muscle, and WAT is not increased by EPA treatment. Moreover, activities of acyl-CoA oxidase and hydroxyacyl-CoA dehydrogenase in the liver and skeletal muscle, respectively, were reduced by EPA, suggesting a minor contribution of β-oxidation to enhanced energy consumption. This discussion is also supported by increased RER by EPA.
Expression of mRNAs for UCPs except UCP-3 in the skeletal muscle is not also increased when treated with EPA. Although UCP-3 mRNA expression in the skeletal muscle is only slightly increased by EPA (experiment 1), EPA fails to enhance expression of UCP-3 mRNA in the skeletal muscle as well as UCP-1 mRNA in BAT in experiment 4, suggesting a minor contribution of UCPs to EPA-induced energy consumption. In this regard, our preliminary data show no significant increase in O2 consumption in the HF + EPA group relative to the HF group (data not shown). These observations, taken together, suggest that the suppressed hepatic lipogenesis is related to enhanced energy consumption in the HF/HS + EPA group. The molecular mechanisms by which EPA enhances energy consumption remain to be elucidated. We also found that EPA only partly restores the otherwise reduced WAT lipolysis in the HF/HS group. Similar to the HF/HS group, β3-AR, TGH-1, and TGH-2 mRNA expression is reduced in the HF group, but EPA fails in the restoration of the reduced gene expression. These observations suggest that EPA restoration of lipolysis is the consequence of the anti-obesity effect of EPA.
The mechanism underlying the anti-obesity effect of EPA has not been extensively studied, and the anti-obesity effect of EPA reported so far is somewhat controversial. Indeed, there is a report showing that EPA does not prevent visceral fat accumulation in rats with HF/HS-induced obesity, where EPA was administered for a much shorter period of time (15). On the other hand, Oh-i et al. (16) reported that EPA promotes body weight gain in rats fed an HF diet through the reduction of brain leptin transport or leptin resistance. The authors used diet without carbohydrate such as sucrose and fructose, when EPA may not exert the anti-obesity effect. In this study, the fatty acid composition was different between the HF/HS and HF diets used. Although the difference might modify the phenotype of our models, this would not invalidate our conclusion on the association between the anti-obesity effect of EPA and suppression of hepatic lipogenesis. In analyzing the anti-obesity effect of EPA, we should be careful of the doses of EPA administered, period of administration, species of animals used, etc., in addition to the proportion of sucrose versus fat in the diet.
Given that hepatic lipogenesis in the HF group was roughly comparable to that in control group, it is likely that hepatic lipogenesis is less associated with HF-induced obesity. This may be related to no appreciable anti-obesity effect of EPA in the HF group, even with a downregulation of hepatic lipogenic genes. In this regard, Kuda et al. (46) reported that treatment with fish oil tends to prevent HF-induced body weight gain, although not with statistical significance. This may be because of the potential difference in the anti-obesity effect between EPA used in this study and fish oil or the doses of administration. On the other hand, EPA reduced hepatic triglyceride in both the HF/HS and HF groups. Rustan et al. (47) reported that EPA inhibits incorporation of other fatty acids into triglyceride, which may explain the EPA-induced reduction of hepatic triglyceride accumulation in the HF group.
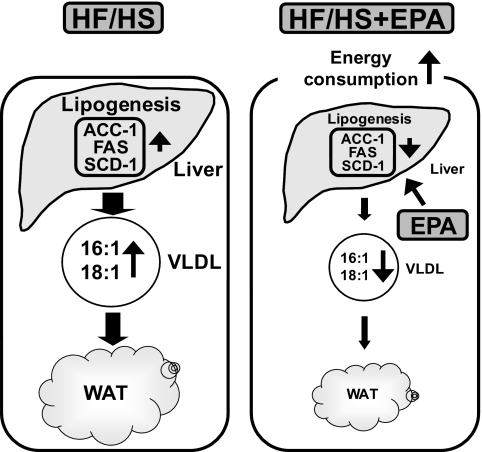
In conclusion, this study is the first demonstration that EPA prevents visceral fat accumulation and obesity, possibly through the suppression of hepatic lipogenesis and enhancement of energy consumption (Fig. 7). Because the metabolic syndrome is often associated with enhanced lipogenesis and steatosis or nonalcoholic fatty liver disease, this study suggests that EPA may be effective to improve visceral fat accumulation and hepatic steatosis in patients with the metabolic syndrome. Our data also suggest that EPA is suited for the treatment of the metabolic syndrome.
FIG. 7.
Possible mechanism underlying the anti-obesity effect of EPA. EPA prevents WAT accumulation in HF/HS-induced obese mice possibly through the suppression of hepatic lipogenesis and enhancement of energy consumption.
Supplementary Material
ACKNOWLEDGMENTS
A.S., H.K., T.N., M.O., M.N., and K.M. are company members of Mochida Pharmaceutical Co. This company sells EPA preparation.
No other potential conflicts of interest relevant to this article were reported.
A.S. researched data, wrote the manuscript, and contributed to discussion. H.K. and Y.O. wrote the manuscript, contributed to discussion, and reviewed/edited the manuscript. T.N., M.O., M.N., K.M., M.I., and T.S. contributed to discussion and reviewed/edited the manuscript.
We thank Takahisa Yamamoto, Yuri Kikuchi, and Keiko Hayasaka for their excellent technical assistance.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement Circulation 2005;112:2735–2752 [DOI] [PubMed] [Google Scholar]
- 2. Matsuzawa Y, Funahashi T, Nakamura T. Molecular mechanism of metabolic syndrome X: contribution of adipocytokines adipocyte-derived bioactive substances. Ann N Y Acad Sci 1999;892:146–154 [DOI] [PubMed] [Google Scholar]
- 3. Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes 2001;50:1844–1850 [DOI] [PubMed] [Google Scholar]
- 4. Kris-Etherton PM, Harris WS, Appel LJ. American Heart Association Nutrition Committee. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002;106:2747–2757 [DOI] [PubMed] [Google Scholar]
- 5. Ando M, Sanaka T, Nihei H. Eicosapentanoic acid reduces plasma levels of remnant lipoproteins and prevents in vivo peroxidation of LDL in dialysis patients. J Am Soc Nephrol 1999;10:2177–2184 [DOI] [PubMed] [Google Scholar]
- 6. Tamura Y, Hirai A, Terano T, Takenaga M, Saitoh H, Tahara K, Yoshida S. Clinical and epidemiological studies of eicosapentaenoic acid (EPA) in Japan. Prog Lipid Res 1986;25:461–466 [DOI] [PubMed] [Google Scholar]
- 7. Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, Petasis NA, Blumberg RS, Serhan CN. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci U S A 2005;102:7671–7676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yamada H, Yoshida M, Nakano Y, Suganami T, Satoh N, Mita T, Azuma K, Itoh M, Yamamoto Y, Kamei Y, Horie M, Watada H, Ogawa Y. In vivo and in vitro inhibition of monocyte adhesion to endothelial cells and endothelial adhesion molecules by eicosapentaenoic acid. Arterioscler Thromb Vasc Biol 2008;28:2173–2179 [DOI] [PubMed] [Google Scholar]
- 9. Mita T, Watada H, Ogihara T, Nomiyama T, Ogawa O, Kinoshita J, Shimizu T, Hirose T, Tanaka Y, Kawamori R. Eicosapentaenoic acid reduces the progression of carotid intima-media thickness in patients with type 2 diabetes. Atherosclerosis 2007;191:162–167 [DOI] [PubMed] [Google Scholar]
- 10. Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K. Japan EPA lipid intervention Study (JELIS) Investigators Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 2007;369:1090–1098 [DOI] [PubMed] [Google Scholar]
- 11. Saito Y, Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K. JELIS Investigators, Japan. Effects of EPA on coronary artery disease in hypercholesterolemic patients with multiple risk factors: sub-analysis of primary prevention cases from the Japan EPA Lipid Intervention Study (JELIS). Atherosclerosis 2008;200:135–140 [DOI] [PubMed] [Google Scholar]
- 12. Satoh N, Shimatsu A, Kotani K, Sakane N, Yamada K, Suganami T, Kuzuya H, Ogawa Y. Purified eicosapentaenoic acid reduces small dense LDL, remnant lipoprotein particles, and C-reactive protein in metabolic syndrome. Diabetes Care 2007;30:144–146 [DOI] [PubMed] [Google Scholar]
- 13. Itoh M, Suganami T, Satoh N, Tanimoto-Koyama K, Yuan X, Tanaka M, Kawano H, Yano T, Aoe S, Takeya M, Shimatsu A, Kuzuya H, Kamei Y, Ogawa Y. Increased adiponectin secretion by highly purified eicosapentaenoic acid in rodent models of obesity and human obese subjects. Arterioscler Thromb Vasc Biol 2007;27:1918–1925 [DOI] [PubMed] [Google Scholar]
- 14. Pérez-Matute P, Pérez-Echarri N, Martínez JA, Marti A, Moreno-Aliaga MJ. Eicosapentaenoic acid actions on adiposity and insulin resistance in control and high-fat-fed rats: role of apoptosis, adiponectin and tumour necrosis factor-α. Br J Nutr 2007;97:389–398 [DOI] [PubMed] [Google Scholar]
- 15. Raclot T, Groscolas R, Langin D, Ferré P. Site-specific regulation of gene expression by n-3 polyunsaturated fatty acids in rat white adipose tissues. J Lipid Res 1997;38:1963–1972 [PubMed] [Google Scholar]
- 16. Oh-I S, Shimizu H, Sato T, Uehara Y, Okada S, Mori M. Molecular mechanisms associated with leptin resistance: n-3 polyunsaturated fatty acids induce alterations in the tight junction of the brain. Cell Metab 2005;1:331–341 [DOI] [PubMed] [Google Scholar]
- 17. Yahagi N, Shimano H, Hasty AH, Amemiya-Kudo M, Okazaki H, Tamura Y, Iizuka Y, Shionoiri F, Ohashi K, Osuga J, Harada K, Gotoda T, Nagai R, Ishibashi S, Yamada N. A crucial role of sterol regulatory element-binding protein-1 in the regulation of lipogenic gene expression by polyunsaturated fatty acids. J Biol Chem 1999;274:35840–35844 [DOI] [PubMed] [Google Scholar]
- 18. Sekiya M, Yahagi N, Matsuzaka T, Najima Y, Nakakuki M, Nagai R, Ishibashi S, Osuga J, Yamada N, Shimano H. Polyunsaturated fatty acids ameliorate hepatic steatosis in obese mice by SREBP-1 suppression. Hepatology 2003;38:1529–1539 [DOI] [PubMed] [Google Scholar]
- 19. Kajikawa S, Harada T, Kawashima A, Imada K, Mizuguchi K. Highly purified eicosapentaenoic acid prevents the progression of hepatic steatosis by repressing monounsaturated fatty acid synthesis in high-fat/high-sucrose diet-fed mice. Prostaglandins Leukot Essent Fatty Acids 2009;80:229–238 [DOI] [PubMed] [Google Scholar]
- 20. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 1957;226:497–509 [PubMed] [Google Scholar]
- 21. Molero JC, Waring SG, Cooper A, Turner N, Laybutt R, Cooney GJ, James DE. Casitas b-lineage lymphoma-deficient mice are protected against high-fat diet-induced obesity and insulin resistance. Diabetes 2006;55:708–715 [DOI] [PubMed] [Google Scholar]
- 22. Nepokroeff CM, Lakshmanan MR, Porter JW. Fatty-acid synthase from rat liver. Methods Enzymol 1975;35:37–44 [DOI] [PubMed] [Google Scholar]
- 23. Osumi T, Hashimoto T. Acyl-CoA oxidase of rat liver: a new enzyme for fatty acid oxidation. Biochem Biophys Res Commun 1978;83:479–485 [DOI] [PubMed] [Google Scholar]
- 24. Hannah VC, Ou J, Luong A, Goldstein JL, Brown MS. Unsaturated fatty acids down-regulate SREBP isoforms 1a and 1c by two mechanisms in HEK-293 cells. J Biol Chem 2001;276:4365–4372 [DOI] [PubMed] [Google Scholar]
- 25. Inoue N, Shimano H, Nakakuki M, Matsuzaka T, Nakagawa Y, Yamamoto T, Sato R, Takahashi A, Sone H, Yahagi N, Suzuki H, Toyoshima H, Yamada N. Lipid synthetic transcription factor SREBP-1a activates p21WAF1/CIP1, a universal cyclin-dependent kinase inhibitor. Mol Cell Biol 2005;25:8938–8947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schweiger M, Schreiber R, Haemmerle G, Lass A, Fledelius C, Jacobsen P, Tornqvist H, Zechner R, Zimmermann R. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J Biol Chem 2006;281:40236–40241 [DOI] [PubMed] [Google Scholar]
- 27. Steiner G, Haynes FJ, Yoshino G, Vranic M. Hyperinsulinemia and in vivo very-low-density lipoprotein-triglyceride kinetics. Am J Physiol 1984;246:E187–E192 [DOI] [PubMed] [Google Scholar]
- 28. Werner A, Havinga R, Bos T, Bloks VW, Kuipers F, Verkade HJ. Essential fatty acid deficiency in mice is associated with hepatic steatosis and secretion of large VLDL particles. Am J Physiol Gastrointest Liver Physiol 2005;288:G1150–G1158 [DOI] [PubMed] [Google Scholar]
- 29. Tanaka T, Yamamoto J, Iwasaki S, Asaba H, Hamura H, Ikeda Y, Watanabe M, Magoori K, Ioka RX, Tachibana K, Watanabe Y, Uchiyama Y, Sumi K, Iguchi H, Ito S, Doi T, Hamakubo T, Naito M, Auwerx J, Yanagisawa M, Kodama T, Sakai J. Activation of peroxisome proliferator-activated receptor delta induces fatty acid beta-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc Natl Acad Sci U S A 2003;100:15924–15929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Son C, Hosoda K, Ishihara K, Bevilacqua L, Masuzaki H, Fushiki T, Harper ME, Nakao K. Reduction of diet-induced obesity in transgenic mice overexpressing uncoupling protein 3 in skeletal muscle. Diabetologia 2004;47:47–54 [DOI] [PubMed] [Google Scholar]
- 31. Wu C, Kang JE, Peng LJ, Li H, Khan SA, Hillard CJ, Okar DA, Lange AJ. Enhancing hepatic glycolysis reduces obesity: differential effects on lipogenesis depend on site of glycolytic modulation. Cell Metab 2005;2:131–140 [DOI] [PubMed] [Google Scholar]
- 32. Madsen L, Petersen RK, Kristiansen K. Regulation of adipocyte differentiation and function by polyunsaturated fatty acids. Biochim Biophys Acta 2005;1740:266–286 [DOI] [PubMed] [Google Scholar]
- 33. Miyazaki M, Flowers MT, Sampath H, Chu K, Otzelberger C, Liu X, Ntambi JM. Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metab 2007;6:484–496 [DOI] [PubMed] [Google Scholar]
- 34. Loftus TM, Jaworsky DE, Frehywot GL, Townsend CA, Ronnett GV, Lane MD, Kuhajda FP. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science 2000;288:2379–2381 [DOI] [PubMed] [Google Scholar]
- 35. Abu-Elheiga L, Oh W, Kordari P, Wakil SJ. Acetyl-CoA carboxylase 2 mutant mice are protected against obesity and diabetes induced by high-fat/high-carbohydrate diets. Proc Natl Acad Sci U S A 2003;100:10207–10212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, Attie AD. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci U S A 2002;99:11482–11486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jiang G, Li Z, Liu F, Ellsworth K, Dallas-Yang Q, Wu M, Ronan J, Esau C, Murphy C, Szalkowski D, Bergeron R, Doebber T, Zhang BB. Prevention of obesity in mice by antisense oligonucleotide inhibitors of stearoyl-CoA desaturase-1. J Clin Invest 2005;115:1030–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Surwit RS, Feinglos MN, Rodin J, Sutherland A, Petro AE, Opara EC, Kuhn CM, Rebuffé-Scrive M. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. Metabolism 1995;44:645–651 [DOI] [PubMed] [Google Scholar]
- 39. Goudriaan JR, Tacken PJ, Dahlmans VE, Gijbels MJ, van Dijk KW, Havekes LM, Jong MC. Protection from obesity in mice lacking the VLDL receptor. Arterioscler Thromb Vasc Biol 2001;21:1488–1493 [DOI] [PubMed] [Google Scholar]
- 40. Gao J, Katagiri H, Ishigaki Y, Yamada T, Ogihara T, Imai J, Uno K, Hasegawa Y, Kanzaki M, Yamamoto TT, Ishibashi S, Oka Y. Involvement of apolipoprotein E in excess fat accumulation and insulin resistance. Diabetes 2007;56:24–33 [DOI] [PubMed] [Google Scholar]
- 41. Mittendorfer B, Patterson BW, Klein S. Effect of sex and obesity on basal VLDL-triacylglycerol kinetics. Am J Clin Nutr 2003;77:573–579 [DOI] [PubMed] [Google Scholar]
- 42. Yamazaki T, Sasaki E, Kakinuma C, Yano T, Miura S, Ezaki O. Increased very low density lipoprotein secretion and gonadal fat mass in mice overexpressing liver DGAT1. J Biol Chem 2005;280:21506–21514 [DOI] [PubMed] [Google Scholar]
- 43. Okada T, Furuhashi N, Kuromori Y, Miyashita M, Iwata F, Harada K. Plasma palmitoleic acid content and obesity in children. Am J Clin Nutr 2005;82:747–750 [DOI] [PubMed] [Google Scholar]
- 44. Paillard F, Catheline D, Duff FL, Bouriel M, Deugnier Y, Pouchard M, Daubert JC, Legrand P. Plasma palmitoleic acid, a product of stearoyl-coA desaturase activity, is an independent marker of triglyceridemia and abdominal adiposity. Nutr Metab Cardiovasc Dis 2008;18:436–440 [DOI] [PubMed] [Google Scholar]
- 45. Rustan AC, Hustvedt BE, Drevon CA. Dietary supplementation of very long-chain n-3 fatty acids decreases whole body lipid utilization in the rat. J Lipid Res 1993;34:1299–1309 [PubMed] [Google Scholar]
- 46. Kuda O, Jelenik T, Jilkova Z, Flachs P, Rossmeisl M, Hensler M, Kazdova L, Ogston N, Baranowski M, Gorski J, Janovska P, Kus V, Polak J, Mohamed-Ali V, Burcelin R, Cinti S, Bryhn M, Kopecky J. n-3 Fatty acids and rosiglitazone improve insulin sensitivity through additive stimulatory effects on muscle glycogen synthesis in mice fed a high-fat diet. Diabetologia 2009;52:941–951 [DOI] [PubMed] [Google Scholar]
- 47. Rustan AC, Nossen JO, Christiansen EN, Drevon CA. Eicosapentaenoic acid reduces hepatic synthesis and secretion of triacylglycerol by decreasing the activity of acyl-coenzyme A:1,2-diacylglycerol acyltransferase. J Lipid Res 1988;29:1417–1426 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.